A reversible zwitterionic SO2-binding organic liquid†
David J.
Heldebrant
*a,
Phillip K.
Koech
b and
Clement R.
Yonker
c
aPacific Northwest National Laboratory, Materials Chemistry and Surface Research Group, Energy and Efficiency Division, Richland, WA 99352, USA. E-mail: david.heldebrant@pnl.gov; Tel: +1 509-372-6359; Fax: +1 509-375-2186
bPacific Northwest National Laboratory, Materials Chemistry and Surface Research Group, Energy and Efficiency Division, Richland, WA 99352, USA. E-mail: phillip.koech@pnl.gov; Fax: +1 509-375-2186; Tel: +1 509-372-6891
cPacific Northwest National Laboratory, Molecular Interactions & Transformations Group, Fundamental and Computational Sciences Directorate, Richland, WA 99352, USA. E-mail: clem.yonker@pnl.gov; Fax: +1 509-376-6660; Tel: +1 509-372-4748
First published on 28th September 2009
Abstract
N,N-Dibutylundecanolamine is a liquid that chemically binds SO2 to form a viscous zwitterionic liquid that contains 35% by wt. SO2 at standard temperature and pressure. SO2 is chemically bound to the alcohol component as an alkylsulfite, which is then stabilized by the amine. The zwitterionic liquid can be reverted to its non-ionic form and recycled by thermally stripping the SO2 under vacuum at temperatures near 70 °C. N,N-Dibutylundecanolamine is a potential flue gas desulfurizing solvent because it is chemically selective to bind SO2 but not basic enough to chemically bind CO2.
Broader contextWith global consumption of energy derived from carbon-based fuels expected to continue rising, capturing greenhouse gases other than CO2 becomes a necessity. SO2 is a major component of acid rain, and is captured using aqueous lime and caustic soda solutions, which are corrosive, and irreversible. While SO2 is considered a waste product, it can be recovered and used in the wine and cement industries rather than being permanently sequestered. It was our goal to design a more environmentally benign solvent system that can reversibly capture SO2 as well as use it as a solvent miscibility trigger. |
Introduction
As fossil fuel consumption continues, there is much attention being focused on capturing acid gas emissions from power plants. We have recently investigated SO2-binding organic liquids (SO2BOLs) as means to reversibly capture and release SO2 selectively over CO2.1 SO2BOLs are mixtures of tertiary amines and alcohols, which bind SO2 as liquid ammonium alkylsulfite salts. Tertiary amines selectively bind SO2 over CO2 because tertiary amines are basic enough to accept a proton from sulfurous and alkylsulfurous acids but not carbonic or alkylcarbonic acids. Other groups have absorbed SO2 physically in ionic liquids2–4 or chemically absorb SO2 in organic systems5–7 or as irreversible aqueous bisulfite or sulfite salts.8–11We believed that a bifunctional tertiary alkanolamine would be able to capture SO2 comparable to our binary SO2BOL system. We present here a reversible zwitterionic liquid produced from the reaction of SO2 with N,N-dibutylundecanolamine (DBUA). The SO2 is chemically bound through the alcohol moiety as an alkylsulfite, which we believe is stabilized by hydrogen bonding through the protonated amine portion of the molecule.
Our recent interest in SO2BOLs led us to explore the possible reaction of tertiary alkanolamines with SO2 to form reversible zwitterionic liquids. Pairing the base and the alcohol on one molecule provides reduced volatility of the base and alcohol components and provides unimolecular phase behaviour compared to bimolecular reversible ionic liquid systems. To our knowledge there are few examples of zwitterionic liquids, and none of them have been shown to be reversible (polar to non-polar).12–14 Our first target molecule was DBUA because we envisioned long alkyl chains keeping the alkanolamine as a liquid before and after exposure to SO2. N,N-Dibutylundecanolamine was synthesized in 88% isolated yield by refluxing dibutylamine and 11-bromoundecanol in ethanol (Fig. 1).
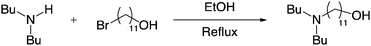 | ||
| Fig. 1 Synthetic route for making DBUA. | ||
The isolated DBUA was a clear and yellow viscous liquid. Spectroscopic characterization of DBUA was performed using both 1H in CDCl3 and 13C NMR of the neat liquid (Table 1). The 1H NMR of the free DBUA in CDCl3 showed the R-CH2-O hydrogens (1 using the numbering scheme in Fig. 2) as a triplet at 3.65 ppm. All three R-CH2-N hydrogens (11,12) were observed as a triplet at 2.4 ppm. Terminal CH3 triplets on the butyl chains (15) were seen at 0.9 ppm. The remaining internal CH2 hydrogens in the butyl and undecyl chains (2–10,13,14) were observed as complex multiplets from 1.2 to 1.6 ppm. The O–H was not observed likely because of overlap of other hydrogen peaks. The infrared (IR) spectrum of DBUA neat on NaCl disks showed a strong and broad O–H band at 3333 cm−1, and aliphatic C–H bands at 2800–3000 cm−1.
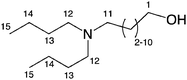 | ||
| Fig. 2 Numerical assignment of DBUA. | ||
Anhydrous DBUA when exposed to one equivalent of SO2 generates a highly viscous yellow liquid, which we assign to be the zwitterion (Fig. 3). Amine and alcohol blends have been shown to increase in viscosity when a liquid alkylsulfite salt is produced.1 After one equivalent of SO2 is chemically absorbed to form the zwitterion, the viscosity then begins to decrease as more SO2 is physically absorbed in the ionic liquid. Physically absorbed SO2 in an ionic liquid acts like a molecular lubricant which has been shown to reduce the viscosity of numerous ionic liquids.2–4 The zwitterion was soluble in CDCl3 and MeCN and was fully characterized by NMR. The 1H NMR spectrum (CDCl3) showed minimal downfield shifting of the R-CH2-O protons (1) from 3.65 to 3.70. Slight shifting of the protons (1) was also observed in our previous studies of alkylsulfites, which we attributed to the shielding of the CH2 protons by the large sulfur atom counteracting the electronegativity of the sulfoxylate anion.1 The three R-CH2-N protons (11,12) on the amine functionality were all observed at 2.95 ppm, 0.5 ppm downfield from the unbound DBUA at 2.40 ppm. The 0.5 ppm downfield shifting is in agreement with other ammonium alkylsulfite salts.1 The spectrum showed a broad singlet centered at 10.2 ppm corresponding to the protonated amine [N–H]+. The viscous liquid is not likely a sulfonamide-like salt because the amine is sterically hindered. The 13C spectrum of the neat zwitterion showed minor upfield shifting of the R-CH2-O (1) carbon by 0.6 ppm and the R-CH2-N (11,12) carbons by 1.6 ppm. The 1H and 13C chemical shifts are in agreement with other liquid alkylsulfite salts.1 High resolution mass spec (+mode analysis) of the DBUA matched the expected DBUA +1 peak, however the zwitterion molecular ion peak was not depleted because SO2 had been cleaved under ionizing conditions and only the DBUA +1 peak was observed.
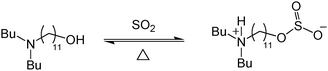 | ||
| Fig. 3 Reaction of DBUA with SO2. | ||
The infrared (IR) spectrum of the neat zwitterion on NaCl plates showed the strong O–H band had been replaced by a weak N–H band at 3384 cm−1. Medium intensity [R3N–H]+ bands were observed at 2646 and 2548 cm−1 similar to trialkylammonium salts.15 Strong ROSO2− bands appeared at 1322, 1170, and 678 cm−1, which are in agreement with other sulfite salts.1,16,17 After SO2 stripping, the ROSO2−, N–H and [R3N–H]+ bands disappeared, and the O–H band reappeared. Crystallographic analysis could not be performed because the zwitterion was a liquid.
DBUA was unreactive towards CO2. An NMR sample (10 mg DBUA in 0.8 mL of CDCl3) was sparged for ten minutes with CO2 and then characterized by 1H NMR. There was no mass increase in the sample associated with CO2 uptake, nor was there a change in the 1H NMR spectrum of DBUA in the presence of CO2. The same NMR sample was then sparged with SO2 for 5 minutes and spectroscopically characterized. The 1H NMR spectrum matched that of DBUA-SO2, indicating SO2 was bound by DBUA. The ability of DBUA to capture SO2 and not CO2 suggests chemically selective desulfurization of gas streams is possible.
One milliliter of DBUA (0.96 grams, 3.3 mmol) absorbed 0.52 grams (8.1 mmol) of SO2, 35% SO2 by weight (0.21 g chemical, 0.31 g physical). The zwitterion did not liberate gas at room temperature, however it did liberate gas as temperature was increased. The physically absorbed SO2 can be removed by heating the zwitterion or by placing it under vacuum at room temperature. The chemically bound SO2 is more difficult to strip. It can be removed at 70 °C with a nitrogen sparge overnight, or a more rapid stripping of SO2 can be done in 40 minutes at 70 °C under vacuum. The DBUA can be quantitatively recovered (3.3 mmol, >99%) after SO2 stripping. The DBUA was exposed to SO2 and subsequently stripped for 2 cycles; both cycles showed absorption of 8.1 mmol of SO2 and complete stripping of SO2 without any significant loss of material. DBUA is predicted to be recycled indefinitely as long as there is no contact with moisture, wherein a thermally stable bisulfite salt would be produced.
The polarity switching of DBUA between its nonionic and zwiterionic forms was demonstrated by placing 0.35 mL of DBUA and 0.35 mL of hexanes (Fig. 4) in a glass vial. DBUA was miscible with hexanes until SO2 was sparged through the solution until one molar equivalent of SO2 had been chemically bound (10 minutes at 0 °C). The hexanes partitioned out into a separate phase when DBUA was converted into its more polar zwitterionic form.
 | ||
| Fig. 4 Left: DBUA and hexanes, Right: DBUA-SO2 and hexanes. | ||
SO2 is chemically bound on the alcohol component of DBUA as an alkylsulfite and not a sulfonamide because of the steric hindrance of the amine functionality. The alkyl chains on the nitrogen prohibit access of the amine to coordinate to SO2, leaving the alcohol as the lone nucleophilic center on the molecule. SO2 reacts with alcohols to form thermodynamically unstable alkylsulfonic acids comparable to SO2 reacting with water to form sulfurous acid. Alkylsulfonic acids are likely to be stabilized in the presence of non-nucleophilic organic bases to form alkylsulfite salts similarly to alkylcarbonic acids which can be stabilized by strong organic bases.1,18–22 We predict that the zwitterion is a liquid because of the large undecanyl group between the cationic and anionic portions of the molecule as well as the butyl groups on the nitrogen. We are currently investigating the binding energies and kinetics of SO2 absorption in comparison to our dual component SO2BOL systems. Investigations of the effect of alkyl chain length of the amines and the alkyl spacer between the amines and alcohol to determine the effect of spacing on the physical properties of the resulting zwitterions.
In conclusion, DBUA reversibly reacts with SO2 to form a room temperature, reversible zwitterionic liquid. The SO2 is chemically bound through the alchohol as an alkylsulfite, which is stabilized by the base component. The zwitterion can contain up to 35% by weight SO2, and can be thermally reversed at 70 °C. The reversible zwitterion can be applied to phase separation chemistry and selective desulfurization of gas streams.
Experimental section
All reactions were run under an atmosphere of argon, unless otherwise indicated. Solvents were transferred by a plastic syringe. Flasks were oven-dried and cooled under a stream of argon. Ethanol was purchased from Fisher and used without further purification. SO2 was purchased from the Aldrich chemical company and used without purification. High-resolution mass spectra (HRMS) were obtained on a LTQ Orbitrap™ Mass Spectrometer from Thermo Scientific Company as m/e (relative intensity). Accurate masses are reported for the molecular ion (M + 1) or a suitable fragment ion. IR measurements were performed on NaCl disks using a Nicolet Magna-750 spectrometer running on OMNIC software. Proton nuclear magnetic resonance (1H NMR) spectra were recorded with a Varian (300 MHz) spectrometer. Chemical shifts are reported in delta (δ) units, parts per million (ppm) downfield from trimethylsilane. Coupling constants are reported in Hertz (Hz). Carbon-13 nuclear magnetic resonance (13C NMR) spectra were recorded with a Varian 300 (75.5MHz) spectrometer. Chemical shifts are reported in delta (δ) units, parts per million (ppm) relative to the singlet of the terminal CH3 on the butyl groups of dibutylamine at 14.2 ppm.11-(N,N-Di-n-butylamino)-1-undecanol (DBUA) was prepared according to a modified literature procedure.23 Dibutylamine (9.4 mL, 55.70 mmol) and 11-bromo-1-undecanol (4.0g, 15.92 mmol) were charged into a clean dry 250 mL flask. Ethanol (100 mL) was added, the resulting solution was heated to reflux under an argon atmosphere for 18 h at which point the reaction mixture was allowed to cool to room temperature and then the solvent was removed in vacuo to afford a white solid. This crude product was dissolved in water (100 mL), and the pH was adjusted to 9.0 using solid Na2CO3. This solution was extracted with CHCl3 (3 × 30 mL), the organic layers were combined, dried over MgSO4, filtered, and then concentrated to provide a pale yellow oil. Excess dibutylamine was distilled off under vacuum at 90 °C. The final product was recovered as a pale yellow oil (4.2g, 88% yield.)
DBUA was syringed into oven-dried round bottom flask fitted with a rubber septa and weighed. The septa was punctured with a needle connected to a N2 bubbler and a polyetheretherketone (PEEK) 1/16″ tube connected to a lecture bottle of SO2. The flask was placed in an ice water bath and then DBUA was sparged with SO2 to form the zwitterion. The formation of DBUA-SO2 was very exothermic and was kept cold until only physically absorbed SO2 was dissolving into the zwitterionic liquid. The sample was continually sparged with SO2 while the flask was warmed to 25 °C. Once the uptake had finished, the flask was quickly vacuumed and refilled with N2 three times to remove any gaseous SO2 in the flask. The flask was then weighed to record the SO2 gravimetric uptake at 25 °C.
Stripping of SO2 was performed by placing DBUA-SO2 and a 1″ stir bar in a flask fitted with a jacketed condenser. The flask was then placed in an oil bath set to 70 °C under vacuum. The flask was heated under vacuum for 40 minutes while stirring. The flask was then cooled and weighed.
References
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Chem.–Eur. J., 2009, 15, 7619–7627 CrossRef CAS.
- Y. Wang, H. Pan, H. Li and C. Wang, J. Phys. Chem. B, 2007, 111, 10461–10467 CrossRef CAS.
- L. J. A. Siqueira, R. A. Ando, F. F. C. Bazito, R. M. Torresi, P. S. Santos and M. C. C. Ribeiro, J. Phys. Chem. B, 2008, 112, 6430–6435 CrossRef CAS.
- J. L. Anderson, J. K. Dixon, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B: Lett., 2006, 110, 15059–15062 Search PubMed.
- L. R. Drake, S. C. Stowe and A. M. Partansky, J. Am. Chem. Soc., 1946, 68, 2521–2524 CrossRef CAS.
- N. Jiang, D. Vinci, C. L. Liotta, C. A. Eckert and A. Ragauskas, Ind. Eng. Chem. Res., 2008, 47, 627–631 CrossRef CAS.
- D. Vinci, M. Donaldson, J. P. Hallett, E. A. John, P. Pollet, C. A. Thomas, J. D. Grilly, P. G. Jessop, C. L. Liotta and C. A. Eckert, Chem. Commun., 2007, 1427 RSC.
- A. B. M. Heesinik and W. P. M. Vanswaaij, Chem. Eng. Sci., 1995, 50, 2983–2996 CrossRef.
- A. Bandyopadhyay and M. N. Biswas, Chem. Eng. Commun., 2006, 193, 1562–1580 CrossRef CAS.
- R. J. Gleason, M. Richmanand P. E. Cooke, U.P. 06/176796. T., Ed.; Research-Cottrell, Inc.: USA, 1982.
- E. R. Lindgren, D. W. Pershing, D. A. Kirchgessner and D. C. Drehmel, Environ. Sci. Technol., 1992, 26, 1427–1433 CrossRef CAS.
- D. Fang, Z.-h. Fei and Z.-l. Liu, Monatsh. Chem., 2008, 139, 799–803 CrossRef CAS.
- A. Narita, W. Shibayama and H. Ohno, J. Mater. Chem., 2001, 11, 1057 RSC.
- H. Ohno. Electrochemical Aspects of Ionic Liquids; 1 ed.; John Wiley & Sons, 2005 Search PubMed.
- R. M. Silverstein, G. C. Bassler and T. C. Morrill. Spectroscopic Identification of Organic Compounds; 5th ed.; John Wiley and Sons: New York, 1991 Search PubMed.
- I. C. Hisatsune and J. Heickle, Can. J. Chem., 1975, 53, 2646 CrossRef CAS.
- M. Albrecht, M. Lutz, A. M. M. Schreurs, E. T. H. Lutz, A. L. Spek and G. VanKoten, J. Chem. Soc., Dalton Trans., 2000, 3797–3804 RSC.
- D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, J. Org. Chem., 2005, 70, 5335–5338 CrossRef CAS.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Energy Environ. Sci., 2008, 1, 487–493 RSC.
- P. G. Jessop, D. J. Heldebrant, X. W. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS.
- P. G. Jessop, D. J. Heldebrant, X. W. Li, J. Lu, J. P. Hallett, R. S. Jones, P. Pollet, C. A. Thomas, C. A. Eckert and C. L. Liotta, Abstr. Pap. Am. Chem. Soc., 2005, 229, U971–U971.
- L. Phan, D. Chiu, D. J. Heldebrant, H. Huttenhower, E. John, X. W. Li, P. Pollet, R. Y. Wang, C. A. Eckert, C. L. Liotta and P. G. Jessop, Ind. Eng. Chem. Res., 2008, 47, 539–545 CrossRef CAS.
- W. Q. Haolong Li and L. Wu, Adv. Mater., 2007, 19, 1983–1987 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and spectral data for DBUA and DBUA-SO2. See DOI: 10.1039/b916550a |
| This journal is © The Royal Society of Chemistry 2010 |
