Implications of human pharmaceutical occurrence in the Sindian river of Taiwan: A strategic study of risk assessment†
Angela
Yu-Chen Lin
*,
Sri Chandana
Panchangam
and
Huan-Yo
Chen
Graduate Institute of Environmental Engineering, National Taiwan University, 71-Chou-Shan Road, Taipei, 106, Taiwan. E-mail: yuchenlin@ntu.edu.tw; Fax: +886-2-3366-9828; Tel: +886-2-33663729
First published on 15th June 2009
Abstract
Contamination of natural aquatic environments with human-use pharmaceuticals poses a significant potential threat to local ecosystems. However studies of the risk assessment of this contamination are limited, in part because currently the environmental concentration prediction is logistically and technically difficult to perform. In this study, 1) a strategic method to determine occurrence and risk of pharmaceutical compounds in an aquatic environment is proposed, and 2) this method is applied to study ten human-use pharmaceuticals in Taiwan's Sindian river, which traverses and supplies drinking water for Taipei, a major metropolitan center. In river-water samples collected over a three-week period, concentrations of NSAIDs (acetaminophen, diclofenac, ibuprofen, naproxen, and ketoprofen), steroids (estrone, 17α-ethinylestradiol and 17β-estradiol), the anti-hypertensive agent propranolol, and the lipid regulator gemfibrozil were found at ng/L to µg/L levels; night-time concentrations were often doubled or even greater, compared to day-time levels. With the exception of the estrogens, predicted environmental concentrations (PECs) of the target pharmaceuticals agreed well with measured environmental concentrations (MECs). Conclusions: In the Sindian river, which is impacted by both regional and hospital discharges, the pattern of pharmaceutical occurrences and concentrations observed here is due to variations in source release rather than to natural attenuation phenomena. In addition, the environmental risk posed by acetaminophen, ibuprofen, and the estrogens was observed to be imminent, while risk from the other drugs targeted was found to be lower, even when maximum MECs were considered.
Introduction
The appearance of pharmaceuticals in the aquatic environment was first observed as long as three decades ago,1 but only in this decade has their presence raised significant concern for the impact of chronic exposure on local human and wildlife populations. Previous investigators have demonstrated concrete negative effects, including reduced sperm counts in adult male guppies2 and development of antibiotic-resistant bacterial strains.3 The synthetic estrogen 17α-ethynylestradiol has been found to affect reproductive cycles in fish even at 4 ng L−1 concentrations.4 The occurrence and fate of pharmaceuticals in European5–7 and American8,9 aqueous environments have been much more fully characterized than in Asian waters, but some studies have reported the occurrence of pharmaceuticals in Asian aquatic environments as in the ng L−1 to µg L−1 range.10–12 Pharmaceutical data must be considered in the context of a particular region, and data obtained from one locale cannot be applied to another, because both occurrence and fate are constrained by local patterns of medical use, cultural and climatic conditions, available techniques for removal from wastewater, etc.10,13,14The sources and pathways of pharmaceuticals into the environment are well understood.7,15 Pharmaceuticals consumed by humans may undergo biochemical modifications during hepatic or enteric metabolism before excretion in urine or feces16 and eventual arrival into the aquatic environment. Direct release via household toilet flushing is another non-negligible route of drug disposal. Bound et al.13 concluded that the chosen disposal method was unaffected by the human perception on risk associated with pharmaceuticals. Of non-household pharmaceutical contamination sources such as hospitals, sewage treatment plants, wastewater treatment plants (WWTPs), production facilities, animal husbandries and aquacultures – WWTPs, hospitals, and livestock operations have been shown to be the dominate sources.17,18 In Taipei, Taiwan, we previously conducted surveys of pharmaceutical removal efficiencies in WWTPs which revealed that NSAIDs were the main constituents (61–69%) entering WWTP influents, while certain antibiotics restrained the secondary treatment processes.19 In addition, a sweep of 97 pharmaceuticals in 23 Taiwanese potential pharmaceutical contamination source water sites were studied previously.10 The results showed the presence of several pharmaceuticals at µg L−1 level exceeding the predicted no effect concentration (PNEC) values warranting further investigations.
Although regulating the entire assembly of pharmaceuticals is impractical, a clear need exists for targeted risk assessment studies of compounds with the potential for environmental harm. Risk assessment guidelines proposed by the European Medicines Agency (EMEA: formerly the Agency for the Evaluation of Medicinal Products) calculate the predicted environmental concentration (PEC) and signal potential risk when the PEC-to-PNEC ratio (risk quotient) exceeds one.20 With these guidelines in mind, several recent attempts have been made to estimate PEC values for the common pharmaceuticals with high prescription.14,18,21 However, inconsistent findings for rates of pharmaceutical metabolism severely limit the reliability of currently reported PECs.22,23 In addition, only limited data are available for pharmaceuticals' eventual fate; their dilution in the receiving water bodies depend on various factors such as regional climatic conditions, catchment area and flow of the streams.21–24 This may cause underestimation of the predicted concentrations comparing to measured environmental concentrations (MECs), eventually resulting in false-negative error as in the case of carbamazepine and propranolol in the study by Ferrari et al.24 Even though the pharmaceuticals are present in low concentrations in surface waters, the level of toxicity they exhibit is significant as witnessed in the case of Pakistan where the decline in vulture population was attributed to diclofenac.25 The pharmaceuticals are expected to pose more risk due to their mixture/combined toxicity.
Although guidelines for risk assessment of newly registered pharmaceuticals were introduced in the US/EU, they are unlikely to affect the environmental occurrence of already licensed pharmaceuticals that are not regulated by any guidelines.26,27 In addition to the constraints discussed above, the analytical techniques which were available to detect pharmaceutical compounds in various complex environmental matrices were also limited. However, major advances in analytical chemistry have introduced reliable and accurate techniques such as liquid chromatography tandem mass spectrometry (LC-MS/MS) and solid phase extraction (SPE), which have minimized solving previous analytical limitations, lowered detection limits, and increased sensitivity to target compounds.6
This study investigated the occurrence of ten pharmaceuticals (five NSAIDs, three estrogens, an anti-hypertensive and a lipid-regulator) in Taiwan's Sindian river using LC-MS/MS. Risk assessment for the pharmaceuticals detected was performed and PECs were evaluated using a method based on removal rates by various routes. PECs and MECs were compared to validate the method itself and its usefulness for risk assessment in an aquatic environment.
Material and methods
Chemicals
HPLC-grade methanol and disodium ethylenediaminetetraacetate (EDTA-2Na) were purchased from Mallinckrodt Baker (Phillipsburg, NJ, USA). ACS-grade formic acid, estrone, and 17α-ethinylestradiol were obtained from Riedel-de Haën, Germany. Acetaminophen, gemfibrozil, ibuprofen, ketoprofen, naproxen and 17β-estradiol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Diclofenac and propranolol were obtained from United States Pharmacopeia (Rockville, MD, USA). Sodium hydroxide was purchased from Nacalai Tesque (Kyoto, Japan) and sulfuric acid from Fluka, Switzerland. The purity of the target compound standards was >99%. Stock standard solutions of individual compounds (1000 mg L−1) were prepared in methanol and stored at −20 °C in amber glass bottles for ≤15 days. Working solutions were prepared by diluting stock solutions to desired concentrations (0.01, 0.1, 1 and 10 mg L−1) prior to each analytical run. The chemicals used and their physiochemical properties are listed in Table 1.| Compound | CAS No. | Log Kowa | MW | Structure |
|---|---|---|---|---|
| a The log Kow values are from the references 28, 33, 46, and 47. | ||||
| Acetaminophen | 103-90-2 | 0.46 | 151.2 |

|
| Ibuprofen | 15687-27-1 | 3.97 | 206.3 |

|
| Naproxen | 22204-53-1 | 3.18–3.24 | 230.3 |
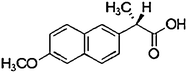
|
| Ketoprofen | 22071-15-4 | 3.12–3.16 | 254.3 |

|
| Diclofenac | 15307-79-6 | 4.51 | 318.13 |
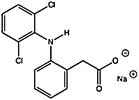
|
| Estrone | 53-16-7 | 3.13 | 270.37 |
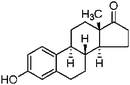
|
| 17α-Ethynylestradiol | 57-63-6 | 3.67 | 296.4 |

|
| 17β-Estradiol | 50-28-2 | 4.01 | 272.39 |

|
| Propranolol | 525-66-6 | 1.20–3.48 | 295.8 |

|
| Gemfibrozil | 25812-30-0 | 4.77 | 250.3 |

|
Site descriptions
The Sindian river runs through densely-populated, metropolitan Taipei and belongs to the Dan-Shui river basin, the largest basin in northern Taiwan. The Sindian river merges with the Da-Han river in Banqiao district near Jiangzicui and flows into the Dan-Shui river. The Sindian is 82 km long, with a 910 km2 drainage area. This river supplies drinking water for 97% of the local population, which, according to Taiwan Water Department data, comprises more than 4 million people. Samples were collected from six different locations along the river: 1) at water gate No. 1 on the Jing-Mei river (A1; 121°32′13″ E, 024°59′31″ N); 2) the intersection of the Sindian and Jing-Mei rivers (A2; 121°32′01″ E, 025°00′11″ N); 3) under Yong-Fu Bridge (A3; 121°31′37″ E, 025°00′42″ N); 4) near Zhong-Zheng Bridge (A4; 121°31′09″ E, 025°01′13″ N); 5) under Hwa-Zhong Bridge (A5; 121°29′42″ E, 025°00′36″ N); 6) and under Hwa-Jiang Bridge (A6; 121°29′03″ E, 024°02′02″ N). Fig. 1 depicts river water sampling sites, with hospitals and regional discharge points marked. | ||
| Fig. 1 Map of sampling site. | ||
Sample collection and storage
River water samples were collected in 1 L amber glass bottles, which were pre-washed with tap water (three times), deionized (DI) water (also three times) and rinsed with river water. Samples were collected in triplicate at each location and stored on ice while in the field to inhibit bacterial growth. Samples were vacuum-filtered through 0.45 and 0.22 µm cellulose acetate filter papers (Advantec, Tokyo Roshi Kaisha, Ltd., Japan) and stored at 4 °C until analysis. All collections were performed in December 2007 and January 2008.Solid phase extraction and LC-MS/MS methods
Water samples were concentrated and purified with solid phase extraction (SPE). Oasis HLB cartridges (Waters, Milford, MA, USA) were preconditioned first with 5 mL 100% methanol and then with 5 mL DI water. Water samples were adjusted to pH 7.0 by adding 0.5 N H2SO4 and 0.5 N NaOH. Aliquots of the water samples (250 mL) were loaded onto the HLB cartridges at flow rate 3–6 mL min−1. Cartridges were washed with 6 mL DI water and dried with N2 gas stream. The analytes were eluted with 8 mL of 50% aqueous methanol. The eluates were collected and evaporated to dryness for 1–2 h by nitrogen stream and a heated bath at 37 °C. The dried eluate was dissolved into 0.5 mL of 50% aqueous methanol, and the samples were again filtered through 0.22-µm polytetrafluoroethylene aperture filters (13-mm diameter) before LC-MS/MS analysis.A high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) system (Applied Biosystems API 4000) was used to analyze the samples. The HPLC module included a degasser (Agilent 1100 Series Micro Vacuum Degasser), pump (Agilent 1100 Series Binary Pump) and autosampler equipment (CTC Analytics HTC PAL System). Chromatographic separations were performed with ZORBAC Eclipse XDB-C18 columns (Agilent, Palo Alto, CA, USA, 150 × 4.6 mm, 5 µm). The autosampler was operated at room temperature. The column was equilibrated for 5 min before injection of 20 µL samples. The analyzing time was 10 min for each sample. A binary gradient program was used with 0.1% formic acid in DI water as mobile phase A (MPA) and 0.1% formic acid in 100% methanol as mobile phase B (MPB) in both positive and negative ion modes. The HPLC solvent gradient was operated at 1000 µL min−1 flow rate, starting at 90%:10% MPA:MPB for 0.2 min, gradually approaching 30%:70% MPA:MPB, and finally reaching 10%:90% MPA:MPB at 4.5 min. The gradient reached 5% MPA:95% MPB at 5.0 min, continued until 8.0 min, and by 8.5 min reached 90% MPA:10% MPB which was held constant until the end of the analysis for up to 10 min.
A quadrupole mass spectrometer equipped with electrospray ionization (ESI) interface was used to make mass spectrometric measurements. The analyses were done in positive ion mode for acetaminophen, propranolol, estrone, 17β-estradiol, and 17α-ethinylestradiol, and in negative ion mode for ibuprofen, gemfibrozil, ketoprofen, naproxen, and diclofenac. Ions were acquired in multiple reaction monitoring (MRM) mode with a dwell time of 200 ms. The mass spectrometric conditions applied were: ion spray voltage of 5.5 and −4.5 kV, curtain gas at 10 L h−1, nebulizer gas at 60 L h−1 and turbo gas at 50 L h−1, heated capillary temperature of 550 °C and 450 °C (negative ion mode) and collisionally activated dissociation 5 with interface heater on. The precursor and MRM product ion pairs of the target compounds are shown in Table 2.
| Compound | MRM pairs | Retention time | MDLs | Linearity, r | Accuracy (%) | Precision (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| m/z | (min) | (ng/L) | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | ||
| Acetaminophen | 152 > 93 | 4.13 | 0.2 | 0.9998 | 17.1 | 4.4 | 8.4 | 2.7 | 1.0 | 3.0 |
| 152 > 110 | ||||||||||
| Ibuprofen | 205 > 161 | 7.32 | 10 | 0.9993 | −3.3 | 9.0 | 9.8 | 1.8 | 6.1 | 3.8 |
| Naproxen | 229 > 169.8 | 6.70 | 2 | 0.9922 | −13.3 | −4.6 | 7.4 | 4.2 | 3.7 | 2.5 |
| 229 > 184.9 | ||||||||||
| Ketoprofen | 253 > 208.9 | 6.49 | 5 | 0.9997 | −4.9 | 6.6 | 5.0 | 3.7 | 4.8 | 6.7 |
| 253 > 197 | ||||||||||
| Diclofenac | 294 > 250 | 7.21 | 5 | 0.9991 | 7.4 | 10.6 | 8.3 | 6.2 | 5.9 | 6.9 |
| 294 > 214 | ||||||||||
| Estrone | 271 > 133 | 6.81 | 10 | 0.9985 | 8.3 | 10.6 | 3.2 | 11.0 | 5.2 | 3.7 |
| 271 > 253 | ||||||||||
| 17α-Ethynylestradiol | 279 > 133 | 6.67 | 10 | 0.9998 | −5.6 | 12.6 | 12.6 | 6.3 | 4.0 | 1.9 |
| 279 > 159 | ||||||||||
| 17β-Estradiol | 255 > 133 | 6.78 | 10 | 0.9999 | 11.9 | 7.2 | 5.4 | 13.9 | 8.7 | 4.2 |
| 255 > 159 | ||||||||||
| Propranolol | 260 > 116 | 5.31 | 0.2 | 0.9999 | 18.4 | 16.2 | 14.8 | 2.6 | 3.9 | 6.0 |
| 260 > 183 | ||||||||||
| Gemfibrozil | 249 > 120.9 | 7.74 | 0.2 | 0.9987 | −4.7 | 0.6 | 11.3 | 5.1 | 7.1 | 9.3 |
| 249 > 126.9 | ||||||||||
Detection, quantification and method validation
Due to the lack of isotopically labeled standards for each compound, our study used matrix-matched external calibration to reduce analyte suppression/enhancement during instrumental analysis. Although this method is often more labor-intensive than the internal calibration method,28 it eliminates the matrix effect which would otherwise confound our analysis. A site for our river water “blank” was carefully selected which was located directly upstream of the sampling locations and further away from residential and industrial areas. Calibration curves were constructed by spiking known standards into river water blanks and subjecting them to the same SPE procedures used for the unspiked river water samples. The relative recoveries of this method are represented by the accuracy and precision data, which were determined by the replicate river blanks (n = 6) spiked at 50 and 500 ng L−1 and subjecting them to the same SPE procedures over the period of 3 days. The range of accuracy at 50 and 500 ng L−1 was −13.3 to 18.4% and −8.0 to 17.8%, respectively. Precision ranged from 1.0 to 13.9% (50 ng L−1) and 0.5 to 6.8% (500 ng L−1). These values are all well within the acceptable range (20%). The method detection limit (MDL) was determined from the minimum detectable concentration of analytes in the linear range with a signal-noise ratio of 3. Quantification of target compounds was performed via HPLC-MS/MS with MRM, using the two most intense characteristic precursor ion/product ion transition pairs. Compounds were identified using the LC retention time ±30% of retention time of a standard. MRM pairs, retention times, MDLs, linearity, precision, and accuracy are presented in Table 2.PEC calculation
The formula generally used to estimate PEC is given by the EMEA guidelines as shown below (eq 1): Rnet in Equation (1) has been modified and elaborated in this study according to the strategic approach that best fits the regional conditions. | (1) |
In this study, the amount of pharmaceutical used (“A”, Table 3) was calculated using data from Taiwan's Bureau of National Health Insurance (NHI). The NHI is an exceptional database that tracks drug use patterns, with which about 91% of health care providers such as hospitals and clinics had contracted. Further, more than 97% of the Taiwanese population was enrolled by the end of 2005.29 For prescription medications, the most recent data available are from the year 2005 (Table 3), with the exception of ketoprofen, for which data from the year 2002 were used with a factor of 4 for the PEC estimation. The ‘A’ value in kg/yr was calculated as a product of prescribed dose to averaged drug content using the NHI medicinal usage data.
| Pharmaceuticals | Prescription in Taiwan, NHI 2005 (× 103) | Amount of pharmaceutical used per annum, A (kg/yr) | Removal by transformation into metabolites, RTM (%) | Removal by natural attenuation, RNA (%), (half-life values from literature) | Net removal, Rnet (%) |
|---|---|---|---|---|---|
| a na: not available. | |||||
| Acetaminophen | 568839 | 142278.0 | 9640 | 0, (>24 h)41 | 76.8 |
| Ibuprofen | 131359 | 52543.8 | 9642 | 0, (14.8 ± 0.7 h)33 | 76.8 |
| Naproxen | 22655 | 3685.9 | 3522 | 60, (1.4 ± 0.10 h)33 | 71.2 |
| Ketoprofen | 7914 | 7914.0 | 2022 | 99, (4.1 ± 0.13 min)33 | 99.16 |
| Diclofenac | 214672 | 6400.9 | 8543 | 60, (4 h)48 | 87.2 |
| Estrone | 15 | 0.0189 | na | 60, (2.3 ± 0.07 h)33 | 60.0 |
| 17α-Ethinylestradiol | 801 | 0.0281 | 7440 | 60, (2.3 ± 0.11 h)33 | 83.68 |
| 17β-Estradiol | 19106 | 8.9821 | 9540 | 60, (2.0 ± 0.14 h)33 | 90.4 |
| Propranolol | 156637 | 3916.0 | 9943 | 60, (1.1 ± 0.04 h)33 | 91.68 |
| Gemfibrozil | 25015 | 11256.9 | 5043 | 0, (14.8 ± 0.70 h)33 | 40.0 |
Removal rate is a critical parameter that depends on factors including conversion of pharmaceuticals in human body, illicit disposal of drugs, and the fate of pharmaceuticals in the aquatic environment after being subjected to natural attenuation processes. Here, the method depicted in Fig. 2 was implemented to find an appropriate Rnet value. In general, human-use pharmaceuticals enter the aquatic environment via one of two major routes. A drug may be consumed and metabolized by a human, who eventually excretes a portion of the remaining parent compound along with its metabolites. Although some metabolites are shown to be as toxic as or even more toxic than the parent compouds,7 due to the fact that limited and sparse metabolite data is available, only parent compounds are considered in this study. Alternatively, unused drugs may be thrown away, having never been consumed or metabolized.
 | ||
| Fig. 2 Methodological approach to calculate the net removal rates. | ||
In Taiwan, medications are available predominantly via prescription. In contrast, the quantity of over-the-counter (OTC) medications consumed is negligible relative to prescription medications and for the purposes of this study may be ignored. Medications are relatively inexpensive, making it easy for Taiwanese patients to obtain too many prescription drugs, which are then stored at home. Upon reaching their expiration dates, these surplus drugs are thrown away into household dustbins or flushed down toilets. This path (route 2, Fig. 2), which depends on local cultural and social patterns of medication use, may involve a significant medication volume; in fact it may be reasonable to conclude that a majority (nearly 80%) of human pharmaceutical release into aquatic streams followed route 1, while the remaining 20% appeared to follow route 2.30 Drugs which were released through route 1 are considered to be removed from the system via their transformation into metabolites (RTM). Pharmaceutical residues which remain in the form of the original parent compound would then be subjected to natural attenuation processes such as hydrolysis, photodegradation, biodegradation, and adsorption, and may undergo further degradation. In surface waters, pharmaceuticals and their residues are most likely to be removed by direct and indirect photolysis.31,32 Sorption and biotransformation are also significant natural attenuation mechanisms.7,22
Their removal rates (RNA) were assigned according to the half-lives reported in the literature. Since the rivers in Taiwan flow at comparatively faster rates and are exposed to little sunlight, a removal rate of 60% was assumed for a half-life <5 h, 30% for half-lives between 5 and 10 h, and no removal for a reported half-life >10 h. However, two exceptions must be taken into account when calculating Rnet: for ketoprofen, whose photolytic half-life is only a few minutes long, RNA is taken to be 99%,33 and for estrone, RTM is assigned as 100%, since metabolite data are unavailable. The net removal rate Rnet was then calculated using the following formula:
 | (2) |
Table 3 shows the RTM, RNA, and Rnet values along with the half-life values obtained from the literature. In order to calculate PEC values, the Rnet value obtained from equation 2 was substituted in to equation 1 (Table 4).
| Pharmaceuticals | PEC | MEC (min–max) | MEC (Median) | PNEC | RQPEC (PEC/PNEC) | RQmin (Minimum MEC/PNEC) | RQmax (Maximum MEC/PNEC) | RQmed (Median MEC/PNEC) |
|---|---|---|---|---|---|---|---|---|
| a RQ was obtained by calculating PEC with half of the MDL value; nd. not detected; na. not available. | ||||||||
| NSAIDs | ||||||||
| Acetaminophen | 848.1 | 8.3–9170 | 1755.0 | 920021 | 0.0922 | 0.0009 | 0.9967 | 0.1908 |
| Ibuprofen | 313.2 | nd–4350 | 231.5 | 500021 | 0.062 | <0.001a | 0.87 | 0.0462 |
| Naproxen | 27.3 | 35.2–270 | 65.5 | 3700021 | 0.0007 | 0.0010 | 0.0073 | 0.0018 |
| Ketoprofen | 1.7 | nd–45.0 | nd | 1560000014 | 0.00000011 | <0.00000016a | 0.0000028 | <0.00000016a |
| Diclofenac | 21.1 | nd–56.5 | 12.9 | 1000021 | 0.0021 | <0.00025a | 0.0057 | 0.0013 |
| Estrogens | ||||||||
| Estrone | 0.0002 | nd–191 | 13.1 | 1844 | 0.000011 | <0.27a | 10.6 | 0.7278 |
| 17α-Ethynylestradiol | 0.0001 | nd–19.5 | nd | 0.0244 | 0.005 | <250a | 975 | <250a |
| 17β-Estradiol | 0.0222 | nd–64.7 | 15.6 | 0.0244 | 1.11 | <250a | 3235 | 780 |
| β-Blocker | ||||||||
| Propranolol | 8.4 | 5.8–39.1 | 13.1 | 50024 | 0.0168 | 0.5800 | 0.0782 | 0.0262 |
| Lipid-regulator | ||||||||
| Gemfibrozil | 173.5 | 66.9–279.0 | 115.2 | 10000045 | 0.001735 | 0.1520 | 0.0028 | 0.001152 |
Results and discussion
Occurrence of pharmaceuticals in the Sindian river
The occurrence of pharmaceuticals was studied in the Sindian river at six sampling locations for three consecutive weeks during the day and night. The concentrations detected at all sampling points are shown in Fig. 3 as a semi-log plot. Most NSAIDs were detected at high concentrations, with acetaminophen ranging from 8.3 to 9170 ng L−1 and ibuprofen with a maximum concentration of 4350 ng L−1. The concentration of acetaminophen measured in the Sindian river was much higher than reported concentrations in river streams in other countries. For example, Boyd et al.34 found only a trace amount of acetaminophen (up to 0.2 ng L−1) in the Mississippi river in New Orleans, while Nakada et al.35 measured a maximum concentration of 52 ng L−1 in the Tone river basin of Japan. This observation of an exceptionally high acetaminophen concentration in our study is not surprising because acetaminophen was the most often prescribed medicine by the NHI in the year 2005. There are no indications that this pattern has changed since then. Another possible cause for the observed high acetaminophen concentrations is the complete absence of treatment plants in the surrounding area. Several researchers have reported that acetaminophen can be effectively removed during secondary treatment processes;36,37 thus, it was concluded that the presence of many nearby legal and illegal regional discharges into the river (which do not undergo treatment processes) would be expected to result in high acetaminophen residuals. In this study, the concentrations of naproxen and diclofenac were observed to be lower than acetaminophen and ibuprofen, with median concentrations of 65.5 ng L−1 and 12.9 ng L−1, respectively. Ketoprofen was almost entirely absent from several samples studied, with a maximum detected concentration of only 45 ng L−1; this may be explained by ketoprofen's extremely photolability.33 Estrogens were mostly absent from the Sindian, with median detected concentrations of 13.05 ng L−1 (estrone) and 15.6 ng L−1 (17β-estradiol), and 17α-ethinylestradiol was not detected at all. Although the NHI reported that 17β-estradiol was used in higher quantities than estrone, the occurrences of estrone and 17β-estradiol were comparable. This could be due to generation of estrone as a by-product during 17β-estradiol degradation, which is considerably accelerated by sunlight and the presence of natural organic matter.38 Propranolol and gemfibrozil were often detected in river waters, but their observed aqueous concentrations were low, with median concentrations of 13.05 ng L−1 and 115.15 ng L−1 respectively. Kim et al.12 detected gemfibrozil at a mean concentration of 6.6 ng L−1 in South Korean waters, while Bound and Voulvoulis39 found no propranolol in UK rivers. Although the concentrations of propranolol and gemfibrozil detected here could be considered fairly low, they are still higher than published levels; future studies should examine the environmental implications of the higher pharmaceutical levels identified here. | ||
| Fig. 3 Semi log plot of pharmaceutical contamination in the Sindian river at the six sampling sites. | ||
As shown in Fig. 3, acetaminophen, ibuprofen, gemfibrozil, and naproxen were most often detected at each sampling point and at the highest concentrations; in contrast, other pharmaceuticals were detected at levels well below 40 ng L−1. The high concentrations of these four compounds may reflect their usage patterns. Closer examination of pharmaceutical occurrences at different sampling points showed that the median concentrations of acetaminophen and naproxen decreased from sampling points A1 to A4 and increased up to sampling point A6, while ibuprofen concentrations displayed the reverse trend. Gemfibrozil concentration increased continuously from A1 to A6. Interestingly, acetaminophen alone displayed concentrations of thousands of ng L−1. Our findings on the pharmaceutical contamination in the Sindian river are likely to have been influenced by uncertainties in local customs of medication prescription, regional discharges, or dilution factors (the Sindian's streamway appears to widen beyond sampling location A3). Sites A5 and A6 may have been impacted more by nearby urban regional discharges (since hospitals were absent), while sampling locations A1 and A2 might have been influenced by both urban regional and hospital discharges. However, the distances between the sampling points are not so far away that any alarming differences in the contamination sources may be identified.
Variation between day/night and among consecutive weeks
In general, concentrations of each compound did not show significant difference between day and night during the three-week period except in the following cases. Both acetaminophen and 17β-estradiol exhibited major swings in median concentrations between day and night in the second week (Table 5). Similarly, the concentration of ibuprofen varied widely over the course of the day. Naproxen and gemfibrozil levels were stable during the first and second weeks but fluctuated in the third week. The estrone concentration varied between days and nights by two orders of magnitude in all three weeks, with relatively high concentrations observed during day time in the first week. Ketoprofen and 17α-ethinylestradiol were not detected (by mean concentration) in either day or night over the three weeks. If derived median concentrations of each compound in each sampling location by day and night during all the three weeks were compared, interestingly, the median concentrations of every compound were higher during the night than the day time. This implies either that photodegradation does not play a significant role in removing pharmaceuticals during the day time, or that source variation during the day determines to a much larger degree the concentrations of target compounds in the Sindian river. It was postulated that on a local scale, regional and hospital discharges of pharmaceuticals as well as human medication intake and corresponding release into receiving waters occur in greater volume at night. Future investigations should certainly include careful studies of mass balance; unfortunately, this analysis could not be undertaken here due to the lack of complete flow discharge data from nearby sources, many of which are illegally released into the river.| Pharmaceuticals | Day/night | First week | Second week | Third week | Median concentration |
|---|---|---|---|---|---|
| a n.d. = not detected. | |||||
| Acetaminophen | Day | 1540.0 | 512.2 | 1730.0 | 1450.0 |
| Night | 2370.0 | 4800.0 | 1930.0 | 2360.0 | |
| Ibuprofen | Day | 92.7 | 303.0 | 365.5 | 119.5 |
| Night | 125.0 | 237.0 | 1635.0 | 237.0 | |
| Naproxen | Day | 50.4 | 63.2 | 65.7 | 57.8 |
| Night | 49.4 | 79.9 | 156.0 | 72.65 | |
| Ketoprofen | Day | n.d. | n.d. | n.d. | n.d. |
| Night | n.d. | n.d. | n.d. | n.d. | |
| Diclofenac | Day | 8.1 | 10.4 | 14.9 | 10.2 |
| Night | 7.3 | 23.3 | 24.3 | 20.3 | |
| Estrone | Day | 12.3 | n.d. | n.d. | 5.7 |
| Night | n.d. | 56.5 | 29.6 | 21.3 | |
| 17α-Ethilnylestradiol | Day | n.d. | n.d. | n.d. | n.d. |
| Night | n.d. | n.d. | n.d. | n.d. | |
| 17β-Estradiol | Day | 7.9 | 5.3 | 24.4 | 11.0 |
| Night | 5.0 | 44.5 | 24.6 | 24.6 | |
| Propranolol | Day | 13.9 | 8.4 | 10.6 | 11.6 |
| Night | 13.6 | 31.8 | 12.2 | 14.6 | |
| Gemfibrozil | Day | 109.8 | 95.7 | 91.5 | 97.2 |
| Night | 107.8 | 126.5 | 194.5 | 126.5 |
Comparisons of PECs and MECs
The PEC is considered to be an indication of the expected concentration of a given pharmaceutical in the aquatic environment. The amount of pharmaceutical initially present in (or added to) the environment, its distribution, probable manner and rate of environmental degradation and removal (whether forced or natural) were considered here. A perfect estimate of PEC eliminates the need to actually measure environmental concentrations when monitoring pharmaceutical occurrence patterns. The PEC is also a critical parameter in risk assessment. PECs were estimated for all target pharmaceuticals using the methods depicted in Fig. 2. The comparison of PECs and MECs was made in this study to evaluate the feasibility of this strategic PEC estimation.Values for PECs and minimum, maximum and median MECs are shown in Table 4. Almost all of the PECs fell within the experimentally detected concentration range, with almost perfect prediction of the median MECs of diclofenac, ibuprofen, gemfibrozil and propranolol (MECs: 12.9, 231.5, 115.2, and 13.1 ng L−1 and PECs: 21.1, 313.2, 173.5, and 8.4 ng L−1). PECs of naproxen and acetaminophen, popular over-the-counter (OTC) analgesics, were about half of their median MECs but still fallen within the detection range. This discrepancy may be explained by the fact that we did not consider OTC purchase of these widely available drugs. Ketoprofen was almost undetectable in many samples, but the PEC was one order of magnitude below the maximum MEC value, which was considered an acceptable prediction. Estrogen levels were underestimated in most of the cases. This could be due to underestimation of the quantity of drugs used or overestimation of removal by transformation (RTM) and natural attenuation processes (RNA). In this study, pharmaceutical removal by wastewater treatment was not considered, despite its importance in PEC estimation,14,22 as there were no wastewater treatment plants situated along the stretch of river near our sampling areas. In an earlier study, Lin et al.10 did not detect any estrogens in hospital effluents in Taiwan; this finding in combination with the fact that they are prescribed in considerable quantity indicates that estrogens are effectively removed by currently used treatment processes. Furthermore, the estrogens (with the exception of 17α-ethinylestradiol) occur naturally and could enter the river through natural excretion and plant decomposition processes. In general, with the exception of estrogens, our method for predicting environmental concentrations of these pharmaceuticals in Taiwan's aquatic environment yielded results which closely matched measured values, strongly supporting the methodology adopted in this study.
Environmental risk assessment
Risk assessment of pharmaceuticals in the surface waters of Taiwan's Sindian river was performed according to the guidelines set by EMEA. The risk quotient (Table 4) was evaluated as the ratio of PEC (RQPEC), minimum MEC (RQmin), median MEC (RQmed) or maximum MEC (RQmax) values to the PNEC. The PNEC is generally derived from ecotoxicity data. Even though it may be more rigorous to use chronic ecotoxicity data, due to financial and time constraints several researchers have employed acute toxicity data.24 The lowest PNECs reported were compiled from the peer-reviewed literature (EC50, NOEC, LOEC, and LC50) and given in Table 4.When RQs were calculated with minimum MECs, none of the pharmaceuticals would be predicted to pose risk, as the RQmins were well below one. When RQmed and RQPEC were analyzed, 17β-estradiol posed potential risk, with a calculated RQmed and RQPEC of 780 and 1.11, respectively. The RQmax of estrone (10.6), 17α-ethinylestradiol (975), and 17β-estradiol (3235) were alarmingly high, signifying a major potential risk. The RQmax of acetaminophen (0.9967) and ibuprofen (0.87) were close to one, indicating a non-negligible risk. When RQs were calculated using PECs, the estrogens appeared to pose little risk, with RQPEC of 0.000011 (estrone) and 0.005 (17β-estradiol). In certain cases PECs may underestimate the underlying risk of the pharmaceuticals, which can cause a false-negative error (as for estrone and 17β-estradiol). A false-negative error is a situation in which a chemical that poses significant risk to the environment is falsely identified as non-hazardous. Overall, acetaminophen, ibuprofen, and the three estrogens emerge in Sindian river as risk posing pharmaceuticals while other drugs in this study are far safer even by their maximum MEC. It is important to note that no pharmaceutical contaminant is present in isolation in the environment; instead the true state of affairs should always be viewed as an assembly of pharmaceuticals with unpredictable toxicities and complex synergistic or antagonistic interactions.
In conclusion, we have assessed the risk from the selected human pharmaceuticals in the Sindian river. A novel method for evaluating Rnet which is based on removal rates of multiple degradative processes, and predicts experimentally measured concentrations with a high degree of accuracy was proposed in this study. This approach may be implemented in the future to monitor the occurrence and risk assessment of other human pharmaceuticals in the Sindian river. Since no WWTPs were located in the study area, inclusion of a factor accounting for removal of pharmaceuticals by hospital treatment processes into the PEC calculation (equation 2) would improve the accuracy and broader applicability of this approach.
Acknowledgements
This work was partially funded by the National Science Council, Taiwan, under project number NSC95-2218-E-002-058.References
- A. W. Garrison, J. D. Pope and F. R. Allen, GC/MS analysis of organic compounds in domestic wastewater. In: Keith, L. H. (Ed), Identification and Analysis of organic pollutants in water. Ann Arbour Science, Minneapolis, 1976, pp. 517–566 Search PubMed.
- E. Baatrup and M. Junge, Environ. Health Perspect., 2001, 109, 1063 CrossRef CAS.
- K. Kümmerer, Chemosphere, 2009 DOI:10.1016/j.chemosphere.2008.006.
- R. Lange, T. H. Hutchinson, C. P. Croudace, F. Siegmund, H. Schweinfurth, P. Humpe, G. H. Panter and J. P. Sumpter, Environ. Toxicol. Chem., 2001, 20, 1216 CrossRef CAS.
- T. Ternes, Wat. Sci. Technol., 2007, 55, 327 Search PubMed.
- S. D. Richardson, Anal. Chem., 2007, 79, 4295 CrossRef CAS.
- S. Mompelat, B. LeBot and O. Thomas, Environ. Int., 2008 DOI:10.1016/j.envint.2008.10.008.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202 CrossRef CAS.
- A. K. Sarmah, M. T. Meyer and A. B. A. Boxall, Chemosphere, 2006, 65, 725 CrossRef CAS.
- A. Y. C. Lin, T. H. Yu and C. F. Lin, Chemosphere, 2008, 74, 131 CrossRef CAS.
- S. Managaki, A. Murata, H. Takada, B. C. Tuyen and N. H. Chiem, Environ. Sci. Technol., 2007, 41, 8004 CrossRef CAS.
- S. D. Kim, J. Chao, I. S. Kim, B. J. Vanderford and S. A. Snyder, Wat. Res., 2007, 41, 1013 CrossRef CAS.
- J. P. Bound, K. Kitsou and N. Voulvoulis, Environ. Toxicol. Pharmacol., 2006, 21, 301 CrossRef CAS.
- J. L. Santos, I. Aparicio and E. Alonso, Environ. Int., 2007, 33, 596 CrossRef CAS.
- T. A. Ternes, M. Stumpf, J. Mueller, K. Haberer, R. D. Wilken and M. Servos, Sci. Tot. Environ., 1999, 225, 81 Search PubMed.
- S. Perez and D. Barcelo, Trends. Anal. Chem., 2007, 387, 1225.
- M. J. Gomez, M. Petrovic, A. R. Fernandez-Alba and D. Barcelo, J. Chrom. A, 2006, 1114, 224 CrossRef CAS.
- Jones, N. Voulvoulis and J. N. Lester, Wat. Res., 2002, 36, 5013 CrossRef CAS.
- A. Y. C. Lin, T. H. Yu, S. K. Lateef and J. Hazar, Mater, 2009 DOI:10.1016/j.jhazmat.2009.01.108.
- European Agency for the Evaluation of Medicinal Products, EMEA, 2003. CPMP Safety Working Party. Note for guidance on environmental risk assessments of non-genetically modified organism (non-GMO) containing medicinal products for human use. CPMP/SWP/4447/00. 24 June, 2003, London. UK Search PubMed.
- C. Carlsson, A. K. Johansson, G. Alvan, K. Bergman and T. Kuhler, Sci. Tot. Environ., 2006, 364, 67 Search PubMed.
- D. Bendz, N. A. Paxeus, T. R. Ginn and F. J. Loge, J. Hazard. Mater., 2005, 122, 195 CrossRef CAS.
- S. Castiglioni, R. Fanelli, D. Calamari, R. Bagnati and E. Zuccato, Regul. Toxicol. Pharmacol., 2004, 39, 25 CrossRef CAS.
- B. Ferrari, R. Mons, B. Vollat, B. Fraysse, N. Paxeus, R. L. Giudice, A. Pollio and J. Garric, Environ. Toxicol. Chem., 2004, 23, 1344 CrossRef CAS.
- J. L. Oaks, M. Gilbert, M. Z. Virani, R. T. Watson, C. U. Meteyer, B. A. Rideout, H. L. Shivaprasad, S. Ahmed, M. J. I. Chaudhry, M. Arshad, S. Mahmood, A. Ali and A. A. Khan, Nature, 2004, 427.
- FDA, 1995. Guidance for industry for the submission of an environmental assessment in human drug applications and supplements. Food and Drug Administration, Rockville, USA Search PubMed.
- EU, 1994. Assessment of potential risks to the environment posed by medicinal products for human use, excluding products containing live genetically modified organisms. EU Ad Hoc Working Party, III/5504/94 Draft 4. European Commission, Brussels, Belgium Search PubMed.
- M. Gros, M. Petrovic and D. Barcelo, Talanta, 2006, 70, 678 CrossRef CAS.
- Department of Health, 2008. http://dohlaw.doh.gov.tw/Chi/FLAW/FLAWDAT0202.asp.
- G. Vanesse, Medications return program: Draft 2008 Pharmaceuticals annual report submitted to Ministry of Environment, Victoria, Canada. June 30, 2008, http://www.medicationsreturn.ca/ar2007.pdf Search PubMed.
- S. K. Khetan and T. J. Collins, Chem. Rev., 2007, 107, 2319 CrossRef CAS.
- A. Nikolaou, S. Meric and D. Fatta, Anal. Bioanal. Chem., 2007, 387, 1225 CrossRef CAS.
- A. Y. C. Lin and M. Reinhard, Environ. Toxicol. Chem., 2005, 24, 1303 CrossRef CAS.
- G. R. Boyd, J. M. Palmeri, S. Zhang and D. A. Grimm, Wat. Res., 2003, 311, 135 CAS.
- N. Nakada, K. Komori, Y. Suzuki, C. Konishi, I. Houwa and H. Tanaka, Wat. Sci. Technol., 2007, 56, 133 Search PubMed.
- M. J. Gomez, M. J. M. Bueno, S. Lacorte, A. R. Fernandez-Alba and A. Aguera, Chemosphere, 2007, 66, 993 CrossRef CAS.
- C. Miege, J. M. Choubert, L. Ribeiro, M. Eusebe and M. Coquery, Wat. Sci. Technol., 2008, 57, 49 Search PubMed.
- D. M. Leech, M. T. Snyder and R. G. Wetzel, Sci. Tot. Environ., 2008 DOI:10.1016/j.scitotoenv.2008.11.018.
- J. P. Bound and N. Voulvoulis, Wat. Res., 2006, 40, 2885 CrossRef CAS.
- A. C. Johnson, A. Belfroid and A. Di Corcia, Sci. Tot. Environ., 2000, 256, 163 Search PubMed.
- H. Yamamoto, Y. Nakamura, S. Moriguchi, Y. Nakamura, Y. Honda, I. Tamura, Y. Hirata, A. Hayashi and J. Sekiwaza, Wat. Res., 2009, 43, 351 CrossRef CAS.
- D. Ashton, M. Hilton and K. V. Thomas, Sci. Tot. Environ., 2004, 333, 167 Search PubMed.
- T. A. Ternes, Wat. Res., 1998, 32, 3245 CrossRef CAS.
- K. Press-Kristensen, A. Ledin, J. E. Schmidt and M. Henze, Sci. Tot. Environ., 2007, 373, 122 Search PubMed.
- M. Isidori, A. Nardelli, L. Pascarella, M. Rubino and A. Parrella, Environ. Int., 2007, 33, 635 CrossRef CAS.
- R. A. Trenholm, B. J. Vanderford, J. C. Holady, D. J. Rexing and S. A. Snyder, Chemosphere, 2006, 65, 1990 CrossRef CAS.
- Z. L. Zhang and J. L. Zhou, J. Chromatogr. A, 2007, 1154, 205 CrossRef CAS.
- D. Schowanek and S. Webb, Toxicol. Lett., 2002, 131, 39 CrossRef CAS.
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |
