A multi-year survey of organic disinfection by-products in drinking waters of Castilla y León, Spain. The need and difficulty to comply with the legal limit of 2009†‡
Rafael J.
Garcia-Villanova
*a,
Belén Blanca
Mera
a,
Ana M.
González Paramás
a,
J. Miguel
Hernández Hierro
a,
Ramón Ardanuy
Albajar
b and
Ivania M.
Toruño Fonseca
ac
aDepartamento de Química Analítica, Nutrición y Bromatología, Facultad de Farmacia, Universidad de Salamanca, Campus “Miguel de Unamuno”, E-37007, Salamanca, Spain. E-mail: rgvill@usal.es; Fax: +34 923294515; Tel: +34 923294537
bDepartamento de Estadística, Facultad de Ciencias, Universidad de Salamanca, Spain
cFacultad de Química, Universidad Nacional Autónoma de León, León, Nicaragua
First published on 7th September 2009
Abstract
This article explains the general difficulties of the Spanish source waters and climatic conditions regarding a control of trihalomethanes (THMs), as reflected by the case of Castilla y León, and how the median values of 75 and 163 μg L−1 of years 1999 and 2002 gave way to the more moderate of 31 and 47 μg L−1 of years 2006 and 2007, respectively —both the latter being measured during the warmer season. Particular circumstances such as raw surface water—with frequently high total organic carbon (T.O.C) values—being the source for 80% of population served, the moderate-to-high water temperatures during the warm seasons and the high chlorine dosages frequently applied account for such as high levels. The median global value (n = 98) for raw water T.O.C. was 4.26 mg L−1 (90th percentile of 9.81 mg L−1) and a median T.O.C. removal of 30% was observed during the treatment, but with an enormous variety (a 90th percentile in the order of 70%). Regression analysis associated the variables raw water temperature, prechlorination dosage and raw and finished water T.O.C. with the THMs measured in the finished waters and in the distribution systems. A certain linear correlation exists between THMs and haloacetic acids (HAAs) contents. However, a shift on their profile is noticeable with the temperature of the water, so that above 11.12 °C, THMs concentration tends to be higher than that of HAAs, and vice versa.
Environmental impactDuring recent decades, Spain has recorded in its drinking waters one of the highest concentrations of chlorination by-products within the E.U. A few singularities account for this, as studied by us for the case of Castilla y León. First, the frequent employment of surface waters—some 80% of the population served; this generally entails a high content of natural organic matter in the source waters, which sometimes may be very reactive. Secondly, the climatic conditions, with high water temperatures in the warm seasons, which accelerates the reaction kinetics of chlorine with this complex and varied organic matter. Third, the particular design of the water treatment plants and the way they were operated, usually with very high chlorine dosages. |
Introduction
Before the current Drinking Water Directive of the E.U.1 came into force (1998), and to a lesser extent during the past ten years, an enormous variety of the levels reported for disinfection by-products (DBPs) in drinking water, as trihalomethanes (THMs), has been observed from country to country. The origin of the source water, either surface or ground water, and the climate are determining factors, so that, in general, northern countries exhibit much lower values than southern ones.Broadly speaking, the levels reported within the E.U. have been rather low. In The Netherlands, with a very stringent regulation for DBPs, only 20% of its population is served with chlorinated and/or ozonated water, and the levels of THMs are usually below 20 μg L−1.2 In Germany,3 a survey among 23 utilities treating waters from various sources concluded that 90% of the distribution systems (D.S.) had levels below 25 μg L−1. Two multiyear studies in the Midlands and north of England4 reported mean levels of 46 μg L−1 and an area with a frequent maximum level of 140 μg L−1 that fell to less than 90 μg L−1 over the years. Multiyear studies carried out in Greece5,6 also reported low levels for 15 towns, except for the city of Athens, supplied from surface water, with values sometimes nearing 100 μg L−1. A monitoring of 35 Finnish7 waterworks showed that, surprisingly, mean values for haloacetic acids (HAAs) were of 108 μg L−1 while those for THMs were of 26 μg L−1.
Regarding Spain, a highly documented case is that of Barcelona's drinking water. Because of the high total organic carbon (T.O.C) content and the varying bromide discharges from a salt mine upstream in the River Llobregat source water, up to 715 μg L−1 and 336 μg L−1 THMs in finished and tap waters, respectively, were reported.8 Over 200 μg L−1 were still recorded9 but slowly decreasing over the years to an average of 75 μg L−1 (41 to 122 μg L−1).10 At the other extreme, source water of Madrid11 has the best quality (very low T.O.C.) and, besides this, the pioneering employment of chloramination, some forty years ago, has enabled it to maintain levels below 40 μg L−1. Finally, a study of very different source waters of four provinces12 reported average THM values in drinking waters of the provinces of Barcelona (86 μg L−1) and Alicante (63 μg L−1) against those of Asturias (22 μg L−1) and Tenerife (8 μg L−1), while HAA ranges were generally half.
To study the factors that influence the DBP formation, statistical analysis has been extensively employed, using those parameters upstream at a water system likely to affect it. Varying conclusions have been achieved, mainly depending on the particular composition of the water, the climatic conditions and the treatment.10,13–18 Besides chlorine dose and water temperature, the T.O.C. content and its nature has an enormous influence. When using conventional coagulation, T.O.C. percentage removals from below 30% to 50% have been reported.16,19–22
The current Drinking Water Directive laid down a legal limit of 100 μg L−1 for the sum of four THMs (chloroform, bromodichloromethane, dibromochloromethane and bromoform), allowing a delay in its application until January 2009. Meanwhile an interim value of 150 μg L−1 would be applied from January 2004. This 10 year phase out was sought by the Spanish Government representative23 in light of a report on the exposure of the European population to THMs carried out 3 years earlier.24 The Spanish representative argued the need to introduce major operational changes in the treatment practices for many utilities. No other country requested this.
This article explains the general difficulties of the Spanish source waters and climatic conditions regarding a control of THMs, as reflected by the case of Castilla y León, and how the permanently high levels have been reduced over the years. An abundant set of data was recorded at the first phase of the study for the water systems selected—most of them, but not all, with the highest records of DBP concentrations. The aim of this work has been to try to understand, in real full-scale systems, what conditions can account for these high levels measured. This large data set has permitted a statistical analysis to correlate the physical and chemical raw water characteristics, the climatic conditions and the treatment practices versus the THMs and HAAs measured.
Methodology
Geographical area under study
Castilla y León, with 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 Km2, is the largest region in the E.U. Most of its extension is a plateau, with an average altitude of 850 m, dominated by a continental climate. Winters are cold (average temperature of 4 °C) with frequent night frosts and summers are fairly short and mild (average temperature of 21 °C) and a frequently large gradient of temperature from day to night (some 20 °C in spring). An important part of its soil can be classified as of both prairie and temperate forest, with cereal growing plains, moors and mountains. As is the general case for Spain, surface waters are the source for large and mid-size supplies in Castilla y León, some 80% of the population served.
224 Km2, is the largest region in the E.U. Most of its extension is a plateau, with an average altitude of 850 m, dominated by a continental climate. Winters are cold (average temperature of 4 °C) with frequent night frosts and summers are fairly short and mild (average temperature of 21 °C) and a frequently large gradient of temperature from day to night (some 20 °C in spring). An important part of its soil can be classified as of both prairie and temperate forest, with cereal growing plains, moors and mountains. As is the general case for Spain, surface waters are the source for large and mid-size supplies in Castilla y León, some 80% of the population served.
Sample collection strategies
One of the purposes of this study was to investigate the levels of THMs in drinking waters from a large enough number of populations that employed surface waters as a source, in order to assist the regional Health Authority in following up the adaptation of the water treatment plants (WTPs) for compliance with the forthcoming regulation. The study began in 1999, but was restricted to the capitals (10 WTPs) of the 9 provinces, with an enlargement to a number of 14 towns (15 WTPs) in 2002. A more complete study was performed on the water quality and operational parameters of the WTPs during these years. Since a tendency to include HAAs in regulatory requirements was noticeable by that time, analyses of both THMs and HAAs were performed during the first year of study. In the years 2006 and 2007 a further extension of sample collection was made to a total of 36 water systems, but the study was restricted merely to a monitoring of THMs. The population served was now of 1.4 million, which represents 56% of the region's people and some 70% of the population served with surface waters. The collection of samples in the early years (1999 and 2002) was concentrated principally in the warm and hot seasons, although a few were also taken in winter as a reference. Samples of finished water (at the outlet of WTPs) and from two points of the D.S. related to the WTPs (close and remote points from WTPs) were taken. Since the town of Valladolid is supplied simultaneously from two WTPs, no differentiation can be made from the mixed water of its D.S. Thus, it was taken only from one point of it. Then, in the years 2006 and 2007, the collection was made only in the D.S. and restricted to the peak period of THM formation generally observed by us, i.e. one sample at the end of August and a second one in the middle/end of September.The selection of the exact days of collection was made so as to be as representative as possible of normal operational conditions in each WTP. Thus, samples were never taken during or following infrequently heavy rains—which entails a high runoff of T.O.C. and high doses of chlorine and coagulant. For the same reason, in those WTPs where ozonation was infrequently applied (only a few weeks per year) collection excluded these days.
Overview of treatment plants
At the beginning of the study all the existing plants were designed for a conventional treatment, thus based on a simultaneous pre-chlorination-coagulation-flocculation in pulsator or accelator type clarifiers, a rapid sand filtration and a post-chlorination and pH-correction. Coagulant reagents were based on alum; exceptionally one of the WTPs alternated it with ferric chloride and a minority regularly used polyelectrolyte as a coagulant aid. In addition to chlorine, some of them employed a combination of oxidants, but not permanently—in fact ozone was used for only a few weeks per year. At that time, only two practised filtration with activated carbon. This is an estimation of the population served (in parentheses) with water treated with various disinfectants at the beginning of this study: chlorine gas (60%); ozone + chlorine gas (12%); permanganate + chlorine gas (9.8%); chlorine gas + sodium hypochlorite (8.7%); ozone + chlorine gas + chlorine dioxide (6.5%); sodium hypochlorite 3%; chloramines (0%). Modifications, of which granular carbon filtration is one which has been broadly adopted, were introduced progressively during the ensuing years.Analytical and statistical methods
Duplicate samples of water were taken in two similar containers, i.e. 120 ml amber vials sealed with a rubber plug, one for THMs and the other for HAAs and T.O.C. determinations. A spatula tip of ammonium chloride was added before filling them to quench the reaction, eliminating the residual free chlorine. A 25 ml amber vial was employed for the T.O.C. analysis of raw water. All of them were sent refrigerated and processed before 48 h.Trihalomethanes. A single liquid–liquid extraction with n-pentane into the sampling vial itself, with no head-space, and then analysis by capillary GC/ECD.25 Detection limits are: chloroform (0.53 μg L−1), chlorodibromomethane (0.91 μg L−1), bromodichloromethane (0.37 μg L−1) and bromoform (0.92 μg L−1). Precision, as standard deviation, ranged from 0.082 μg L−1 (for CH3Cl) to 5.92 μg L−1 (for CH3Br), and as variation coefficient from 8.7 to 18.3%, respectively.
Haloacetic acids. The Official Method 6251B26 was employed as modified by us at the extraction step, so that OASIS® HLB Plus/225 mg cartridges from Waters were used for the solid-phase extraction and subsequent elution with methyl-tert-butyl ether. Detection limits are: monochloroacetic MCAA (0.021 μg L−1), dichloroacetic DCAA (0.074 μg L−1), trichloroacetic TCAA (0.015 μg L−1), monobromoacetic MBAA (0.045 μg L−1), dibromoacetic acid DBAA (0.047 μg L−1), tribromoacetic TBAA (0.410 μg L−1) and bromochloroacetic BCAA (0.074 μg L−1). Precision, as standard deviation, ranged from 0.12 μg L−1 (for MCAA) to 5.38 μg L−1 (for DBAA), and as variation coefficient from 8.35 to 19.4%, respectively.
All standards were from Sigma Aldrich and the GC/ECD apparatus was a Hewlett Packard 5890 Series II.
Total organic carbon (T.O.C.). Mineralization at 680 °C and IR measurement in a Shimadzu TOC 5000. A previous sonication was made for raw water. Detection limit: 0.5 mg L−1; precision: 6–10%, as variation coefficient.
Free/total chlorine. Colorimetry with N,N′-diethyl-p-phenylenediamine (DPD) at 515 nm (field measurement).
Periodical recalibrations were made for all the instruments.
The statistical treatment of data was made with the help of the SPSS computer program, 15.0 version.
Results and discussion
A common drawback in all the WTPs studied is that primary disinfection takes place at the same time as coagulation. This, generally, entails a major contribution to the final THM content. However, the efficiency of the T.O.C. reduction during the coagulation and sand filtration will determine on this final content of the finished water and, furthermore, of the D.S. water. Tables 1 and 2 summarise the data set of operational and water quality parameters recorded for the 9 and 14 main locations (15 WTPs), respectively. If we look at the levels of THMs and/or HAAs studied in either of these years, some 8 locations stand out. Before applying statistical regression methods, the following observations are notable. In some cases, both high chlorine dosages and high values of free chlorine are observed. As reported24–27 and observed here, high chlorine dosages were applied in Spain as compared to others reported for Germany,3 Canada,7 Finland16 and even Greece.18| No. sampling days | No. D.S. sampling points | Chlorine doses | Raw water | Finished water | Distribution system | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prechl. | Postchl. | T | pH | TOC | Free chl. | TOC | THM | HAA | Free chl. | TOC | THM | HAA | |||
| mg L−1 | mg L−1 | °C | mg L−1 | mg L−1 | mg L−1 | μg L−1 | μg L−1 | mg L−1 | mg L−1 | μg L−1 | μg L−1 | ||||
| a Preoxidation with KMnO4 and postozonation. b Postdisinfection with ClO2 simultaneously to chlorine. c Pre- and postozonation. d Rechlorination in distribution system. | |||||||||||||||
| Ávila | 8 | 4 | 6.82 (5.60–8.33) | 1.20 (0.47–1.85) | 12.5 (4.5–21.0) | 7.08 (6.47–7.51) | 8.71 (6.89–15.54) | 1.17 (0.83–1.42) | 2.76 (1.77–3.80) | 106 (42–169) | 104 (18–154) | 0.89 (0.09–1.60) | 2.45 (1.57–3.91) | 96 (29–175) | 102 (7–159) |
| Burgosa | 3 | 1 | 0.58 (0.25–1.20) | 0.66 (0.47–0.90) | 12.6 (9–15.6) | 9.51 (9.14–9.33) | 1.35 (0.97–1.71) | 0.46 (0.33–0.61) | 0.89 (0.73–1.08) | 19 (13–30) | 15 (7–23) | 0.36 (0.32–0.43) | 1.32 (0.53–2.54) | 27 (22–30) | 14 (7–21) |
| León | 3 | 2 | 1.81 (1.77–1.86) | 0.85 (0.64–1.00) | 7.8 (5.0–11.3) | 7.95 (7.81–8.14) | 1.99 (1.15–2.84) | 1.08 (0.64–1.36) | 2.04 (1.60–2.68) | 21 (13–27) | 19 (10–26) | 0.90 (0.41–1.63) | 1.77 (1.05–2.73) | 28 (17–38) | 32 (26–39) |
| Palencia | 3 | 2 | 1.57 (1.00–2.00) | 1.00 (1.00–1.00) | 12.0 (4.0–19.0) | 8.01 (7.80–8.34) | 2.25 (1.65–3.40) | 0.24 (0.14–0.40) | 1.21 (0.79–1.99) | 11 (2–15) | 8 (4–12) | 0.67 (0.18–0.98) | 1.24 (0.69–2.24) | 21 (6–44) | 17 (12–25) |
| Salamanca b,d | 8 | 7 | 3.66 (2.10–5.20) | 0.47 (0.35–0.69) | 15.0 (8.0–22.2) | 7.23 (6.57–8.80) | 3.69 (2.64–5.04) | 1.07 (0.39–1.90) | 2.44 (1.41–3.20) | 54 (15–167) | 60 (43–83) | 0.56 (0.04–1.78) | 2.52 (0.21–6.91) | 71 (17–318) | 66 (28–101) |
| Segovia | 3 | 2 | 2.43 (2.20–2.60) | 0.30 (0.10–0.50) | 6.8 (3.0–11.8) | 7.18 (6.93–7.51) | 2.27 (2.09–2.62) | 0.50 (0.34–0.66) | 2.06 (1.81–2.34) | 64 (12–130) | 46 (7–71) | 0.28 (0.04–0.41) | 2.18 (1.65–2.74) | 47 (14–67) | 48 (3–73) |
| Soria c | 3 | 2 | 2.60 (1.80–3.00) | 0.85 (0.70–0.94) | 13.0 (10.0–15.5) | 7.11 (6.91–7.41) | 3.13 (2.34–4.67) | 0.81 (0.72–0.87) | 2.86 (1.83–4.35) | 47 (38–61) | 32 (28–37) | 0.11 (0.03–0.21) | 1.80 (0.99–2.91) | 69 (53–96) | 37 (27–47) |
| Valladolid (WTP-1) | 3 | 1 | 8.00 (3.00–11.00) | 1.10 (0.80–1.50) | 11.0 (3.0–17.0) | 8.29 (8.20–8.40) | 3.96 (2.55–5.88) | 1.17 (0.98–1.46) | 4.99 (2.42–8.55) | 64 (8–130) | 93 (31–130) | 1.16 (0.98–1.49) | 4.06 (1.33–7.07) | 53 (18–70) | 113 (27–180) |
| Valladolid (WTP-2) | 3 | 1 | 2.70 (1.80–3.50) | 0.30 (0.20–0.50) | 11.5 (3.5–17.0) | 8.03 (7.40–8.69) | 3.38 (3.18–3.57) | 1.24 (0.98–1.49) | 2.02 (1.23–3.50) | 54 (4–87) | 52 (23–70) | ||||
| Zamoraa,d | 4 | 2 | 8.25 (4.50–13.00) | 1.63 (1.20–2.00) | 13.9 (6.0–20.5) | 7.93 (7.51–8.21) | 5.61 (4.25–7.93) | 1.35 (1.06–1.65) | 4.85 (2.55–8.91) | 70 (53–114) | 42 (14–115) | 0.35 (0.06–1.27) | 2.85 (1.62–4.56) | 131 (65–287) | 42 (10–129) |
| No. sampling days | No. D.S. sampling points | Chlorine doses | Raw water | Finished water | Distribution system | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prechl. | Postchl. | T | pH | TOC | Free chl. | TOC | THM | Free chl. | TOC | THM | |||
| mg L−1 | mg L−1 | °C | mg L−1 | mg L−1 | mg L−1 | μg L−1 | mg L−1 | mg L−1 | μg L−1 | ||||
| a Preoxidation with KMnO4 and postozonation. b Postdisinfection with ClO2 simultaneously. c Pre- and postozonation. d Rechlorination in distribution system. | |||||||||||||
| Ávila | 5 | 2 | 6.05 (5.30–6.50) | 1.28 (0.72–1.70) | 14.7 (7.4–20.5) | 7.17 (7.02–7.30) | 10.00 (3.91–12.99) | 1.78 (1.50–2.00) | 5.21 (2.65–6.75) | 101 (86–131) | 0.24 (0.00–0.63) | 6.78 (4.74–10.92) | 194 (169–228) |
| Benavente | 4 | 2 | 2.00 (2.00–2.00) | 1.50 (1.50–1.50) | 15.75 (9.0–21.1) | 7.86 (7.78–7.95) | 5.80 (4.99–6.71) | 0.61 (0.50–0.80) | 2.08 (1.15–3.03) | 183 (96–244) | 0.63 (0.46–0.90) | 1.76 (0.29–3.25) | 239 (167–306) |
| Burgos a | 3 | 1 | 0.55 (0.55–0.55) | 0.55 (0.55–0.55) | 15.4 (15.1–15.7) | 8.16 (7.31–8.74) | 1.82 (1.53–2.08) | 0.51 (0.42–0.61) | 1.73 (1.18–2.38) | 29 (15–53) | 0.31 (0.22–0.40) | 1.19 (0.55–1.95) | 58 (45–83) |
| Cuellar | 2 | 1 | — | — | 15 (13.0–17.0) | 7.59 (7.20–7.97) | 3.92 (3.77–4.07) | 0.75 (0.70–0.80) | 3.33 (3.21–3.44) | 48 (47–49) | 0.35 (0.50–0.20) | 3.59 (3.07–4.11) | 66 (58–75) |
| Laguna de Duero | 4 | 1 | 3.78 (0.60–8.00) | 2.08 (0.40–4.00) | 15.1 (8.8–21.0) | 8.55 (8.32–8.79) | 2.98 (1.51–4.87) | 0.77 (0.60–1.09) | 1.52 (0.73–3.10) | 185 (101–265) | 0.50 (0.40–0.70) | 0.78 (0.23–2.29) | 222 (163–315) |
| León | 3 | 1 | 1.98 (1.94–2.00) | 0.77 (0.73–0.83) | 13.5 (13.0–14.5) | 7.71 (7.70–7.71) | 5.11 (4.01–5.95) | 0.51 (0.40–0.63) | 4.20 (3.27–4.76) | 67 (52–86) | 0.60 (0.91–0.40) | 4.55 (3.77–5.02) | 59 (85–40) |
| Medina del Campo | 4 | 1 | 5.15 (3.60–6.50) | 2.05 (1.70–2.50) | 13.7 (7.4–18.0) | 7.59 (7.09–7.82) | 8.62 (7.90–9.67) | 2.35 (1.90–2.70) | 7.35 (6.07–8.39) | 147 (78–261) | 0.53 (0.30–0.70) | 6.95 (5.79–8.28) | 212 (146–368) |
| Palencia | 5 | 2 | 1.63 (1.30–2.00) | 1.13 (1.10–1.20) | 14.1 (8.7–19.0) | 8.06 (7.90–8.22) | 5.52 (3.45–11.12) | 1.04 (0.90–1.39) | 4.83 (2.36–11.86) | 53 (37–71) | 0.63 (0.20–0.92) | 4.65 (2.49–10.73) | 92 (41–135) |
| Salamanca b,d | 5 | 3 | 4.27 (2.10–6.90) | 1.53 (1.40–1.80) | 19.1 (11.0–23.0) | 7.13 (6.89–7.23) | 4.45 (4.28–4.61) | 1.82 (1.10–3.00) | 2.80 (2.61–3.07) | 113 (67–156) | 0.38 (0.10–0.68) | 3.08 (2.27–5.04) | 130 (46–176) |
| Segovia | 3 | 2 | 2.03 (1.25–2.80) | 0.50 (0.50–0.50) | 14.1 (12.6–16.0) | 6.93 (6.00–7.49) | 3.62 (2.51–5.00) | 0.61 (0.40–0.72) | 3.77 (2.97–4.55) | 147 (79–246) | 0.45 (0.20–0.73) | 3.56 (2.51–4.52) | 122 (75–174) |
| Soria c | 3 | 2 | — | 3.6 (3.6–3.6) | 17.9 (15.0–21.1) | 6.80 (6.78–6.82) | 4.98 (4.65–5.30) | 0.59 (0.52–0.66) | 2.96 (2.64–3.20) | 51 (44–57) | 0.24 (0.03–0.40) | 2.67 (1.61–3.22) | 83 (64–102) |
| Valladolid (WTP-1) | 5 | 1 | 6.80 (4.00–12.00) | 2.55 (1.00–5.00) | 15.5 (8.1–21.7) | 8.14 (8.03–8.20) | 4.95 (0.77–11.47) | 0.85 (0.10–1.24) | 1.54 (0.19–3.13) | 182 (42–411) | 0.82 (0.50–0.98) | 4.51 (3.34–5.60) | 160 (56–279) |
| Valladolid (WTP-2) | 5 | 1 | 2.33 (1.10–3.50) | 0.70 (0.60–0.90) | 16.2 (10.7–21.2) | 7.81 (7.55–8.05) | 4.95 (3.49–6.33) | 0.97 (0.80–1.30) | 4.36 (3.22–5.39) | 73 (45–103) | |||
| Venta de Baños | 4 | 2 | 11.0 (4.0–17.0) | 10.8 (3.0–17.0) | 13.7 (8.7–18.0) | 8.10 (7.80–8.41) | 4.76 (1.41–11.05) | 0.83 (0.47–1.00) | 3.18 (2.08–4.01) | 134 (88–225) | 0.86 (0.79–0.90) | 3.49 (1.14–5.36) | 170 (115–260) |
| Zamora a,d | 5 | 2 | 10.50 (8.50–11.0) | 1.70 (1.50–1.75) | 16.6 (8.0–21.7) | 8.03 (7.50–8.60) | 8.23 (2.86–15.09) | 1.08 (0.28–2.01) | 6.31 (2.44–16.05) | 279 (175–371) | 0.12 (0.04–0.21) | 4.72 (2.47–7.44) | 349 (210–553) |
Besides this, in a number of cases, either the high T.O.C. value in raw water (Ávila, Benavente, Medina del Campo and Zamora) or the inefficiency in removing it (Medina del Campo, Valladolid WTP-1 and WTP-2 and Zamora) are expected to contribute to this. The high alkalinity of source water, hardly lowered at any stage, would be a reason for the high records of Laguna de Duero and Benavente even in the autumn, since the rest of the parameters and operational conditions seem reasonable. Finally, it is noteworthy that the water system of Salamanca is the one with the highest raw water temperature (up to 23 °C), a parameter that is expected to contribute to a high level of DBPs. However, this was not the case, since for this water system we had demonstrated14,15 that although the formation is accelerated at the time of higher water temperature (end of summer), a certain removal of THMs must happen at the same time, most probably owing to volatilisation.
In the year 2002, a restriction on the sampling programme was made in order to concentrate it in the seasons in which the climatic, physico-chemical and operational conditions of the waters favoured the production of THMs. Thus, samples were taken between May and November. This is why a general rise in the figures of THMs and parameters such as temperature and T.O.C. and, to a lesser extent, chorine dosages is noticeable (Table 2 and Fig. 1 and 2).
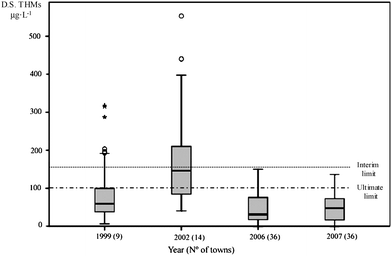 | ||
| Fig. 1 Box-plot depiction of concentrations of THMs in the D.S. of all the water systems (towns) throughout the years of study. Outlier values are indicated as *. | ||
 | ||
| Fig. 2 Box-plot with values of TOC measured in years 1999 (10 WTPs) and 2002 (15 WTPs) for raw water (a) (n = 98), finished water (b) (n = 99) and two points of D.S. (c) (n = 223). Outliers values are indicated as *. | ||
In the years 2006 and 2007 a further extension of sample collection was made to a total of 36 water systems (72 determinations each year), but merely for a monitoring of THMs in the D.S. during the peak period of formation. The evolution of the 15 main water systems studied is depicted in Fig. 3. A dramatic shift in the profile of THMs is noticeable from the year 2002 to 2006–07. The global evolution for the four years of study can be seen in Fig.1, where the outliers and enormous range of values in the years 1999 and 2002 have disappeared in 2006–07. It is noteworthy that all of them were, at any time, within the interim legal limit of 150 μg L−1. Thus, the median THM values of 75 and 163 μg L−1 for the years 1999 and 2002 had given way to the more moderate values of 31 and 47 μg L−1 for the years 2006 and 2007, respectively.
 | ||
| Fig. 3 Evolution of levels of THMs in 14 towns of Castilla y León through the years 2002, 2006 and 2007. For comparison purposes, in this Figure for each year two samples of finished water taken at the time of higher THMs formation have been selected, i.e. one by the end of August (stronger colours) and the other by the middle/end of September (lighter colours). Dotted lines indicate interim and ultimate legal limits allowed until January 2004 and 2009, respectively). | ||
Regarding the speciation, chloroform was the most abundant in all water systems, except in Zamora and Benavente, where bromodichloromethane was in most cases.
T.O.C. values and removal rates
The box-plot of years 1999 and 2002 shows higher T.O.C. contents and wider scattering of values (and outliers) for the latter year (Fig. 2). The average and range for raw water T.O.C. at each WTP is given in Tables 1 and 2. With the exception of four water systems, in general the values are rather high and three of them show average values from 8.23 to 10.00 mg L−1 in the year 2002. As for all the conventional surface WTPs, the coagulation/filtration stage of all those studied here was mostly intended for inorganic turbidity removal and not for softening or for T.O.C. reduction. However, as is generally known, a certain rate of removal is also achieved, especially for the hydrophobic fraction of the natural organic matter. The global data for raw and finished water T.O.C. and percent removal in our two year study are given in Table 3. It can be noticed that the median global value (n = 98) for raw water T.O.C. was 4.26 mg L−1, with a 90th percentile of 9.81 mg L−1. A median T.O.C. removal of 30% (90th percentile in the order of 70%) is achieved, but with an enormous variability—sometimes the process is even responsible for T.O.C. entering the treated water instead of removing it, as denoted by the negative data of Table 3. A most extensive report on this subject was made earlier19 for surface raw waters of 100 WTPs serving 116 million U.S. residents, where the median and 90th percentile T.O.C. was of 3.9 and 8.4 mg L−1, respectively. Using conventional dosages of coagulant and chlorine as the only disinfectant, they reported, in effluent water, a median and 90th percentile T.O.C. of 2.6 and 4.3 mg L−1, which means percentage T.O.C. removals of 33% and 49%, respectively, somewhat comparable to ours. More recently, very similar full-scale systems with T.O.C. water values in the order of 3 mg L−1 have been reported 30%16 and 45%22 T.O.C. removal.| TOC/mg L−1 | Percentage TOC removal (global and by ranges of raw water TOC) | ||||||
|---|---|---|---|---|---|---|---|
| Raw water (n = 98) | Finished water (n = 99) | Global (n = 97) | TOC: <2 mg L−1 (n = 13) | TOC: 2–4 mg L−1 (n = 30) | TOC: 4–8 mg L−1 (n = 37) | TOC: >8 mg L−1 (n = 17) | |
| Media | 5.06 | 3.34 | 24.45 | 5.82 | 11.14 | 33.02 | 43.57 |
| Median | 4.26 | 2.95 | 31.01 | 11.35 | 14.04 | 37.11 | 53.24 |
| S.D. | 3.20 | 2.35 | 43.45 | 38.38 | 56.98 | 30.85 | 32.01 |
| Range | 14.77 | 15.86 | 318.16 | 118.96 | 315.93 | 192.51 | 114.39 |
| Min. | 0.77 | 0.19 | −235.29 | −66.84 | −235.29 | −109.65 | −35.56 |
| Max. | 15.54 | 16.05 | 82.86 | 52.12 | 80.64 | 82.86 | 78.83 |
| 90th percentile | 9.81 | 6.07 | 68.59 | 51.93 | 68.73 | 68.89 | 75.67 |
The percentage reduction of T.O.C. is generally higher with a higher initial T.O.C. content, so that waters with low T.O.C. (2–4 mg L−1) may have a removal of 20–40%, whereas for those with high T.O.C. (>8 mg L−1) removal might be 30–50%, depending on the alkalinity and on the humic content of its organic matter.20 From our study, a separate assessment by ranges of raw water T.O.C. is presented in Table 4. As forecasted, T.O.C. removal efficiency is increased from a median value of 11% to 53% (90th percentiles of 52% to 76%), respectively, as the raw water T.O.C increased.
| Independent variables | Dependent variables | Regression for the global data set |
|---|---|---|
| Raw water TOC | Finished water THMs | n = 98, r = 0.277, p = 0.0057 |
| Raw water TOC | D.S. THMs | n = 220, r = 0.332, p = 0.0000 |
| Finished water TOC | Finished water THMs | n = 223, r = 0.430, p = 0.0000 |
| Prechlorination dosage | Finished water THMs | n = 91, r = 0.399, p = 0.0001 |
| Prechlorination dosage | D.S. THMs | n = 209, r = 0.467, p = 0.0000 |
| Raw water temperature | Finished water THMs | n = 99, r = 0.421, p = 0.0000 |
| Raw water TOC, raw water temperature, prechlorination dosage | Finished water THMs | n = 203, R = 0.567, p = 0.0000 |
| D.S. THMs | n = 203, R = 0.622, p = 0.0000 |
An in-depth study on the aquatic organic matter and on the high THM formation potentials of the raw waters of five of these water systems has been completed recently.28 An advance of results permits the conclusion that either the high T.O.C. values, or the high reactivity of this T.O.C. or a combination of both are major factors that contribute to the high THMs formation potentials found. The abundant prairie soils and, to a lesser extent, the forest soils of the River Duero basin are pointed out as the source of this abundant and/or reactive organic matter.
Regression analyses
In order to search for correlations between THM concentrations and those parameters upstream likely to have an influence on it, linear regression analysis was performed with the data set of the years 1999 and 2002. Fifteen tests were performed for each regression: 14 for each of water systems studied plus a global one for the whole data set. The following 10 variables were tested: raw and finished water TOC; prechlorination and postchlorination dosages; raw water temperature; raw, finished water and D.S. pH; finished water and D.S. free chlorine. First, simple regression tests were made systematically with each independent variable and the dependent finished water THMs and D.S. THMs. Then, those variables with a statistical significance (p < 0.05) were submitted for multiple regression tests with the same dependent variables. In Table 4 are shown the variables that correlated with p < 0.05 in both simple and multiple regression tests for the global set of data. A high level of significance was obtained for both simple and multiple linear regressions. Stepwise regression found a simultaneous linear correlation of the three variables (raw water TOC, raw water temperature and prechlorination dosage) with both finished (R = 0.567) and D.S. (R = 0.622) water THMs, in both cases with a confidence level of p = 0.0000. This is in agreement with the conclusions reported by other authors.10,13–17In spite of the small set of individual data for each of the 14 water systems, significant correlations were also found in a number of them when they were tested separately for linear regression. The parameters raw water temperature (of 6 water systems), prechlorination dosage (of 3 water systems) and finished water TOC (of 4 water systems) were found to correlate (p < 0.05) with the THMs measured in finished and D.S. Waters. Raw water TOC correlated with p > 0.05, no doubt owing to the low number of cases. Regarding the application of multiple regression tests, 9 water systems were found to select from one to three variables, the most frequent being again raw water temperature (6 water systems, R = 0.676 through 0.850), followed by postchlorination dosage (3 water systems, R = 0.623 through 0.952) and finished or D.S. water TOC (2 water systems, R = 0.623 through 0.965).
For years, it has been said that THMs are the most abundant group of DBPs, followed by HAAs—concentrations of which are said to be almost permanently half of those of THMs. More recently, it has been reported that the rate of THMs to HAAs may vary significantly depending on the water system and the season, so that they may not be always correlated.22,29 An exploration of this was tried for the data from the year 1999. Since a certain interconversion30,31 is believed to exist between them from formation to D.S., the data for THMs and HAAs in finished water (FWTHMs, FWHAAs) and D.S. (DSTHMs and DSHAAs), expressed in μg L−1, were employed separately and the following two equations were obtained:
| FWTHMs = 31.8183 + 1.2021 FWHAAs (n = 41, r = 0.6146, p = 0.0000) |
| DSTHMs = 67.1536 + 0.6778 DSHAAs (n = 129, r = 0.3108, p = 0.0003) |
Somewhat different equations were obtained for the cases of Salamanca (n = 56, r = 0.2770, p = 0.0391) and Segovia (n = 6, r = 0.9710, p = 0.0013).
Since a tendency to higher rate of THMs vs. HAAs was observed by the summer (see Fig. 1), linear regression was tested for the difference (THMs minus HAAs) vs. temperature. The following equation was obtained:
| (THMs − HAAs) = −33.8008 + 3.0386T (n = 167, r = 0.3071, p = 0.0000), |
so when this difference is zero, T = 11.12 °C. Consequently, at temperatures below 11.12 °C, the HAA concentration is higher than that of THMs, and vice versa. In our study the number of cases above and below this temperature were n = 99 and 68—a balanced number of cases. This result would suggest that a higher temperature enables chemical and/or microbial decomposition of HAAs into THMs, one of the mechanisms reported to contribute to the THMs formation.
Conclusions
At the moment that the current Directive 98/83/CE came into force, very high levels of THMs and HAAs were frequently present in Spanish drinking waters and particularly in the region of Castilla y León. Special circumstances, such as raw surface water (with frequently high T.O.C. values) being the source for 80% of the population served, the moderate-to-high water temperatures during the warm seasons and the high chlorine dosages frequently applied, account for such high levels. These enormous values have been reduced over the years.The median global value (n = 98) for raw water T.O.C. at the 15 WTPs of our two year study was 4.26 mg L−1. T.O.C. removal efficiency was found to be of 30%, but with an enormous variability and this removal was increased as the raw water T.O.C was increased.
Simple and multiple regression analyses showed that the variables raw water temperature, prechlorination dosage and raw water TOC were correlated with the presence of THMs with a varying regression coefficient, but with a high level of significance. A certain correlation exists between THM and HAA contents. However, a shift on their profile is noticeable with the temperature of the water, so that above 11.12 °C the THM concentration tends to be higher than that of HAA, and vice versa. A hypothesis is given that a higher temperature enables chemical and/or microbial conversion of HAAs into THMs.
Acknowledgements
This article is a part of the results of two research projects financed by the Plan Nacional de I + D (Ref. AMB95-0508) and by the Consejería de Educación (Junta de Castilla y León). The authors want to emphasise also their gratefulness to the Consejería de Sanidad for the complimentary support and especially to Mr Enrique Estrada and the Environmental Health Inspectors for the collection of samples in the years 2006 and 2007. Thanks are also given to the firms and staff of the WTPs for their collaboration during years 1999 and 2002: AQUAGEST (Zamora), Aguas de Valladolid and AQUALIA (Ávila and Salamanca).References
- EEC, 1998, Council Directive 98/83/EC of 3 November 1998. Official Journal of the European Communities, L 330/32, 5.12.98.
- A. M. van Dijk-Looijaard and J. van Genderen, Food Chem. Toxicol., 2000, 38, S37 CrossRef CAS.
- U. Müller, Specialized conference on drinking water distribution with or without disinfectant residual., International Water Services Association. Mülheim an der Ruhr, Germany, 1998 Search PubMed.
- T. Keegan, H. Whitaker, M. T. Nieuwenhuijsen, M. B. Toledano, P. Elliott, J. Faweell, M. Wilkinson and N. Best, Occup. Environ. Med., 2001, 58(7), 447 CrossRef CAS.
- S. K. Golfinopoulos, Chemosphere, 2000, 41, 1761 CrossRef CAS.
- A. A. Kampioti and E. G. Stephanou, Water Res., 2002, 36, 2596 CrossRef CAS.
- T. K. Nissinen, I. T. Miettinen, P. J. Martikainen and T. Vartiainen, Chemosphere, 2002, 48, 9 CrossRef CAS.
- F. Ventura and J. Rivera, Bull. Environ. Contam. Toxicol., 1985, 35, 73 CrossRef CAS.
- J. L. Armenter, J. M. Fernández, XXIII Jornadas Técnicas AEAS (1). Asociación Española de Abastecimientos de Agua y Saneamiento, Salamanca, Spain, 2003 Search PubMed.
- S. Platikanov, X. Puig, J. Martín and R. Tauler, Water Res., 2007, 41, 3394 CrossRef CAS.
- F. Ramírez Quirós, XXIII Jornadas Técnicas AEAS (1), Asociación Española de Abastecimientos de Agua y Saneamiento, Salamanca, Spain, 2003 Search PubMed.
- C. M. Villanueva, M. Kogevinas and J. O. Grimalt, Water Res., 2003, 37, 953 CrossRef CAS.
- H. Arora, M. W. Lechevallier and K. L. Dixon, J. Am. Water Works Assoc., 1997, 89(6), 60 CAS.
- R. J. Garcia-Villanova, C. Garcia, M. P. Garcia, J. A. Gomez and R. Ardanuy Albajar, Water Res., 1997, 31, 1299 CrossRef.
- R. J. Garcia-Villanova, C. Garcia, M. P. Garcia, J. A. Gomez and R. Ardanuy Albajar, Water Res., 1997, 31, 1405 CrossRef CAS.
- M. J. Rodríguez and J. B. Sérodes, Water Res., 2001, 35(6), 1572 CrossRef CAS.
- R. J. García-Villanova, B. Blanca Mera, A. González Paramás, J. A. Gómez Bárez and R. Ardanuy Albajar, Journal Européen d'Hidrologie, 2001, 32(1), 55 Search PubMed.
- S. K. Golfinopoulos and G. B. Arhonditsis, Chemosphere, 2002, 47, 1007 CrossRef CAS.
- S. W. Krasner, D. M. Owen, J. E. Cromwell, in: Water disinfection and natural organic matter. Characterization and control., ed. Roger A. Minear and Gary L. Amy, ACS Symposium Series, 1996, 649, pp. 11–23 Search PubMed.
- M. C. White, J. D. Thompson, G. W. Harrington and P. C. Singer, J. Am. Water Works Assoc., 1997, 89(5), 65.
- K. Bel-Ajy, M. Abbaszadegan, E. Ibrahim, D. Verges and M. LeChevalier, J. Am. Water Works Assoc., 2000, 92(10), 45.
- M. J. Rodríguez, J. B. Sérodes and P. Levallois, Water Res., 2004, 38, 4367 CrossRef CAS.
- M. Palau, Spanish Government representative for the negotiations of Directive 98/83/CE, personal communication, 1998.
- Joint Research Centre, Exposure of the European Population to Trihalomethanes in Drinking Water (2). Luxembourg Office for Official Publications of the European Communities, Luxembourg, 1997 Search PubMed.
- C. García, P. G-Tiedra, A. Ruano, J. A. Gómez and R. J. García-Villanova, J. Chromatogr., A, 1992, 605, 251 CrossRef CAS.
- Awwa-Apha-Wep, Official Method 6251B. Standard Methods for the Examination of Water and Wastewater, ed. L. S. Clesceri, A. E. Greenberg and A. D. Eaton, Washington, DC, USA, 19th edn, 1995 Search PubMed.
- European Environment Agency and WHO Regional Office for Europe, Water and Health in Europe, WHO Regional Publications, European Series No. 93, 2002 Search PubMed.
- I. M. Toruño Fonseca, PhD thesis, Universidad de Salamanca, 2007.
- K. J. Lee, B. H. Kim, J. E. Hong, H. S. Pyo, S.-J. Park and D. W. Lee, Water Res., 2001, 35(12), 2861 CrossRef CAS.
- X. Zhang and R. Minear, Water Res., 2002, 36, 3665 CrossRef CAS.
- W. Bayless and R. Andrews, Journal of Water and Health, 2008, 6(1), 15 Search PubMed.
Footnotes |
| † Part of a themed issue dealing with water and water related issues. |
| ‡ Electronic supplementary information (ESI) available: Map of Castilla y León, Spain. See DOI: 10.1039/b911269c |
| This journal is © The Royal Society of Chemistry 2010 |
