Changes in abundance of heterotrophic and coliform bacteria resident in stored water bodies in relation to incoming bacterial loads following rain events†
Anthony Richard
Martin
,
Peter John
Coombes
,
Tracey Lee
Harrison
and
R.
Hugh Dunstan
*
School of Environmental and Life Sciences, University of Newcastle, NSW, Australia. E-mail: hugh.dunstan@newcastle.edu.au
First published on 9th June 2009
Abstract
Microbial properties of harvested rainwater were assessed at two study sites at Newcastle on the east coast of Australia. The investigation monitored daily counts of heterotrophic bacteria (HPC), total coliforms and E. coli during a mid-winter month (July). Immediately after a major rainfall event, increases in bacterial loads were observed at both sites, followed by gradual reductions in numbers to prior baseline levels within 7 days. Baseline HPC levels ranged from 500–1000 cfu/mL for the sites evaluated, and the loads following rain peaked at 3590–6690 cfu/mL. Baseline levels of total coliforms ranged from 0–100 cfu/100 mL and peaked at 480–1200 cfu/100 mL following rain. At Site 1, there was no evidence of E. coli loading associated with the rain events assessed, and Site 2 had no detectable E.coli colonies at baseline, with a peak load of 17 cfu/100 mL following rain which again diminished to baseline levels. It was concluded that rainfall events contributed to the bacterial load in rainwater storage systems, but processes within the rainwater storage ensured these incoming loads were not sustained.
Introduction
Australia is one of the driest of inhabited continents that is subject to cycles of higher and lower rainfall patterns. It has one of the most variable rainfall patterns in the world. Much of Australia's water infrastructure has been developed during periods when rainfall was plentiful and supply could easily meet demand. Lower rainfall regimes that were consistent with the cycle of droughts in Australia, economic development and population growth with subsequent increases in urban development has placed immense pressure on regional urban water resources reliant on rainfall runoff into inland dams.1 Most Australian cities have recently been, or currently are, subject to water restrictions. The harvesting of roof-collected rainwater has been highlighted as a means of reducing demand at the allotment scale, increasing regional water security and providing economic benefits to the community.2 With recently introduced subsidies towards retrofitting rainwater harvesting systems in urban settlements, many local government authorities have also made rainwater tanks mandatory on all newly constructed and renovated dwellings.Recent studies have given significant insight into the microbial and chemical characteristics of stored rainwater and collected roof runoff.3–15 Although water quality was shown to differ significantly based on various geographical, environmental and tank-design factors, none of the investigations looked at the microbial ecosystem on a continuous basis for defined periods of time.
Assessments based on random, snapshot or event based sampling may falsely represent bacterial loads within stored water. The micro-ecological dynamics of the system may alter survivability of incoming bacteria by nutrient deprivation and competitive exclusion. Assessments of discrete bacterial loads do not provide understanding of the survivability of bacteria entering the system and the processes that may act to regulate the survival of bacteria. A recent study in 2008 by Han and Mun16 investigated particulate sedimentation rates within two rainwater storage tanks, and found as retention time increased, particle numbers decreased. Evidence of a phenomenon that acts to improve harvested rainwater quality has also been proposed.4,7,17
This study sampled water from two rainwater tanks as unique case studies to investigate the changes in abundance of heterotrophic and coliform bacteria resident in the stored water bodies in relation to incoming bacterial loads following rain events over a 31 day period of daily sampling. Collected waters from one rainwater harvesting system were effectively unused over the period. The other system was integrated into the mains water supply for the house with mains water trickle top up of the tank when rainwater supplies were low, and an often high rate of daily water use. The project set out to determine whether rainfall provided a mechanism to facilitate bacterial loading of stored rainwater, and whether or not any increases in bacterial numbers were sustainable after rainfall events. The typically low nutrient microbial ecosystem in rainwater tanks was expected to be characterised by a dynamic and diverse community of bacteria.18 This would suggest an equilibrium composition of bacteria with magnitude of numbers tightly governed by physical conditions and a high level of resource competition.
Materials and methods
Study site location
Both study sites were residential dwellings located in Newcastle, on the eastern coast of Australia and were approximately 10.7 kilometres apart. Table 1 outlines several key design characteristics of the study sites.| Site 1 | Site 2 | |
|---|---|---|
| Catchment surface/age | Galvanised Iron, 30 + yrs | Concrete tile, 5 yrs |
| RWT construction/size | 2 × 2.2 kL interconnected tanks, galvanised iron | 2 kL tank lined with food grade polymer, Colourbond™ steel |
| RWT age | 4 yrs | 6 months |
| Supply | All household water demand | Outdoor use only |
| Mains top-up | YES | NO |
| First-flush device | NO | NO |
The tank configuration at Site 1 was a dual water supply system with a mechanical mains water top-up that initiates when water storage levels in the tank fall below a depth of 0.4 m. The tank tops up with mains water to a maximum water level of 0.4 m when rainwater levels are drawn below 0.4 m. Installed water meters in the rainwater supply system on the downstream side of the pump and in the mains water top up system were used to determine onsite mains and rainwater use. The basic configuration at Site 2 consisted of a stormwater grade downpipe feeding directly from the roof catchments' surface into the storage. This second site did not have a mains water top-up system or water use meters. Both study sites had trees overhanging the roof catchment area, with growth at Site 1 being more prolific and within a closer proximity to the roof catchment area.
Sample collection and microbial analysis
Rainwater samples from each of the tanks base outlets and surface levels were collected daily from each of the two study sites for a period of 31 days comprising a total of 124 samples. Site 1 was a rainwater storage system that was subject to infrequent top up with chlorinated mains water and was fully integrated to supply of all water uses, whilst Site 2 had minimal water usage with no input of mains water. A total of 250 mL of harvested rainwater was collected directly from the top layer of the water column and the nearest point of supply from the tanks base outlet. Collected base outlet samples from Site 1 were taken from the dwellings outdoor tap approximately five meters from the rainwater tank and serviced with a pump via galvanised iron plumbing. Base outlet collections for Site 2 were gravity fed directly from the base of the tank without the intervention of a pump.Collected rainwater samples were transported to the laboratory in a chilled-cold box and processed within two hours of collection. Tank water temperature data was recorded daily from both sampling points with the aid of a portable Navi D-54 pH/Temperature meter (Horiba Ltd, Japan). Concentrations of total coliform bacteria and E. coli were determined using the m-ColiBlue24® membrane filtration system (Millipore, Cat #M00PMCB24, Bedford, Massachusetts). One hundred millilitres of each sample were filtered in triplicate onto cellulose esters membranes via vacuum filtration. The membranes were then incubated for 24 hours within sterile petri dishes containing absorbent pads soaked with 2 mL of m-ColiBlue24® broth. The subsequent culture of colonies indicative of E. coli appeared blue in colour, whilst total coliforms appeared red. Figs. 3 & 4 (see Results and discussion section) represent average values obtained for each sampling day for Total coliforms and E. coli respectively. The 1st July m-ColiBlue sample from the Site 1 tap outlet was lost due to misadventure.
Total heterotrophic plate counts (HPC) were also determined for each collection at incubation temperatures of both 25 °C and 37 °C. Briefly, a total of 1mL of rainwater was aseptically plated in replicate from the base and surface levels of the water tank, onto nutrient agar (Oxoid, Australia) and incubated at 25 °C and 37 °C for a maximum of 48 hrs. The plate replicates for each incubation temperature and sampling level were counted with daily average values presented in Table 2 (see Results and discussion section).
All climatic data were obtained from the Australian Bureau of Meteorology website found at http://www.bom.gov.au. The source of mains ‘top-up’ water at Site 1 was not subject to any of the bacterial investigations.
Results and discussion
Fig. 1 illustrates the storage levels for both tanks over the sampling period as well as the monitored usage and mains water top-ups for Site 1. The storage level data showed that the utilisation of harvested rainwater varied considerably between the study sites where water from the tank at Site 1 was used to supply all household demand with an average volume of 233 ± 108 L used daily. In contrast, the volume of harvested rainwater at Site 2 was sustained at 100% of tank capacity for the first 18 days of sampling and did not fall below 80% of tank capacity during the study. Average daily temperature measurements from the tap outlet level were 11.3 ± 1.5 °C and 10.1 ± 1.2 °C for Site 1 and Site 2 respectively.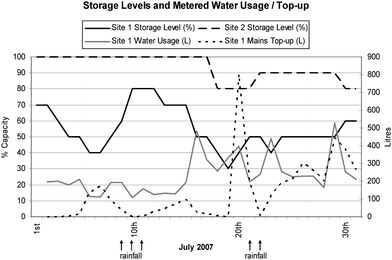 | ||
| Fig. 1 Rainwater storage capacity levels at both sites and daily metered water usage and mains water top-up volumes for Site 1. Arrows indicate days when rainfall was recorded as shown in detail in Fig. 2. | ||
Current Australian Drinking Water Guidelines (ADWG) do not make recommendations for maximum allowable concentrations of HPC as it is of little sanitary value. However, HPC is often used to determine the integrity of a distribution system or the efficacy of water treatment. Significant increases in heterotrophic bacterial counts may be an early indicator of contamination. Similarly, there are no current Australian guideline values for total coliform bacteria in drinking water (although levels of E. coli are used) as recommended by the ADWG due to their common inhabitancy of soil and water. The determination of coliform bacteria in harvested rainwater is often performed for operational monitoring regimes, and can be indicative of faecal contamination or the structural failing of a storage system in contact with surrounding soil.
Heterotrophic plate count
The HPC microbial analyses of the water samples from both sites revealed that there was a consistently greater morphological diversity of colony types observed following incubation at 25 °C compared with incubations at 37 °C. Furthermore, there was a 5-fold increase in bacterial counts when samples were incubated at 25 °C compared with 37 °C. These results suggested that the prevalent bacteria in the water tanks were better suited to growth at the lower temperatures. Therefore, all the analyses of HPC reported for this investigation represent data acquired from the 25 °C incubations. This outcome was interpreted to reflect that most of the bacteria in the rainwater tank ecosystems consisted of those adapted to lower temperature regimes consistent with the relatively stable temperature range of the contained water body and are likely of an environmental origin. These bacteria would presumably be more readily able to survive the oligotrophic, nutrient limited conditions that prevailed in the water tank environment due to the lack of light and restricted entry of particulate organic matter, insects and animals.Both study sites were subject to similar climatic weather patterns as shown in Fig. 2, with July's monthly rainfall totalling 19 mm and 29.4 mm for Sites 1 and 2 respectively. The impact of rainfall was clearly reflected by an insurgence of bacteria within the stored rainwater at both study sites. Rainfall encountered on the 9th of July following an eight day dry weather interval saw HPC values increase by 118% and 143% from the surface and base levels at Site 1, and 317% and 138% at Site 2 respectively. These increases in microbial abundance were attributed to a direct load from roof runoff into the tanks. Post-rainfall dry periods saw a reduction in bacterial populations to pre-rainfall concentrations at both sites within 7 days. During the 31 day period, at least one event of elevated bacterial counts was observed in the skim samples from either tank at separate times independent of rainfall. The origin of this bacterial load was not clear, but it was not detected in subsequent skim samples, nor was it detected in the base level samples. The observed elevations in bacterial numbers at the water surface of the storages may be associated with small inflows of condensation from the roof catchments, and inner tank surfaces.
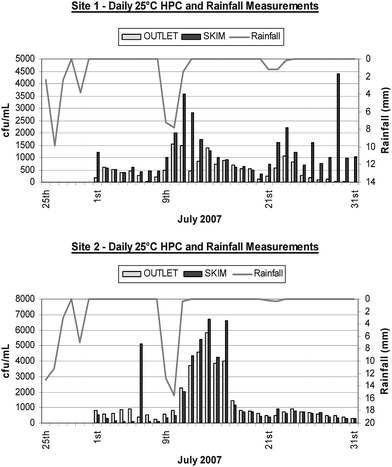 | ||
| Fig. 2 Daily heterotrophic plate counts for both surface skim samples and the outlet taps for both sites recorded from samples incubated at 25 °C. | ||
Total coliforms
The incidence of rain events coincided with an increase in numbers of total coliforms observed in the skim samples for both study sites as shown in Fig. 3. These patterns were consistent with an increased load derived from the collection of roof runoff entering the storage system. Bacterial loads may have originated from the collection surfaces and/or the falling rain as suggested by Evans et al. (2006).13 The counts of total coliforms from tank outlet samples at Site 2 were similar to those observed in the skim samples and both gradually declined towards a base level of counts after the rainfall events. However, at Site 1, counts at the tank outlet were substantially lower than the counts from skim samples, which may have resulted from a dilution effect via the regular throughput of water and from chlorine disinfection provided by the mains water top-up used for the integrated supply of water.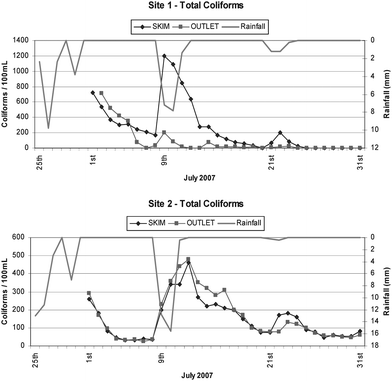 | ||
| Fig. 3 Total coliform counts from both the skim and tap outlet samples from the rainwater tanks at both sites. | ||
Although many of the bacteria grouped as “total coliforms” can be found in the intestinal tract of humans and other animals, the coliform group of bacteria comprise a broad range of microorganisms that are ubiquitous in aqueous and soil environments. Many of the bacteria included in the coliform group have been regarded as non-faecal coliforms.19 The recovery of total coliforms may indicate the presence of organic material such as decaying vegetation as a source of probable contamination.
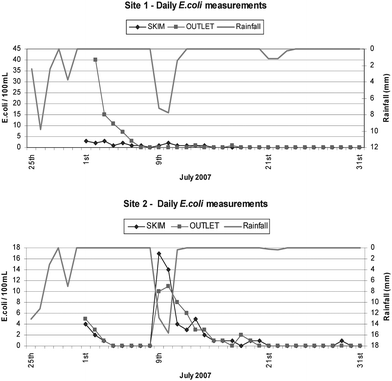 | ||
| Fig. 4 Levels of the faecal indicator organism E. coli from both the skim and tap outlet samples from the rainwater tanks at both sites. | ||
Despite the influx of continued rainfall events, E. coli proved incapable of establishing itself as a resident member of the stored bacterial community under these climatic conditions. These results suggested that the stored rainwater did not provide a sustainable environment for the propagation of the introduced E. coli. In fact, the load of viable bacterial contaminants appeared to be quickly eliminated via a decay process involving either removal from the water body (sedimentation or cell death) or conversion to non-culturable forms of bacteria. The net outcome would appear to represent an equilibration of microbial communities within the stored rainwater to maintain a viable stasis under oligotrophic conditions. Presumably the E. coli were less well adapted to grow under the nutrient limited conditions than the resident heterotrophic bacteria.
Local guideline values in Australia stipulate E. coli to be absent from all drinking waters, as it's regarded the most specific indicator of recent faecal contamination. In this study, E. coli was present from the base outlet on fewer occasions at Site 1 compared to Site 2 with growth observed on seven, and thirteen of the 31 sampling days respectively (see Table 2). The activities of birds and other small animals have generally been speculated to be the principal source of faecal contamination of tank water. This was not assessed in the present study, however, the data indicated that the E. coli were not always present as a component of bacterial load, even when total coliform numbers were high for a given event. This would suggest that the observed total coliforms may have originated from environmental sources such as soil/organic debris, and not from direct faecal contamination. Interestingly, the household occupants at Site 1 did not report episodes of gastrointestinal illness resulting from the consumption of rainwater in the four years since the installation of the onsite tanks. Such an observation reflects findings reported by the Australian government's “Guidance on the use of Rainwater tanks” where despite the prevalence of faecal bacteria, reports of illness associated with rainwater tanks are relatively infrequent.20
| Heterotrophic Plate Count | Total Coliforms | E. coli | ||||||
|---|---|---|---|---|---|---|---|---|
| cfu/mL | cfu/100 mL | cfu/100 mL | ||||||
| Base | Surface | Base | Surface | Base | Surface | |||
| 25 °C | 37 °C | 25 °C | 37 °C | |||||
| Site 1 | 511 ± 427 | 80 ± 67 | 1198 ± 956 | 198 ± 132 | 85 ± 174 | 260 ± 326 | 2.52 ± 7.77 | 0.65 ± 0.91 |
| f = 7/31 days | ||||||||
| Site 2 | 1304 ± 1460 | 298 ± 254 | 1473 ± 2034 | 110 ± 88 | 158 ± 132 | 147 ± 108 | 1.77 ± 3.07 | 1.87 ± 3.91 |
| f = 13/31 days | ||||||||
Harvested rainwater usage and mains water top-up
The rainwater tank configuration at Site 1 included a mechanical mains water top-up system. Input was measured on a daily basis with the use of a water meter fitted directly to the dual tank supply as illustrated in Fig. 1. The basic tank configuration at Site 2 did not have meters for the determination of water usage. However, the rainwater tank was at full capacity prior to the commencement of sampling, and allowed approximate usage to be gauged by incremental reductions of 10% (∼185 L) from maximum capacity levels. The mains water top-up system at Site 1 operated on 26 of the 31 sampling days with a peak input of 803 L on the 20th July.Although the impact of chlorinated water on the integrity of a bacterial community can be difficult to measure, the results of this investigation showed a clear reduction in heterotrophic bacteria from the tanks point of supply on days where >200 L of mains water was infused (see 20th, 24th–31st July in Fig. 2). This reduction in resident flora was not observed for skim level measurements, and despite an average mains water input of 282 ± 89 L for the final eight days of sampling, heterotrophic bacterial measurements appeared to exceed baseline microbial concentrations for previous dry weather intervals when little to no mains water was added. The low numbers of total coliforms and heterotrophs observed during the last 7 days from base level collections at Site 1, may represent a dilution effect from the continual mains top up as well as a potential influence of the chlorine on susceptible bacteria. It is proposed that the distribution of bacteria in the water tank would not be uniform due to stratification based on incoming water densities and particle distributions.16
Finally, the relative density of dissolved solids in mains water to harvested rainwater may see the more dense mains water (top up) concentrating towards the base of the tank, influencing population dynamics at the lower level.
Conclusions
The results of this investigation suggest roof runoff generated from rainfall events acted as a primary source of bacterial loading of the rainwater tanks at the study sites. Bacterial numbers, including total coliforms, increased following the harvest of roof runoff but these numbers declined to baseline levels at the temperature regimes of the mid-winter month. The faecal coliform indicator, E. coli, was not always delivered into the stored waters during rainfall events, and when it was, its presence was found to be unsustainable.At an allotment scale, the carriage of heterotrophic bacteria via rainfall towards storage is unavoidable, regardless of geography or location. The occupants at Site 1 have since pruned or removed branches overhanging the catchment area to minimise the accumulation of organic debris which might support the growth of bacteria. Consideration of ‘point of use’ devices such as tap water filters would also be encouraged to address the occasions when contamination did occur. Although the parameters of this winter study saw both sites unable to support microbial expansions in storage post rainfall, future research is needed to investigate this phenomenon in warmer conditions and diverse locations.
Acknowledgements
Anthony Martin received a joint Industry/Newcastle University Research Scholarship. This work was jointly funded by the University of Newcastle and BlueScope Water.References
- P. J. Coombes and M. E. Barry, The relative efficiency of water supply catchments and rainwater tanks in cities subject to variable climate and the potential for climate change, Australian Journal of Water Resources, 2008, 12, 85–100 Search PubMed.
- P. J. Coombes, G. Kuczera and J. D. Kalma, Economic, water quantity and quality impacts from the use of a rainwater tank in the inner city, Australian Journal of Water Resources, 2003, 7, 101–110 Search PubMed.
- B. N. Uba and O. Aghogho, Rainwater quality from different roof catchments in the Port Harcourt district, Rivers State, Nigeria, J. Wat. Suppl.: Res. & Technol – AQUA, 2000, 49, 281–288 Search PubMed.
- P. J. Coombes, G. Kuczera, J. D. Kalma and R. H. Dunstan, Rainwater quality from roofs, tanks and hot water systems at Figtree Place, Proceedings of the 3rd International Hydrology and Water Resource Symposium, Perth, Australia, 2000 Search PubMed.
- G. Simmons, V. Hope, G. Lewis, J. Whitmore and G. Wanzhen, Contamination of potable roof-collected rainwater in Auckland, New Zealand, Water Res., 2001, 35, 1518–1524 CrossRef CAS.
- D. J. Lye, Health risks associated with consumption of untreated water from household roof catchment systems, Journal of the American Water Resources Association, 2002, 38, 1301–1306 Search PubMed.
- A. T. Spinks, P. Coombes, R. H. Dunstan and G. Kuczera, Water quality treatment processes in domestic rainwater harvesting systems, Proceedings of the 28th International Hydrology and Water Resources Symposium, Wollongong, Australia, 2003 Search PubMed.
- A. Vasquez, M. Costoya, R. M Pena, S. Garcia and C. Herrero, A rainwater quality monitoring network: a preliminary study of the composition of rainwater in Galicia (NW Spain), Chemosphere, 2003, 51, 375–386 CrossRef.
- M. Chang, M. W. McBroom and R. S. Beasley, Roofing as a source of nonpoint water pollution, Journal of Environmental Management, 2004, 73, 307–315 CrossRef.
- R. H. Kim, S. Lee, S. K. Kim and S. G. Kim, Pollutants in rainwater runoff in Korea: Their impacts on rainwater utilization, Environ. Technol., 2005, 26, 411–420 CrossRef CAS.
- E. L. Villarreal and A. Dixon, Analysis of a rainwater collection system for domestic water supply in Ringdansen, Norrkoping, Sweden, Building and Environment, 2005, 40, 1174–1184 CrossRef.
- S. E. Abbott, J. Douwes and B. P. Caughley, A survey of the microbiological quality of roof-collected rainwater of private dwellings in New Zealand. Proceedings of the Water 2006 International Conference, Auckland, New Zealand, 2006 Search PubMed.
- C. A. Evans, P. J. Coombes and R. H. Dunstan, Wind, rain and bacteria: the effect of weather on the microbial composition of roof-harvested rainwater, Water Res., 2006, 40, 37–44 CrossRef CAS.
- S. May and R. T. Prado, Experimental evaluation of rainwater quality for non-potable applications in the city of Sao Paulo, Brazil, Urban Water, 2006, 3, 145–151 Search PubMed.
- E. Sazalki, A. Alexopoulos and M. Leotsinidis, Rainwater harvesting, quality assessment and utilization in Kefalonia Island, Greece, Water Res., 2007, 41, 2039–2047 CrossRef.
- M. Y. Han and J. S. Mun, Particle behaviour consideration to maximise the settling capacity of rainwater storage tanks, Water Sci. Technol., 2008, 58, 73–79 Search PubMed.
- W. Wirojanagud, Rainwater Quality: Pathogens and Heavy Metals, Proceedings for the 5th International Rainwater Catchment Systems Conference, Keelung, Taiwan, 1991 Search PubMed.
- C. A. Evans, P. J. Coombes, R. H. Dunstan, T. Harrison, A. Martin and A. Morrow, Rainwater tanks and microbial water quality: are the indications clear?, Australian Journal of Water Resources, 2008, 12, 143–152 Search PubMed.
- American Public Health Association (APHA), Standard methods for the examination of water and wastewater, ed. L. Clescert, A. Greenburg and A. Eaton, APHA, AWWA, WPCF, Washington DC, 20th edn., 1998 Search PubMed.
- “Guidance on use of rainwater tanks”. EnHealth Council, Australian Government, ISBN 0 642 82443 6 (http://enhealth.nphp.gov.au/council/pubs/pdf/rainwater_tanks.pdf).
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |
