Cyclic tetrapyrrole based molecules for dye-sensitized solar cells
Xiao-Feng
Wang
a and
Hitoshi
Tamiaki
*b
aEnvironmental and Renewable Energy Systems Division, Graduate School of Engineering, Gifu University, Yanagido 1-1, Gifu, 501-1193, Japan
bDepartment of Bioscience and Biotechnology, Faculty of Science and Engineering, Ritsumeikan University, Kusatsu, Shiga, 525-8577, Japan. E-mail: tamiaki@se.ritsumei.ac.jp
First published on 9th November 2009
Abstract
In this Perspective, recent progress on dye-sensitized solar cells (DSSCs) based on cyclic tetrapyrrole type sensitizers including porphyrins, (bacterio)chlorins, and phthalocyanines has been reviewed. Cyclic tetrapyrrole type molecules have been studied extensively with respect to their photochemical and photophysical properties as well as to various applications. The photophysical properties of tetrapyrrole molecules can be readily controlled upon molecularly structural modification. Their low-lying singlet states are also suitable for studying excited state dynamics in the electron donor–acceptor systems. Here, we selected the most representative porphyrin, (bacterio)chlorin, and phthalocyanine sensitizers to discuss how those structural modifications on the cyclic tetrapyrrole rings affect the performance of DSSCs. The most important factors that strictly determine the power conversion efficiencies of DSSCs based on tetrapyrrole type sensitizers are also discussed in detail.
 Xiao-Feng Wang | Xiao-Feng Wang received his MS degree from Jilin University (Professor Xueqi Fu), received his PhD in physical chemistry in 2006 from Kwansei Gakuin University in Japan (Professor Yasushi Koyama). In 2005, he earned the Chinese Government Award for Outstanding Self-Financed Students Abroad. He conducted his postdoctoral research at Kwansei Gakuin University (2006–2008), National Institute of Advanced Industrial Science and Technology (2008–2009), and Gifu University (2009) working on biological, organic, and inorganic materials for solar energy conversion. From 2007–2010, he is the principle researcher of two research projects granted by JST and MEXT of Japan for developing chlorophyll-based solar cells. |
 Hitoshi Tamiaki | Hitoshi Tamiaki received his Doctor of Science from Kyoto University in 1986, then worked in the same organic chemistry laboratory (Professor Maruyama), although spending 1991–1992 at Max-Planck-Institut für Strahlenchemie in Mülheim, Germany (Professor Schaffner and Professor Holzwarth). He moved to Ritsumeikan University in 1993 and is currently a Distinguished Research Professor of Bioorganic Chemistry. He received the 2006 Japanese Photochemistry Association Award. His research interests span from the elucidation of photosynthetic energy and electron transfer in a molecular level using natural systems and artificial models to the construction of photoactive nano(bio)devices. |
Broader contextExchanging traditional fossil fuels as power supply with renewable energy is a global consensus. However, the process of producing renewable energy is not always ‘green’. For example, manufacturing Si-based solar batteries needs lots of energy. Dye-sensitized solar cells (DSSCs) are alternatives to such solar batteries, because they are ‘green’ in fabrication. In DSSCs, a dye sensitizer plays a key role in light-harvesting and electron-transferring processes. Dye sensitizers based on cyclic tetrapyrroles, especially chlorophylls, are well known for their important functions in photosynthesis. Natural photosynthesis has been evolved over billions of years with adaptation of gradually changed Earth's climate and environment. Apparatus in the photosynthetic initial stage mainly require elements of H, C, N, O, Mg, and Fe, which are easily available and reusable. The philosophy behind the present research is to develop DSSCs by using chlorophyllous dyes as a ‘gift’ from natural photosynthesis, to minimize risks that may come from the synthetic processes or environmental contamination as well as to maximize utilization efficiency of solar energy including lights in the near-infrared region. |
Introduction
Over-use of fossil fuels as an energy resource has now led to human society close to an energy and environmental crisis. Solar energy as one of the renewable energies is clean, and could be used in most regions on the surface of Earth. Among those devices that can convert solar energy into electricity, dye-sensitized solar cells (DSSCs) have attracted considerable attention because they are cost-effective and easy to produce. In a DSSC, a dye sensitizer plays the key role in light-harvesting and energy conversion (see Scheme 1). To date, the solar energy-to-electricity conversion efficiency (η) for the best DSSC is over 11.1% when the traditional Ru-complex is employed as a sensitizer.1 Besides Ru-complexes, cost-effective organic dyes are also developed for high efficiency DSSCs.2 To the best of our knowledge, the highest η values for DSSCs appearing in the literature as of August 2009 and based on a series of typical organic sensitizers were reported to be 4.8% for cyanines,3 5.2% for hemicyanines,4 6.8% for perylenes,5 7.7% for oligothiophenes,6 8.2% for coumarines,7 8.6% for triarylamines,8 9.5% for indolines9 and 9.8% for polyenes.10 Although the conversion efficiencies of DSSCs based on these organic sensitizers are close to those based on Ru-complexes, the absence of their absorbance at the near infrared region has always led to limited photocurrent generation. Therefore, the importance of efficiently using the near infrared light of solar energy acts as a driving force in developing cyclic tetrapyrrole sensitizers in DSSCs.11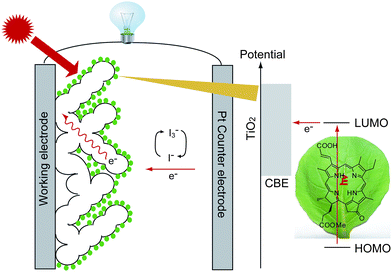 | ||
| Scheme 1 Schematic presentation of the working mechanism of a dye-sensitized solar cell based on chlorophyll a derivative as sensitizer. | ||
Cyclic tetrapyrroles, which include porphyrins (Pors), (bacterio)chlorins ((B)Chls), and phthalocyanines (Pcs), are a large group of organic molecules possessing special photochemical and photophysical properties. The basic structures of these target compounds are depicted in Fig. 1. The spectral properties of these sensitizers can be explained in terms of Gouterman's four-orbital model. The four orbitals are HOMO − 1, HOMO, LUMO and LUMO + 1, termed a2u, a1u, egx and egy.12 A major photophysical feature of this set of sensitizers is that they can more efficiently use the solar energy at the near infrared region with their Q absorption bands. Moreover, Pors and (B)Chls possess some intrinsic properties in photosynthesis including light-harvesting, and energy- and electron-transferring processes.13Fig. 2 shows the chemical structures of typical Chl pigments used in natural photosynthesis, chlorophylls a/b, c and bacteriochlorophyll a having a Chl, Por and BChl π-system, respectively. Compared to Pors and (B)Chls, Pcs are much less important in natural systems, but have earlier been applied in p–n junction type organic solar cells.14
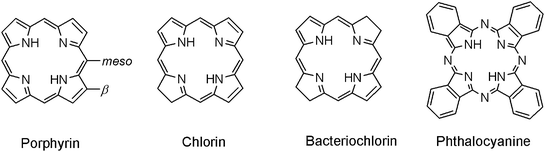 | ||
| Fig. 1 Four basic structures of cyclic tetrapyrrole sensitizers. | ||
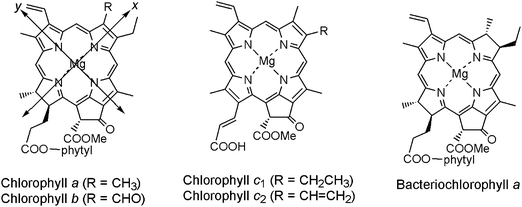 | ||
| Fig. 2 Molecular structures of naturally occurring chlorophyll pigments. | ||
Here, we will first study DSSCs based on synthetic Por sensitizers. These Por sensitizers can be placed in two classes, i.e., meso-Por and β-Por (left in Fig. 1), based on the position where the peripheral substituent has carboxy group(s) attached to a substrate surface.15 Then, we continue to introduce recent works on developing high efficiency DSSCs based on natural chlorophyll derivatives having mainly (B)Chl macrocycles.16 We compare first the different effects of central metals on Por and Chl macrocycles. These results deepened our knowledge of the most important character of a tetrapyrrole sensitizer in DSSCs. This Perspective was also involved in the recent development of transparent DSSCs based on phthalocyanines.17 In the last part of this Perspective, we summarize how to design a highly efficient dye sensitizer based on the cyclic tetrapyrrole macrocycles, and further, the possible strategy of using multiple dye sensitizers is discussed.
Compared to other recently published reviews on DSSCs,18 the present Perspective is focused on the above sensitizers with defined molecular structures. We describe the efficiency of Por and Chl sensitizers by providing some experimental evidence. Finally, we also attempt to give clearer answers to the question of how we can further develop highly efficient DSSCs with dye sensitizers based on different tetrapyrrole rings.
Dye-sensitized solar cells based on synthetic porphyrins
Studies on excited state dynamics of efficient DSSCs based on Ru-complexes suggest that the rate of electron injection from such dye sensitizers to a semiconductor electrode, such as TiO2, was extremely fast and efficient, namely, femtosecond in time scale and ∼100% in electron injection efficiency. Such a high electron injection rate is largely dependent on the electron distributions of the molecules at both excited and ground states. In a typical Ru-complex, N719, for example, electrons are readily localized at the position of the two COOH groups near the TiO2 surface in the LUMO orbital and at the two SCN ligands far from this surface (for easy interaction with electrolytes) in the HOMO orbital, to allow efficient desired electron injection (from N719* to TiO2) and to slow undesired charge recombination (between N719+˙ and TiO2−˙). In contrast, the Por skeleton possesses D4h symmetry, and π-electrons are basically delocalized over the symmetric structure of porphyrin macrocycle at both the ground and excited states. The highly symmetrical structure of porphyrin molecules is a weak point when they are used as a dye sensitizer in DSSCs. Actually, early studies on this group of sensitizers did not notice this point, so that DSSCs based on this group of sensitizers were usually inefficient.15a–15e The most efficient porphyrin sensitizer until 2004 was Por-1, as shown in Fig. 3, giving rise to the η of up to 3%.15a Although the η value of this sensitizer was moderate, this study still contains some additional useful information for further development of this group of sensitizers. Firstly, the zinc metal in the center of a porphyrin macrocycle not only improves the solubility of the sensitizer, but also influences the electron transfer process between the conduction band edge (CBE) of TiO2 and the chromophore by shifting both LUMO and HOMO levels of the porphyrin sensitizer to higher energy levels. Another point is that four meso-phenyl groups extended the π-system of the sensitizer through their partial π-conjugation and left a space between the porphyrin chromophore and the carboxy group to reduce the rate of charge recombination. The excited state dynamics of the Por-1–TiO2 interface has been studied by time-resolved fast spectroscopy.15a,15b The rates of electron injection and charge recombination for a Por-1 sensitized solar cell were similar to those for a Ru-complex N3 sensitized solar cell. The relatively low conversion efficiency of a Por-1 sensitized solar cell was attributed to the formation of the aggregates of Por-1 molecules which caused serious exciton annihilation in the resulting supramolecule.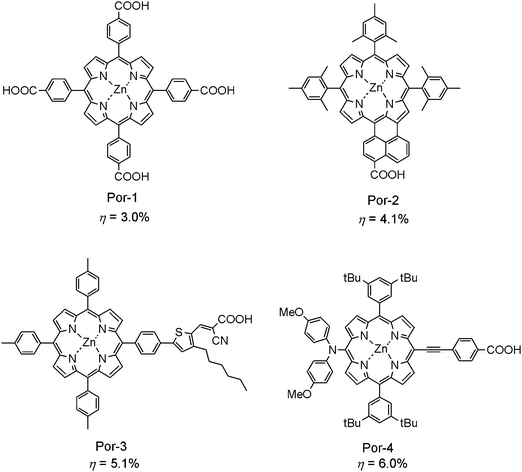 | ||
| Fig. 3 Molecular structures of typical meso-substituted porphyrin sensitizers for DSSCs. | ||
It has been suggested by many studies that an efficient dye sensitizer should possess a low-symmetric structure for efficient charge separation under light illumination.2 Those low-symmetric dye configurations usually consist of an electron donor moiety (D), a π-conjugation bridge (π), and an electron acceptor moiety with anchoring group(s) (A). There are two possible positions on the porphyrin macrocycle for preparation of such low-symmetric dyes, i.e., the meso- and β-positions (see left in Fig. 1). Fig. 3 presents a set of typical porphyrin sensitizers with the extended π-conjugation and the COOH group along the meso-position. Por-2 has a carboxy group at the same position as in Por-1, but the number of carboxy groups has been reduced from 4 to 1.15k Additionally, the naphthyl linker was connected to the neighboring β-position. This change through the π-extension allows a significant improvement in the light-harvesting efficiency (LHE) of the sensitizer at both visible and near infrared regions. The ring-fused Por-2 gives rise to a conversion efficiency of up to 4.1%, which is ∼50% higher than the unfused reference.15k
Electron rich moieties such as polythiophene and polystyrenesulfonate are known to have good electronic conductivity, so they can be good linkers in the D-π-A structure of the organic sensitizers.6 In the case of Por-3,15u a thiophenylene–vinylene group was inserted in between the meso-phenyl group and the carboxy anchoring group. In addition, this sensitizer has a cyano group to improve the electron-withdrawing ability of the anchoring carboxy group. A hydrophobic hexyl group on the thiophene linker is assumed to reduce the charge recombination between TiO2 and triiodide in an electrolyte.6 This sensitizer gives a high η value of up to 5.1%. Unfortunately, the incident photon-to-current conversion efficiency (IPCE) value does not nicely correlate to the observed photocurrent in the I–V curve, suggesting that further reliable studies on this kind of porphyrin sensitizers are necessary. Most recently, Diau and Yeh succeeded in demonstrating a newly synthesized porphyrin sensitizer (Por-4)15s with a typical D–π–A configuration for efficient DSSC. The diarylamine moiety of this dye sensitizer is a strong electron donor, while the carboxy group on the opposite side is a strong electron acceptor. The electrons are located at the diarylamine moiety in the HOMO orbital, and move to the carboxy group in the LUMO orbital. Por-4 gives rise to a η value of up to 6.0%, which is similar to Ru-complex N3 when they are compared under the same experimental conditions.
Compared to porphyrin sensitizers which use meso-substituents for anchoring and/or π-extension, meso-arylporphyrin sensitizers possessing anchoring and π-extending moieties at the β-positions have been studied more extensively. Fig. 4 shows seven typical β-elongated porphyrin sensitizers that have been used in DSSCs. Por-5 is the synthetic porphyrin sensitizer which was first studied in depth from a theoretical aspect, and then examined for photovoltaic performance.15t In the DFT/TD-DFT calculations on Por-5 possessing a vinylene–thiophenylene–vinylene linker, the band gap between the HOMO and LUMO levels is much smaller than the phenylene reference Por-7.15t This narrow band gap was expected to be useful in facilitating electron transfer toward TiO2. However, in the real fabricated DSSCs using these two sensitizers, the η value (1.2%) for thiophenylene-bridged Por-5 was three times lower than the 3.7% for phenylene-bridged Por-7, indicating there must be more factors beyond the relative energy levels of the dye sensitizers to determine the performance of DSSC. Compared to Pors-5 and 7, DSSC based on Por-815g gave a higher efficiency (4.8%), partially because the two m-methyl substituents on the four meso-phenyl group can partially avoid π–π stacking between two neighboring porphyrin molecules attached to the TiO2-surface.
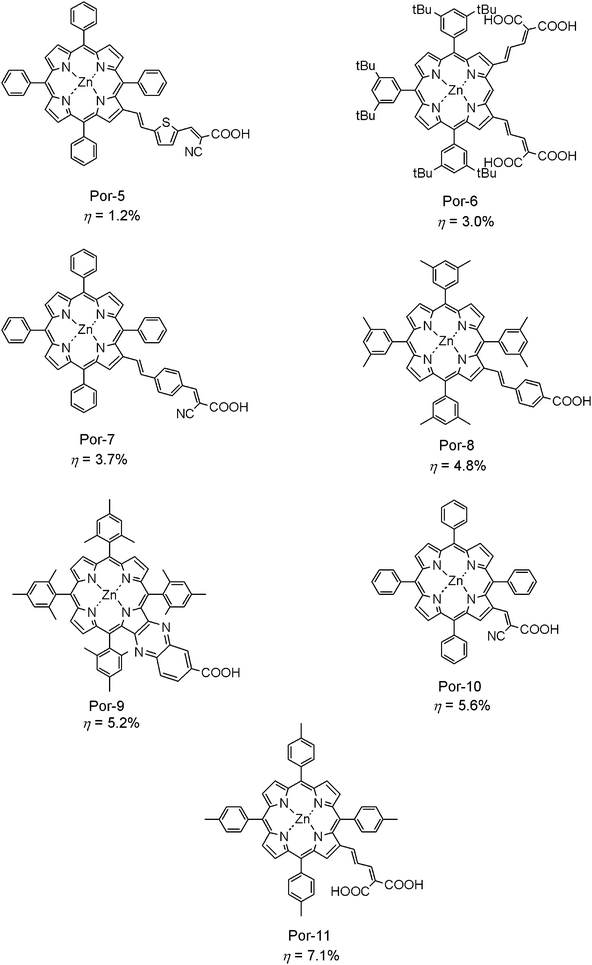 | ||
| Fig. 4 Molecular structures of typical β-substituted meso-arylporphyrins sensitizers for DSSCs. | ||
Shinokubo, Osuka, and Kim synthesized a set of doubly β-functionalized porphyrin sensitizers for DSSCs.15q Among these new porphyrin dyes, Por-6 featuring two malonate moieties gives the highest conversion efficiency of up to 3%. Their work suggests that multiple electron transfer pathways through olefinic side chains at two β-positions enhances the overall electron injection efficiency, and that the moderate distance between the porphyrinyl core π-system and the TiO2 semiconductor layer is important, since it retards the charge recombination process. Also, two neighboring β-positions can be used to elongate the π-conjugation length. Por-9 with a fused quinoxaline linker at the β-positions and a carboxy group gave much higher performance of up to 5.2%,15p and later this value was improved to 6.3% by optimizationhttp://www.bbc.co.uk/go/homepage/int/pr/berlin_wall_1989_strap_9nov09/h1/-/news/1/hi/in_depth/europe/2009/1989_europes_revolution/default.stm of experimental conditions.
The most impressive work on developing β-functionalized meso-arylporphyrins as dye sensitizers for DSSCs was produced by Officer and his co-workers. They found that introduction of both cyano and carboxy groups to π-substituents at the β-substituent sufficiently enhanced the conversion efficiency of the solar cell, and they could obtain a conversion efficiency of up to 5.6% using Por-10.15i Also, they later succeeded in obtaining a much higher conversion efficiency by using Por-11 bearing a malonate anchoring group. The Por-11 enhances the conversion efficiency of up to 7.1%, which is the highest value among porphyrin sensitizers that has been developed for DSSCs so far.15j
Apart from the above-mentioned porphyrin monomer sensitizers, porphyrin dyads are also interesting for DSSC research. In the light-harvesting antenna system of photosynthetic anoxygenic bacteria, many bacteriochlorophyll molecules can form large aggregates to absorb solar energy. However, in DSSCs, a large aggregate of dye molecules may cause serious exciton annihilation. Compared to aggregates, a dyad of porphyrin is, however, not too big in size, but can enhance the LHE of solar cells. Fig. 5 shows a pair of efficient porphyrin dyad molecules that have been used for fabricating DSSCs. Por-1215v is a meso–meso linked porphyrin molecule. The two meso-porphyrin moieties in the dyad are directly bound and are nearly perpendicular to each other. One of the two porphyrin moieties is a zinc complex, while the other is a free base. This difference in the central positions must cause a shift in energy levels of the HOMO and LUMO for each porphyrin moiety favoring energy-transfer to take place between the two moieties and toward the carboxy group, when the dyad is excited with solar irradiation. In contrast, Por-1315w consists of two zinc porphyrin moieties, which were π-conjugated through a phenylene–vinylene at the meso- and β-positions of each porphyrin system. The different electron densities at these meso- and β-positions must act as a driving force for intramolecular electron transfer to take place. The electron-withdrawing cyano group adjacent to an anchoring COOH could help the electron flow to the COOH in a molecule. The conversion efficiencies for these two porphyrin dyads were 3.4% and 3.8%, respectively, although the mechanism of electron injection in DSSCs using these dyads has not yet been revealed. Further studies on this kind of system may provide more information for improving DSSC performance.
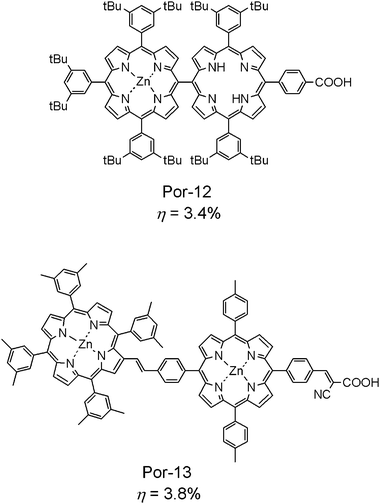 | ||
| Fig. 5 Molecular structures of porphyrin-dyad sensitizers used for DSSCs. | ||
Dye-sensitized solar cells based on naturally originated porphyrin, chlorin and bacteriochlorin sensitizers
The mechanism of DSSCs is partly similar to that of natural photosynthesis involving light-energy migration and charge separation. Thus, it is a good idea to utilize the photosynthetic materials for fabricating DSSCs. Fig. 6 shows the chemical structures of five porphyrin and chlorophyll derivatives, including Cu-mesoporphyrin IX, pheophorbide a, chlorin e6, bacteriopheophorbide a, and bacteriochlorin e6.16a,16b These sensitizers prepared by modifying natural pigments have been studied in DSSCs and have shown low conversion efficiencies. Most carboxy groups in these pigments are not directly conjugated with the π-systems of the cyclic tetrapyrrole ring, except those at the C13-position of chlorin e6 and bacteriochlorin e6. The low conversion efficiencies of DSSCs based on these sensitizers could, therefore, be attributable to the weak interaction between π-electrons in the chromophores and TiO2 films. Based on this consideration, chlorophyll derivatives with a carboxy group directly conjugated to π-electrons of the chromophore are favorable for DSSC sensitizers, and from this viewpoint, chlorophyll c molecules meet the above criteria. The molecular structures of chlorophylls c1 and c2 are shown in Fig. 2. Chlorophyll c molecules are widely distributed in the light-harvesting complexes in some seaweeds and marine planktons including diatoms and brown algae. The η values for DSSCs based on chlorophylls c1 and c2 were 3.4% and 4.6%,16g respectively. The higher conversion efficiency of a chlorophyll c2 sensitized solar cell was attributed to a larger electron density at the position of carboxy group. Therefore, the results on chlorophyll c sensitized solar cells strongly support the idea of developing chlorophyll derivatives having carboxy group(s) conjugated to the cyclic tetrapyrrole ring.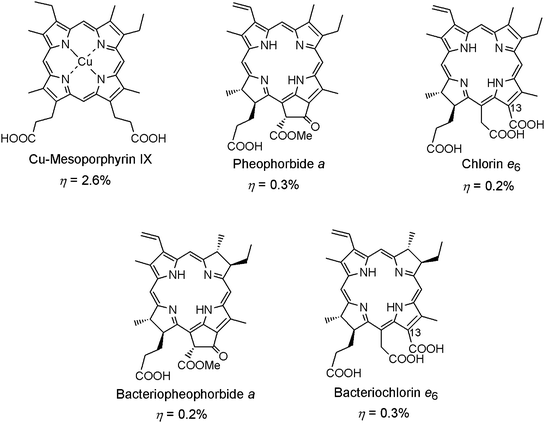 | ||
| Fig. 6 Molecular structures of derivatives of natural pigments related to photosynthesis for DSSC's sensitizers. | ||
The light-harvesting ability is also an important factor for a dye sensitizer. Fig. 7 compares the absorption spectra among three chlorophyll pigments having Por, Chl, and BChl macrocycles, respectively. The intensity of the redmost Q bands increases in the order: Por < Chl < BChl, while that of the B bands decreases in the same order. Thus, compared to Por-based chlorophyll c molecules, (B)Chl-based sensitizers may have higher potential in DSSCs, because they can absorb solar energy in a wider absorption region.
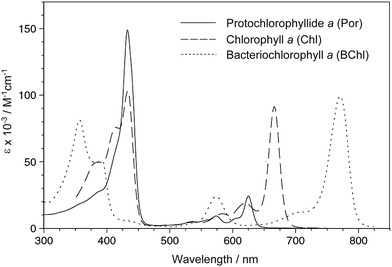 | ||
| Fig. 7 Absorption spectra of naturally occurring chlorophylls with Por, Chl and BChl macrocycles in THF. Protochlorophyllide a is a 17-CH2CH2COOH analog of chlorophyll c1. | ||
To have a strong combination between Chl chromophores and TiO2 semiconductor, the carboxy group needs to directly conjugate with the π-electrons in the Chl macrocycle. The location of the carboxy group could be in the direction of the y- or x-axis (see Fig. 2). The difference in its location will significantly affect the absorption properties and molecular orbitals of Chl sensitizers. In Fig. 8, Chl-1 has an electron-withdrawing π-conjugatable carboxy group at the C3-position on the y-axis, while Chls-2 and 3 have it at the C7- and C8-positions on the x-axis. Therefore, Chl-1 has a much denser Qy band than do Chls-2 and 3. DSSC based on Chl-1 gives much higher photocurrent and performance than those based on Chls-2 and 3. These results proved that the light-harvesting capability of Chl sensitizer is a crucial factor in determining the performance of DSSCs. On the other hand, the photovoltaic performance of Chls-1–3 is much greater than those chlorophyll derivatives presented in Fig. 6, indicating a better combination between the Chls-1–3 and TiO2.16h
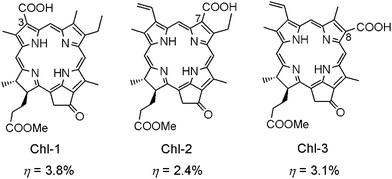 | ||
| Fig. 8 Molecular structures of Chls-1–3 with carboxy group located at C3-, C7- and C8-positions on the Chl macrocycle. | ||
The performance of Chl-1 sensitized solar cells could be further improved by using carotenoids as redox spacers. Chlorophylls and carotenoids always co-exist in natural photosynthetic systems. The intrinsic functions of carotenoids contain light-harvesting, energy- and electron-transferring, and photoprotecting processes. It has been found that when 10% of carotenoid molecules were added in to Chl-1 solution for their adsorption on a TiO2 electrode, an enhanced photocurrent was always obtained. This enhancement was strongly dependent not on the energy level of the low-lying singlet state, but on the one electron-oxidation potential of each carotenoid molecule, suggesting that not energy-transference, but electron-transference has taken place between carotenoid and Chl-1 molecules. The electron-transfer mechanism was further confirmed in time-resolved sub-ps absorption spectra containing Chl-1, carotenoid, and TiO2 nanoparticles in a suspension. In these time-resolved spectra, the decay of the Chl-1 radical cation was always correlated to the rise of carotenoid radical cation, supporting the idea of hole transfer from Chl-1 to carotenoid molecules. The highest conversion efficiency for Chl-1 has been achieved when one of the plant carotenoids, β-carotene, was used as co-adsorbate. However, no trail of energy transfer from these carotenoids to Chl-1 has been observed.16c–16e
A big advantage for cyclic tetrapyrrole sensitizers is that they can choose different coordinating metals. It has been concluded that a central metal like Zn is necessary for a porphyrin sensitizer to perform highly efficiently in DSSC.15f This is the reason that all porphyrin sensitizers listed in the previous section were not free-bases, but coordinated with the Zn2+ ion. However, we also noticed that Chl-1 with a chlorin macrocycle which does not contain any central metal can still give reasonably high performance. Thus, the effect of the central metal on Pors and Chls seems contradictory. In order to clarify this issue, systematical studies on the effect of central metal on sensitizers with both Por and Chl macrocycles are necessary. Fig. 9 shows the chemical structures for (M)Chl-1, (M)Por-c1, and ZnPor-a, having Chl, Por and Por macrocycle, respectively. In the former two sensitizers, substitution effects at the central positions including 2H, Mg, and Zn were compared.
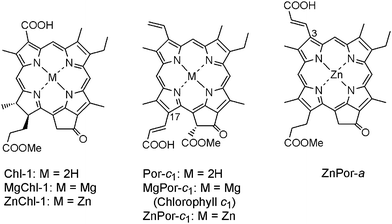 | ||
| Fig. 9 Molecular structures of Chl-1 and Por-c1 having 2H, Zn and Mg at the central position, and of ZnPor-a. | ||
Fig. 10 compares the molecular orbitals and energy levels of HOMO − 1, HOMO, LUMO, and LUMO + 1 for (M)Chl-1 and (M)Por-c1 containing different central metals. For both sensitizers, the energy levels of all these molecular orbitals have been found to be shifted toward a higher energy level upon coordinating with Zn2+ or Mg2+ ions. According to the reported study on the effect of central metals in Por sensitizers,15b this shift of energy level causes reduced charge recombination between TiO2 and the oxidized Por molecules. Based on this theory, enhanced photocurrent must be obtained not only for solar cells based on metallated Por-c1 with a Por macrocycle, but also for those based on metallated Chl-1 with a Chl macrocycle.
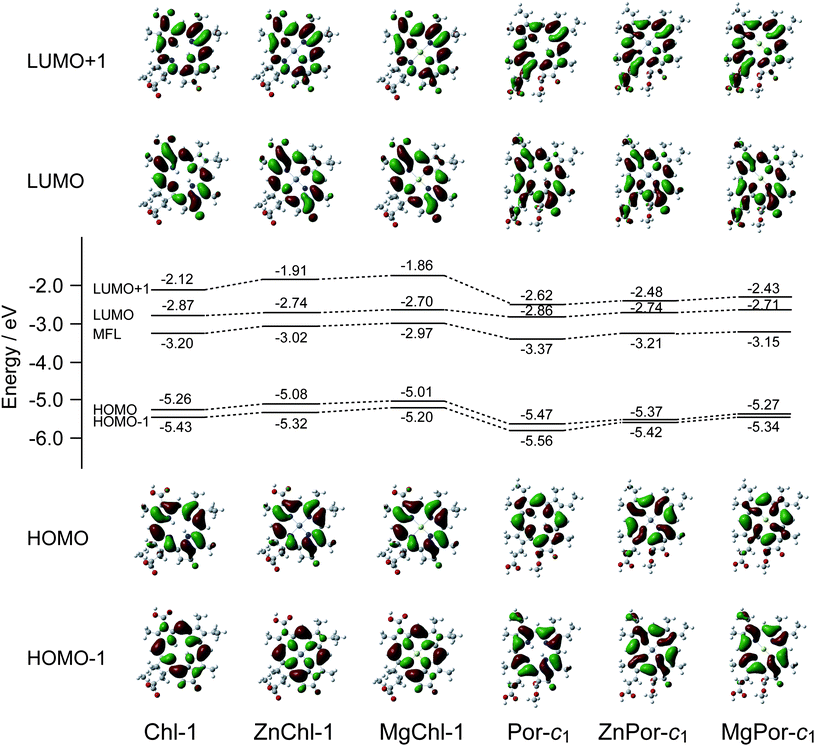 | ||
| Fig. 10 Molecular orbitals of HOMO − 1, HOMO, LUMO, and LUMO + 1, and their energy levels for (M)Chl-1 and (M)Por-c1 having 2H, Zn and Mg at the central position. | ||
The absorption spectra of these (M)Chl-1 and (M)Por-c1 sensitizers in ethanolic solution are shown in Fig. 11. The central metals have caused some different effects on the absorption spectra of Chl- and Por-based sensitizers. In the case of Chl-1, Zn- and Mg-insertion slightly enhanced the intensity of the redmost Qy band and also slightly shifted the peak positions, while it substantially reduced the intensity of the absorption bands at around 500–550 nm where the apparent Qx bands were observed in Chl-1. On the other hand, these central metals in Por-c1 substantially induced shifts of the redmost Q peaks to a longer wavelength region than that of a free base Por-c1 sensitizer. These shifts can endow the metallated Por-c1 sensitizers additional absorption capability in the longer wavelength region. Actually, the solar irradiation is most strong at around 500 nm, where the absorptions of metallated Por and Chl are relatively weak. The Qx bands of the free base Chl pigments are located in this important region. Therefore, DSSCs based on free based Chl sensitizers should provide a larger photocurrent generation than that based on Por sensitizers.
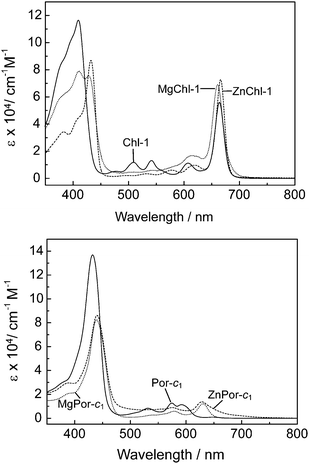 | ||
| Fig. 11 Absorption spectra of (M)Chl-1 (upper) and (M)Por-c1 (lower) having 2H, Zn and Mg at the central position in ethanol. | ||
Fig. 12 shows the incident photon-to-current conversion efficiency (IPCE) profiles and photocurrent-photovoltage (I–V) curves of DSSCs based on (M)Chl-1 and (M)Por-c1 with 2H, Mg, and Zn at the central positions. The IPCE and short-circuit current (Jsc) increases in the order, Zn > Mg > 2H for Por-c1, but in a different order, 2H > Zn > Mg for Chl-1. The ranking order of photocurrent for Por-c1 is in good agreement with the observations in other porphyrin sensitizers. The higher photocurrent for Chl-1 compared to Mg/ZnChl-1 is otherwise hardly explained by the charge recombination mechanism. Judging from the IPCE profiles of the three Chl-1 sensitized solar cells, the stronger Qx peaks of Chl-1 played an important role increasing the response of action spectrum in the 500∼600 nm region, where the intensity of solar irradiation is at the maximum. On the other hand, the Mg and Zn metal can shift the HOMO level of the sensitizer to a higher energy level, and this can reduce charge recombination from TiO2 to the dye cation radical to enhance the photocurrent as suggested in the previous investigation.15b However, at the same time, the shift of the HOMO level will decrease the energy gap between the dye cation radical and the I−/I3− in electrolyte to reduce the regeneration rate of the dye sensitizer, and this will result in a reduced photocurrent of the solar cell. It should be noted that the above two processes contain some trade-off. Actually, a perfect sensitizer should have the HOMO level at a certain position, where the rates of charge recombination and of dye regeneration compromise each other and are in harmony. The entire charge transfer in the solar cell could therefore be optimized. Thus, future development of high efficiency dye sensitizers should take this important factor into account.
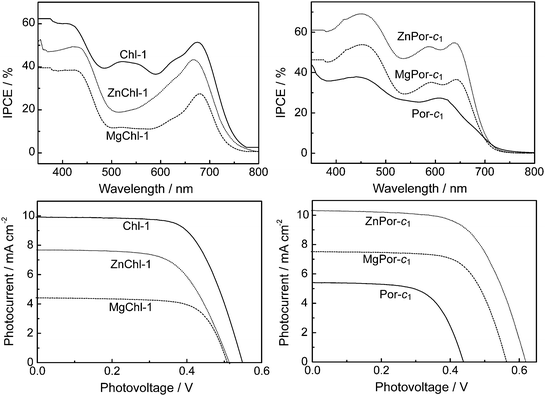 | ||
| Fig. 12 IPCE profiles (upper) and I–V curves (lower) of DSSCs based on (M)Chl-1 (left) and (M)Por-c1 (right) having 2H, Zn and Mg at the central position. | ||
It is an interesting question why the ZnPor-c1 molecule performs so well in DSSC. Can the other zinc porphyrin conjugated with an acrylate residue, ZnPor-a (see right in Fig. 9) result in a similar performance as ZnPor-c1 in DSSC?
In Fig. 13a and b, we compared the molecular orbitals and the energy levels of HOMO − 1, HOMO, LUMO, and LUMO + 1 for ZnPor-c1 and ZnPor-a with 17- and 3-acrylate residues, respectively. Compared to ZnPor-c1, the electron density at the location of carboxy group for LUMO + 1 orbital in ZnPor-a is very low, suggesting a weaker electron combination between ZnPor-a and TiO2. As a result, in Fig. 13c, two sensitizers give rise to completely different photocurrent and photovoltage in the I–V curves, even though their chemical structures are similar. Thus, not only the location of the molecular orbitals, but also the electron density at the carboxy group in the LUMO and LUMO + 1 orbitals of the sensitizer is important in determining the conversion efficiency. Amazingly, naturally occurring chlorophyllous sensitizers have a nearly perfect electron distribution over their cyclic tetrapyrrole rings, and this is a very important point for further development of this kind of sensitizers for DSSCs.
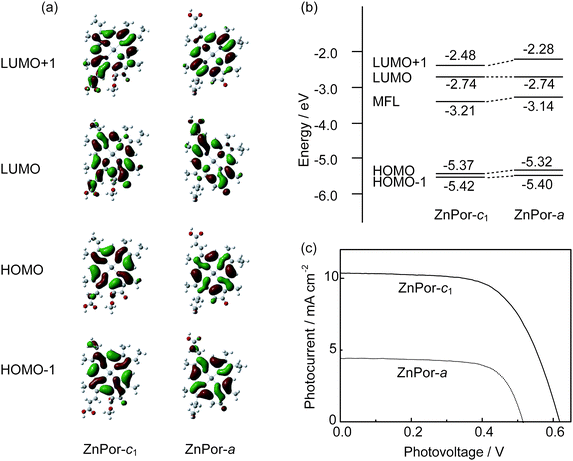 | ||
| Fig. 13 A comparison of the molecular orbitals (a), their energy levels (b), and the photovoltaic performances (c) between ZnPor-c1 and ZnPor-a. | ||
It can be also noted that the absorption spectra for Por and Chl pigments are less overlapped. Fig. 14 shows the absorption spectra of Chl-1 and chlorophyll c2(MgPor-c2) in ethanol solution. The Soret and Q peaks of chlorophyll c2 are nicely interlocked with valleys in the Chl-1 absorption spectrum, so the absorption spectrum of the mixture of two pigments covers a broad wavelength region of 300–700 nm. Therefore, use of both sensitizers together for solar cells would be interesting. In Fig. 15, we co-sensitized Chl-1 with chlorophyll c2 on the TiO2 film in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio to fabricate a solar cell. Compared to DSSCs using either Chl-1 or chlorophyll c2, the co-sensitized solar cell gave a much higher photocurrent and conversion efficiency. An enhancement of conversion efficiency up to 30% has been obtained by co-sensitization. This result suggests that further development of DSSCs based on cyclic tetrapyrrole type sensitizers should also consider the possible co-sensitization to cover as much of the solar spectrum as possible.
1 molar ratio to fabricate a solar cell. Compared to DSSCs using either Chl-1 or chlorophyll c2, the co-sensitized solar cell gave a much higher photocurrent and conversion efficiency. An enhancement of conversion efficiency up to 30% has been obtained by co-sensitization. This result suggests that further development of DSSCs based on cyclic tetrapyrrole type sensitizers should also consider the possible co-sensitization to cover as much of the solar spectrum as possible.
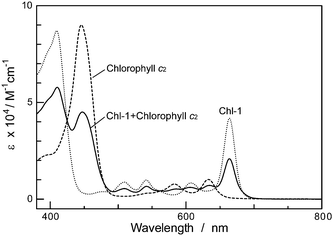 | ||
| Fig. 14 Absorption spectra of Chl-1, chlorophyll c2, and their mixture in ethanol. | ||
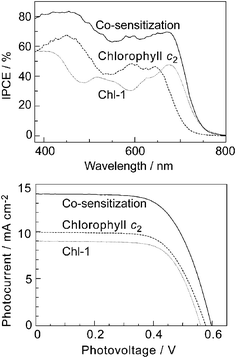 | ||
| Fig. 15 IPCE profiles (upper) and I–V curves (lower) of DSSCs based on Chl-1, chlorophyll c2, and their mixture. | ||
Molecular design based on Chl-1 can lead to further development of better Chl sensitizers. One of the strategies is to extend the π-conjugation length along the y-axis. By this method, we can further increase the molar extinction coefficient of the sensitizer, and simultaneously shift the HOMO orbital to a higher energy level. Chl-4 in Fig. 16 was synthesized based on that strategy, and presented the η value of up to 6.5%. Leaving a vinylene space between the Chl chromophore and carboxy group can be useful in reducing charge recombination. Most importantly, further modification at the C17-propionate residue of Chl-4 with an alkyl substituent, i.e., the dodecyl group, has already led to unprecedented conversion efficiency up to 8.0%.
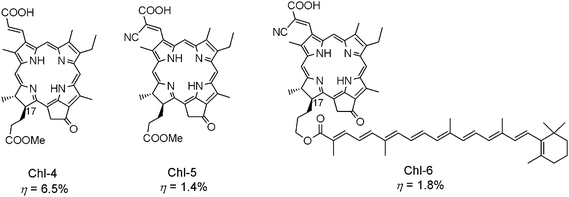 | ||
| Fig. 16 Molecular structures of Chl-4–6 sensitizers for DSSCs. | ||
Compared to Chl-4, Chl-5 with an additional electron withdrawing cyano group in addition to the carboxy group gives much lower conversion efficiency. This is probably due to the energy level of LUMO orbital of this dye sensitizer being shifted to the lower side of the conduction band edge, while introduction of a carotenoid moiety on the C17-substituent in Chl-6 can enhance the conversion efficiency by 30%. This enhancement was attributed to the effects of electron- and energy-transfer reaction from the carotenoid moiety to the Chl macrocycle, as well as to the reduced singlet–triplet annihilation reaction between neighboring dye sensitizers.16f,16i
As shown in Fig. 7, BChl molecules possess a denser and red-shifted Qy band, which usually means a higher light-harvesting capability in this kind of sensitizer. Fig. 17 shows the chemical structures of three typical BChl sensitizers that have been newly developed for DSSCs. The chemical structure of BChl-1 is similar to Chl-1, but possesses a BChl macrocycle. BChl-1 has been compared to Chl-1 in its performance in the DSSC's sensitizer. The conversion efficiency of DSSC based on BChl-1 was 3.7%, which is also similar to that based on Chl-1 (3.8%). Unfortunately, BChl-1 is unstable when deposited on TiO2 films since it undergoes rapid oxidation of the C7–C8 single bond to the double bond. In order to solve this problem and to further improve the performance of a BChl sensitized solar cell, BChl-2 and BChl-3 have been synthesized. Both of them possess an oxo-bacteriochlorin macrocycle, which is much more stable than ordinary bacteriochlorin; furthermore, the π-electrons are extended along the y-axis. This pair of oxo-bacteriochlorin sensitizers gave a much higher photocurrent and conversion efficiency when a suitable electrolyte was used. Interestingly, these oxo-bacteriochlorin sensitizers can readily form J-aggregates on the TiO2 surface. Such a phenomenon has never been observed for other types of Por and Chl sensitizers. The J-aggregates of the synthetic BChl dyes strongly affect the electron lifetime and diffusion coefficient of DSSCs based on such sensitizers. By co-adsorption of the dye with 5 mM chenodeoxycholic acid in solution, the BChl-2 sensitized solar cell exhibited a high conversion efficiency of up to 6.6%.16j Importantly, the solar cell can also give rise to a short-circuit current (Jsc) of more than 18 mA cm−2, which is larger than the values for ordinary organic sensitizers.
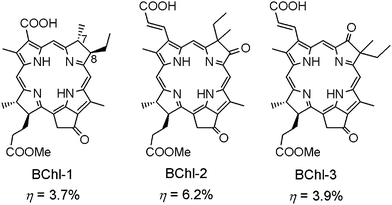 | ||
| Fig. 17 Molecular structures of BChl-1–3 sensitizers for DSSCs. | ||
Dye-sensitized solar cells based on phthalocyanines
Pcs absorb solar energy in both the shorter- and longer-wavelength regions. Moreover, Pcs sensitized TiO2 films are transparent, and this character endows Pcs-sensitized solar cells with a suitable material in their usage as a visible-light transparent window.17a,17b However, the photovoltaic performances of DSSCs based on Pcs are quite poor, compared to those based on Pors and Chls, due to the serious π–π stacking and low light-absorbing capability in the 450–550 nm region. Fig. 18 shows the efficient Pcs that have been developed for DSSC sensitizers. Pc-1 has a carboxy group at one of the four fused benzene rings for binding to the TiO2 surface and the sterically bulky tert-butyl groups disturb its π–π stacking. Using Pc-1 alone as a sensitizer, a maximum conversion efficiency of up to 3.5% was obtained.17c–17f At the same time, the maximum IPCE value at the Q band reached 80%. Furthermore, it has been found that by co-sensitization of Pc-1 with an organic sensitizer, a triarylamine–dithiophene conjugate possessing an acrylate residue on TiO2 film, an enhanced η value can be obtained.17e This result suggests that Pcs are also good candidates for multiple dye co-sensitized solar cells.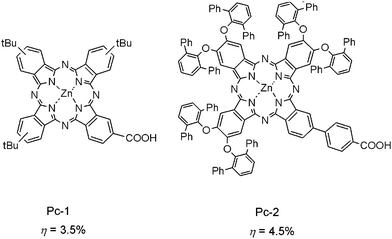 | ||
| Fig. 18 Molecular structures of Pc-1 and 2 sensitizers for DSSCs. | ||
Most recently, a very efficient Pc sensitizer has been synthesized and tested for DSSCs. Pc-2 has a p-carboxyphenyl group attached to a benzene ring which substantially extends π-electrons. Such an extension to a large π-skeleton can sufficiently improve the absorption capability of the sensitizer and six sterically large 2,6-diphenylphenyl groups at the peripheral position avoid aggregation. The Pc-2 sensitized solar cell gives rise to a η value of 4.5%, which is the highest value among Pc sensitized solar cells.17i
Conclusion
Recent advances on dye-sensitized solar cells based on cyclic tetrapyrrole pigments have been reviewed. The highest conversion efficiency of solar cells appearing in the literature to date (August 2009) was 7.1% for Pors, 8.0% for Chls, 6.6% for BChls, and 4.5% for Pcs. It has been found that highly efficient cyclic tetrapyrrole type sensitizers usually possess the following characteristics: 1. a strong combination between the π-electrons in the chromophore and the TiO2 electrode; 2. π-electrons extending out of the cyclic tetrapyrrole ring to enhance the light-harvesting and reduce charge recombination; 3. a suitable HOMO orbital allows the fast forward electron transfer and slow backward charge recombination to take place; 4. they may possess a long alkyl substituent to suppress aggregate formation; 5. relatively broad and intense Q bands to efficiently use solar energy in the visible and near infrared regions; 6. an electron donor and acceptor at both sides of the cyclic macrocycle to facilitate the electron injection toward the TiO2 conduction band. Therefore, it is possible to achieve a high conversion efficiency of up to 10% using a new cyclic tetrapyrrole sensitizer which satisfies all the above requirements. By co-sensitization of different types of cyclic tetrapyrrole molecules, DSSCs based on this group of sensitizers may achieve 12–15% of power conversion efficiency in the near future.Acknowledgements
The authors gratefully acknowledge Dr Shin-ichi Sasaki of Nagahama Institute of Bio-Science and Technology and Dr Osamu Kitao of Institute of Advanced Industrial Science and Technology (AIST) for synthesizing chlorophyllous dye sensitizers and DFT/TD-DFT calculations, respectively. They thank Prof. Yasushi Koyama for his work in initiating and leading the DSSC research project in Kwansei Gakuin University based on principles and materials of photosynthesis, and also appreciate Dr Haoshen Zhou of AIST and Prof. Tsukasa Yoshida of Gifu University for their kind collaboration. This work was partially supported by Grants-in-Aid for Young Scientists (B) (No. 20760606) (to XF.W) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of the Japanese Government and for Scientific Research (B) (No. 19350088) (to H.T) from the Japan Society for the Promotion of Science (JSPS).References
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef CAS; (b) M. Grätzel, Nature, 2001, 414, 338–344 CrossRef CAS; (c) Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638–L640 CrossRef CAS; (d) M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835–16847 CrossRef CAS.
- Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903–2934 CrossRef CAS.
- (a) W.-H. Zhan, W.-J. Wu, J.-L. Hua, Y.-H. Jing, F.-S. Meng and H. Tian, Tetrahedron Lett., 2007, 48, 2461–2465 CrossRef CAS; (b) X. Ma, J. Hua, W. Wu, Y. Jin, F. Meng and H. Tian, Tetrahedron, 2008, 64, 345–350 CrossRef CAS; (c) W. Wu, J. Hua, Y. Jin, W. Zhan and H. Tian, Photochem. Photobiol. Sci., 2008, 7, 63–68 RSC.
- Y.-S. Chen, C. Li, Z.-H. Zeng, W.-B. Wang, X.-S. Wang and B.-W. Zhang, J. Mater. Chem., 2005, 15, 1654–1661 RSC.
- C. Li, J.-H. Yum, S.-J. Moon, A. Herrmann, F. Eickemeyer, N. G. Pschirer, P. Erk, J. Schöneboom, K. Müllen, M. Grätzel and M. K. Nazeeruddin, ChemSusChem, 2008, 1, 615–618 CrossRef CAS.
- N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256–14257 CrossRef CAS.
- Z.-S. Wang, Y. Cui, Y. Dan-oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224–7230 CrossRef CAS.
- (a) S. Kim, J. K. Lee, S. O. Kang, J. Ko, J.-H. Yum, S. Fantacci, F. D. Angelis, D. Di Censo, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 16701–16707 CrossRef CAS; (b) H. Choi, S. O. Kang, J. Ko, G. Gao, H. S. Kang, M.-S. Kang, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2009, 48, 5938–5941 CrossRef CAS.
- S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Péchy and M. Grätzel, Chem. Commun., 2008, 5194–5196 RSC.
- G. Zhang, H. Bala, Y. Cheng, D. Shi, X. Lv, Q. Yu and P. Wang, Chem. Commun., 2009, 2198–2200 RSC.
- (a) A. Hagfeldt and M. Grätzel, Acc. Chem. Res., 2000, 33, 269–277 CrossRef CAS; (b) M. Grätzel, Inorg. Chem., 2005, 44, 6841–6851 CrossRef.
- (a) M. Gouterman, J. Chem. Phys., 1959, 30, 1139–1161 CrossRef CAS; (b) M. Gouterman, J. Mol. Spectrosc., 1961, 6, 138–163 CrossRef CAS.
- (a) D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2001, 34, 40–48 CrossRef CAS; (b) D. M. Guldi, Chem. Soc. Rev., 2002, 31, 22–36 RSC; (c) M. R. Wasielewski, J. Org. Chem., 2006, 71, 5051–5066 CrossRef CAS; (d) H. Scheer, in Chlorophylls, ed. H. Scheer, CRC Press, Boca Raton, 1991, ch. 1 Search PubMed; (e) H. Scheer, in Chlorophylls and Bacteriochorophylls, ed. B. Grimm, R. J. Porra, W. Rüdiger and H. Scheer, Springer, Dordrecht, 2006, ch. 1 Search PubMed; (f) H. Tamiaki, R. Shibata and T. Mizoguchi, Photochem. Photobiol., 2007, 83, 152–162 CAS.
- C. W. Tang, Appl. Phys. Lett., 1986, 48, 183–185 CrossRef CAS.
- (a) S. Cherian and C. C. Wamser, J. Phys. Chem. B, 2000, 104, 3624–3629 CrossRef CAS; (b) Y. Tachibana, S. A. Haque, I. P. Mercer, J. R. Durrant and D. R. Klug, J. Phys. Chem. B, 2000, 104, 1198–1205 CrossRef CAS; (c) T. Ma, K. Inoue, H. Noma, K. Yao and E. Abe, J. Photochem. Photobiol., A, 2002, 152, 207–212 CrossRef; (d) F. Odobel, E. Blart, M. Lagrée, M. Villieras, H. Boujtita, N. E. Murr, S. Caramori and C. A. Bignozzi, J. Mater. Chem., 2003, 13, 502–510 RSC; (e) D. F. Watson, A. Marton, A. M. Stux and G. J. Meyer, J. Phys. Chem. B, 2004, 108, 11680–11688 CrossRef CAS; (f) W. M. Campbell, A. K. Burrell, D. L. Officer and K. W. Jolley, Coord. Chem. Rev., 2004, 248, 1363–1379 CrossRef CAS; (g) M. K. Nazeeruddin, R. Humphry-Baker, D. L. Officer, W. M. Campbell, A. K. Burrell and M. Grätzel, Langmuir, 2004, 20, 6514–6517 CrossRef CAS; (h) L. Schmidt-Mende, W. M. Campbell, Q. Wang, K. W. Jolley, D. L. Officer, M. K. Nazeeruddin and M. Grätzel, ChemPhysChem, 2005, 6, 1253–1258 CrossRef; (i) Q. Wang, W. M. Campbell, E. E. Bonfantani, K. W. Jolley, D. L. Officer, P. J. Walsh, K. Gordon, R. Humphry-Baker, M. K. Nazeeruddin and M. Grätzel, J. Phys. Chem. B, 2005, 109, 15397–15409 CrossRef CAS; (j) W. M. Campbell, K. W. Jolley, P. Wagner, K. Wagner, P. J. Walsh, K. C. Gordon, L. Schmidt-Mende, M. K. Nazeeruddin, Q. Wang, M. Grätzel and D. L. Officer, J. Phys. Chem. C, 2007, 111, 11760–11762 CrossRef CAS; (k) M. Tanaka, S. Hayashi, S. Eu, T. Umeyama, Y. Matano and H. Imahori, Chem. Commun., 2007, 2069–2071 RSC; (l) J. Rochford, D. Chu, A. Hagfeldt and E. Galoppini, J. Am. Chem. Soc., 2007, 129, 4655–4665 CrossRef CAS; (m) J. R. Stromberg, A. Marton, H. L. Kee, C. Kirmaier, J. R. Diers, C. Muthiah, M. Taniguchi, J. S. Lindsey, D. F. Bocian, G. J. Meyer and D. Holten, J. Phys. Chem. C, 2007, 111, 15464–15478 CrossRef CAS; (n) A. Forneli, M. Planells, M. A. Sarmentero, E. Martinez-Ferrero, B. C. O'Regan, P. Ballester and E. Palomares, J. Mater. Chem., 2008, 18, 1652–1658 RSC; (o) S. Eu, S. Hayashi, T. Umeyama, Y. Matano, Y. Araki and H. Imahori, J. Phys. Chem. C, 2008, 112, 4396–4405 CrossRef CAS; (p) S. Hayashi, M. Tanaka, H. Hayashi, S. Eu, T. Umeyama, Y. Matano, Y. Araki and H. Imahori, J. Phys. Chem. C, 2008, 112, 15576–15585 CrossRef CAS; (q) J. K. Park, H. R. Lee, J. Chen, H. Shinokubo, A. Osuka and D. Kim, J. Phys. Chem. C, 2008, 112, 16691–16699 CrossRef CAS; (r) M. P. Balanay and D. H. Kim, Phys. Chem. Chem. Phys., 2008, 10, 5121–5127 RSC; (s) C.-W. Lee, H.-P. Lu, C.-M. Lan, Y.-L. Huang, Y.-R. Liang, W.-N. Yen, Y.-C. Liu, Y.-S. Lin, E. W.-G. Diau and C.-Y. Yeh, Chem.–Eur. J., 2009, 15, 1403–1412 CrossRef CAS; (t) S. J. Lind, K. C. Gordon, S. Gambhir and D. L. Officer, Phys. Chem. Chem. Phys., 2009, 11, 5598–5607 RSC; (u) Y. Liu, N. Xiang, X. Feng, P. Shen, W. Zhou, C. Weng, B. Zhao and S. Tan, Chem. Commun., 2009, 2499–2501 RSC; (v) J. T. Dy, K. Tamaki, Y. Sanehira, J. Nakazaki, S. Uchida, T. Kubo and H. Segawa, Electrochemistry, 2009, 77, 206–209 CAS; (w) D. L. Officer, Presentation at the 3rd International Conference on the Industrialization of DSC, Nara, Japan, 2009 Search PubMed.
- (a) A. Kay and M. Grätzel, J. Phys. Chem., 1993, 97, 6272–6277 CrossRef; (b) Y. Amao and Y. Yamada, Biosens. Bioelectron., 2007, 22, 1561–1565 CrossRef CAS; (c) X.-F. Wang, J. Xiang, P. Wang, Y. Koyama, S. Yanagida, Y. Wada, K. Hamada, S. Sasaki and H. Tamiaki, Chem. Phys. Lett., 2005, 408, 409–414 CrossRef CAS; (d) X.-F. Wang, Y. Kakitani, J. Xiang, Y. Koyama, F. S. Rondonuwu, H. Nagae, S. Sasaki and H. Tamiaki, Chem. Phys. Lett., 2005, 416, 229–233 CrossRef CAS; (e) X.-F. Wang, M. Arihiro, Y. Koyama, H. Nagae, S. Sasaki, H. Tamiaki and Y. Wada, Chem. Phys. Lett., 2006, 423, 470–475 CrossRef CAS; (f) X.-F. Wang, Y. Koyama, Y. Wada, S. Sasaki and H. Tamiaki, Chem. Phys. Lett., 2007, 439, 115–120 CrossRef CAS; (g) X.-F. Wang, C.-H. Zhan, T. Maoka, Y. Wada and Y. Koyama, Chem. Phys. Lett., 2007, 447, 79–85 CrossRef CAS; (h) X.-F. Wang, Y. Koyama, H. Nagae, Y. Wada, S. Sasaki and H. Tamiaki, J. Phys. Chem. C, 2008, 112, 4418–4426 CrossRef CAS; (i) X.-F. Wang, O. Kitao, H. Zhou, H. Tamiaki and S. Sasaki, Chem. Commun., 2009, 1523–1525 RSC; (j) X.-F. Wang, O. Kitao, H. Zhou, H. Tamiaki and S. Sasaki, J. Phys. Chem. C, 2009, 113, 7954–7961 CrossRef CAS.
- (a) J. He, G. Benkö, F. Korodi, T. Polívka, R. Lomoth, B. Åkermark, L. Sun, A. Hagfeldt and V. Sundström, J. Am. Chem. Soc., 2002, 124, 4922–4932 CrossRef CAS; (b) J. He, A. Hagfeldt, S.-E. Lindquist, H. Grennberg, F. Korodi, L. Sun and B. Åkermark, Langmuir, 2001, 17, 2743–2747 CrossRef CAS; (c) E. Palomares, M. V. Martínez-Díaz, S. A. Haque, T. Torres and J. R. Durrant, Chem. Commun., 2004, 2112–2113 RSC; (d) P. Y. Reddy, L. Giribabu, C. Lyness, H. J. Snaith, C. Vijaykumar, M. Chandrasekharam, M. Lakshmikantam, J.-H. Yum, K. Kalyanasundaram, M. Grätzel and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2007, 46, 373–376 CrossRef CAS; (e) J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358–8362 CrossRef CAS; (f) J.-H. Yum, S.-R. Jang, R. Humphry-Baker, M. Grätzel, J.-J. Cid, T. Torres and M. K. Nazeeruddin, Langmuir, 2008, 24, 5636–5640 CrossRef CAS; (g) S. Eu, T. Katoh, T. Umeyama, Y. Matano and H. Imahori, Dalton Trans., 2008, 5476–5483 RSC; (h) B. C. O'Regan, I. López-Duarte, M. V. Martínez-Díaz, A. Forneli, J. Albero, A. Morandeira, E. Palomares, T. Torres and J. R. Durrant, J. Am. Chem. Soc., 2008, 130, 2906–2907 CrossRef CAS; (i) K. Yasuta, R. Goto, S. Mori and M. Kimura, 76th Annual Meeting on Electrochemistry, Kyoto, Japan, 2009, 1A27 Search PubMed.
- (a) N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338–2345 CrossRef CAS; (b) Z. Chen, F. Li and C. Huang, Curr. Org. Chem., 2007, 11, 1241–1258 CrossRef CAS; (c) T. W. Hamann, R. A. Jensen, A. B. F. Martinson, H. V. Ryswyk and J. T. Hupp, Energy Environ. Sci., 2008, 1, 66–78 RSC; (d) A. Mishra, M. K. R. Fischer and P. Bauerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS; (e) Z. Ning and H. Tian, Chem. Commun., 2009, 5483–5495 RSC; (f) H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009 DOI: 10.1021/ar900034t; (g) G. Calogero, G. D. Marco, S. Caramori, S. Cazzanti, R. Argazzi and C. A. Bignozzi, Energy Environ. Sci., 2009, 2, 1162–1172 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
