Transport properties of PFSA membranes with various ion exchange capacities for direct methanol fuel cell application
Lei
Li
*,
Fangjian
Shang
,
Li
Wang
,
Supeng
Pei
and
Yongming
Zhang
*
School of Chemistry and Chemical Engineering, Shanghai Jiaotong University, Shanghai, 200240, China. E-mail: lilei0323@sjtu.edu.cn; ymzhang@sjtu.edu.cn; Fax: +86-21-54742567; Tel: +86-21-34202613
First published on 9th November 2009
Abstract
The transport properties of PFSA for direct methanol fuel cell application were investigated, including water uptake, proton conductivity, methanol permeability and selectivity of perfluorosulfonic acid (PFSA) membranes as a function of the ion exchange capacity.
Broader contextDirect methanol fuel cells (DMFCs) are being considered as a possible solution to replace the current battery as the dominant power provider for portable electronic application, but they currently experience significant power density and efficiency losses due to high methanol crossover through proton exchange membranes (PEMs). Currently, Nafion® perfluorosulfonic acid (PFSA) membrane made by DuPont is the most frequently used PEM in DMFC. However, it is a poor barrier to methanol crossover, which limits its wide application in DMFC. In this communication, our synthesized PFSA polymers with a similar chemical structure to Nafion® were used as membrane materials, and PFSA membranes with various ion exchange capacities (IECs) from 0.36 mmol g−1 to 1.17 mmol g−1 were prepared. The relationship between the IECs of PFSA membranes and their transport properties (including water uptake, proton conductivity and methanol permeability) for DMFC applications was investigated. |
Direct methanol fuel cells (DMFCs) have attracted considerable attention as portable power sources due to their high energy, simple system design, low operating temperature, and convenient fuel storage and supply.1 The proton exchange membrane (PEM) is one of the most critical components in the DMFC. In general, PEMs with high proton conductivity and low methanol permeability are desirable for efficient DMFC operation. Currently, the perfluorosulfonic acid (PFSA) membrane such as Nafion® made by DuPont is the major membrane used in proton exchange membrane fuel cells. It has good chemical and thermal stability, high proton conductivity. However, Nafion® membrane is a poor barrier to methanol crossover, which limits its wide application in DMFC. The methanol crossover to the cathode not only reduces fuel efficiency, but also forms a mixed potential at the cathode, thus resulting in a lower cell performance.2
Various efforts have been made in different directions aimed at PEMs with high proton conductivity and low methanol permeability. Several methods to modify Nafion® membranes were reported. Composite membranes such as sol–gel derived Nafion/silica,2 Nafion/zirconium phosphate3 and Nafion/caesium ion4 membranes have been investigated. Tang et al. prepared ultrathin Nafion/expanded polytetrafluoroethyene membranes.5 Similarly, Nafion-impregnated electrospun polyvinylidene fluoride composite membranes were manufactured for DMFCs.6 Another direction is to prepare some new PEMs based on non-fluorinated polymers, such as polybenzimidazole, polyamide, poly(ether imide), polysulfone, poly(phenylene sulfide), poly(ether ether ketone) and polyphenylquinoxaline.7,8 Other efforts include works on membranes of partially fluorinated polymers9,10 and inorganic–organic composites.11
For PFSA-based membranes, because of their high hydrophobicity of perfluorinated backbone and their high hydrophilicity of the sulfonic acid groups, it would cause hydrophobic/hydrophilic domains to form into PFSA membranes, especially in the presence of water. The sulfonic acid groups aggregate to form a hydrophilic ion-cluster domain. These hydrophilic ion-cluster domains are interconnected into PFSA membranes. Not only protons and water molecules can pass through these domains, methanol can also permeate them. It is the main reason which causes PFSA membranes to have a poor barrier capacity to methanol crossover. It is well known that the size and the extent of the interconnected ion-cluster domains into PFSA membrane are controlled by the density of sulfonic acid groups of PFSA polymers, i.e. ion exchange capacity (IEC) of polymers. Until now, however, most of researches were concentrated on Nafion® commercial membranes such as Nafion® 117 and Nafion® 115 which have a fixed IEC of 0.91 mmol g−1. To the best of our knowledge, research on PFSA membranes with other IECs for DMFC has not been reported. Recently, our group successfully synthesized PFSA polymers with various IECs, and also brought them into the market through cooperation with industry. Therefore, it provides an opportunity for us to investigate the relationship between the membrane's structure and the transport behaviours of proton and methanol in PFSA membranes having various IECs. In this communication, PFSA membranes with various IECs, from 0.36 mmol g−1 to 1.17 mmol g−1, were prepared, and their water uptake, proton conductivity, methanol permeability and selectivity for DMFC were investigated.
In our experiments, a proper amount of PFSA precursor (molecular weight approx. 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) resins with different IECs, whose chemical structure was shown in Scheme 1, was first hot-pressed at 280 °C and 3.5 MPa for 15 min. Then, the precursor membranes were immersed into 8 M NaOH solution and were refluxed at 100 °C for 48 h to chemically convert the –SO2F groups to –SO3Na groups of polymers. After the treatment, the membranes were immersed into 1 M H2SO4 aqueous solution and refluxed at 80 °C for 6 h. The immersion process was repeated 5 times to completely convert the –SO3Na groups to –SO3H groups. The thickness of the resultant PFSA membranes was about 180 ± 5 μm. The IECs of our PFSA membranes were 0.36, 0.69, 0.80, 0.89, 0.93, 0.99, 1.05 and 1.17 mmol g−1, respectively.
000 Da) resins with different IECs, whose chemical structure was shown in Scheme 1, was first hot-pressed at 280 °C and 3.5 MPa for 15 min. Then, the precursor membranes were immersed into 8 M NaOH solution and were refluxed at 100 °C for 48 h to chemically convert the –SO2F groups to –SO3Na groups of polymers. After the treatment, the membranes were immersed into 1 M H2SO4 aqueous solution and refluxed at 80 °C for 6 h. The immersion process was repeated 5 times to completely convert the –SO3Na groups to –SO3H groups. The thickness of the resultant PFSA membranes was about 180 ± 5 μm. The IECs of our PFSA membranes were 0.36, 0.69, 0.80, 0.89, 0.93, 0.99, 1.05 and 1.17 mmol g−1, respectively.
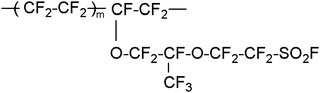 | ||
| Scheme 1 The chemical strcture of perfluorosulfonic acid precursor (m is about 5–7). | ||
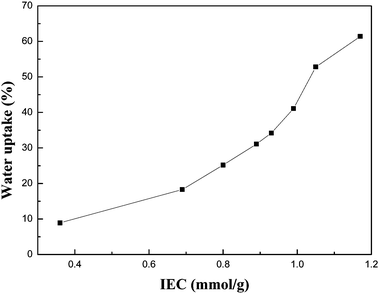
Water uptake in the PFSA membranes was determined as follows: the dry PFSA membranes were immersed in H2O at room temperature for 24 h until there was no further weight gain, then wiped with blotting paper and weighed. The water uptake of the PFSA membranes as a function of the IECs of PFSA is shown in Fig. 1. In our experiments, the water uptake of Nafion® 117 membrane was 36 wt%, which agrees with the reported data in the literature.12 It showed that the water uptake of our membranes increased with increment of the IECs of PFSA and reached 61.4 wt% for a PFSA membrane with 1.17 mmol g−1. A similar behavior was observed in some partially fluorinated or non-fluorinated PEMs.7–10 With increment of the IECs, the density of sulfonic acid groups of PFSA increased. Therefore, the polymer becomes more hydrophilic and absorbs more water. From Fig. 1, it is also clear that the water uptake of PFSA membranes did not increase linearly with increasing IEC. Compared with the membrane having the lowest IEC value (0.36 mmol g−1), the membrane with the highest IEC value (1.17 mmol g−1) had a water uptake about 7 times greater; however, the IEC value only increased less than 4 times. It may involve clustering or agglomeration of the ion-cluster domains. Clustered ionomers absorb more water, therefore a large water uptake may be suggestive of the presence of ion-rich regions,13 where proton transfer is particularly fast.
The proton conductivity and methanol permeability of our PFSA membranes measured at room temperature are shown in Fig. 2. In our experiments, the proton conductivity of membranes in normal direction was measured by using two-electrode AC impedance method, and the methanol permeability was determined by using a diaphragm diffusion cell.14 In our experiments, the proton conductivity and methanol permeability of Nafion® 117 membrane were 18.4 mS cm−1 and 16.4 × 10−7 cm2 s−1, respectively. Both the proton conductivity and methanol permeability of our membranes increased with increasing IEC of the PFSA membranes. Similar trends were also observed in some partially fluorinated or non-fluorinated PEMs.7–10 From Fig. 2, it can be easily seen that neither the proton conductivity nor the methanol permeability increased linearly with increasing IEC of the PFSA. There is an inflexion in both the plots. Below the inflexion, both the proton conductivity and methanol permeability increased slowly with increasing IEC values; however, they increased sharply above the inflexion. Moreover, the inflexion was observed at a different IEC value in each plot. The inflexion in the plot of the proton conductivity vs. the IEC of PFSA was observed at 0.89 mmol g−1, and the point in the plot of the methanol permeability at 0.93 mmol g−1. As mentioned before, proton and methanol molecules are mainly transported from the interconnected hydrophilic ion-cluster domains into PFSA membranes. The size of these ion-cluster domains and the extent of interconnected these ion-cluster domains increases with increasing density of sulfonic acid groups in PFSA, i.e. the IEC of polymer. Above the inflexion, the ion-clusters would absorb more water, therefore the sizes of ion-cluster domains and the interconnecting ion channels would become much larger. Therefore, proton and methanol molecule transfer would be fast. Since the size of proton or hydro-proton molecules is smaller than that of methanol or hydro-methanol molecules, the inflexion value observed in the proton transfer was smaller than that of value in methanol transfer.
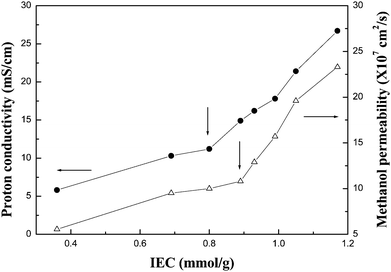 | ||
| Fig. 2 Proton conductivity and methanol permeability of PFSA membranes with various IECs at room temperature. | ||
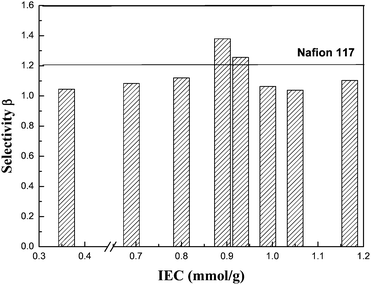
High proton conductivity (σ) and low methanol permeability (P) are two of the essential characteristics which a PEM must possess in order to be validly proposed for use in DMFC. We combined the two into a factor β [= log(σ/P)] as the characteristic parameter of a membrane, so that different membranes can be more easily compared. The practical significance of this parameter is explained in quantitative terms in the literature.15 The selectivity values of our PFSA membranes with various IECs and a Nafion® 117 membrane are shown in Fig. 3. It can be found that our PFSA membranes with 0.89 mmol g−1 and 0.93 mmol g−1 have higher selectivities than that of the Nafion® 117 membrane.
In summary, PFSA membranes with various IECs were prepared by hot-pressed PFSA precursor resins to membranes, and their water uptake, proton conductivity and methanol permeability for direct methanol fuel cell applications were investigated. Since the size of proton and methanol molecules is different, it caused the inflexion point in the plots of the IECs of PFSA vs. proton conductivity or methanol permeability to be observed at different IEC values. The highest selectivity value of our PFSA membranes was obtained at an IEC of 0.89 mmol g−1, which was also higher than that of the Nafion® 117 membrane. Therefore it seems that the PFSA membrane with 0.89 mmol g−1 would be a candidate membrane for DMFC applications. The optimization of the membrane formation processing such as pressing pressure and temperature, processing time and annealing, and the preparation and performance testing of membrane electrode assemblies based on our membranes in DMFC are under way.
Acknowledgements
This work was supported by the Sino-Canada International Project (2008DFA61590) and the National Natural Science Foundation of China (20904031).Notes and references
- A. K. Sahu, G. Selvarani, S. Pitchumani, P. Sridhar and A. K. Shukla, J. Electrochem. Soc., 2007, 154, B123 CrossRef CAS.
- N. Miyake, J. S. Wainright and R. F. Savinell, J. Electrochem. Soc., 2001, 148, A905 CrossRef CAS.
- C. Yang, S. Scrinivasan, A. S. Aricò, P. Cretì, V. Baglio and V. Antonucci, Electrochem. Solid-State Lett., 2001, 4, A31 CrossRef CAS.
- V. Tricoli, J. Electrochem. Soc., 1998, 145, 3798 CrossRef CAS.
- H. Tang, M. Pan and Z. Wan, J. Appl. Polym. Sci., 2008, 110, 2227 CrossRef CAS.
- S. W. Choi, Y.-Z. Fu, Y. R. Ahn, S. M. Jo and A. Manthiram, J. Power Sources, 2008, 180, 167 CrossRef CAS.
- J. A. Kerres, J. Membr. Sci., 2001, 185, 3 CrossRef CAS.
- V. Neburchilov, J. Martin, H. Wang and J. Zhang, J. Power Sources, 2007, 169, 221 CrossRef CAS.
- A. Mokrini, M. A. Huneault and P. Gerard, J. Membr. Sci., 2006, 283, 74 CrossRef CAS.
- M. Sankir, Y. S. Kim, B. S. Pivovar and J. E. McGrath, J. Membr. Sci., 2007, 299, 8 CrossRef CAS.
- A. F. Ismail, N. H. Othman and A. Mustafa, J. Membr. Sci., 2009, 329, 18 CrossRef CAS.
- F. N. Büchi and G. G. Scherer, J. Electrochem. Soc., 2001, 148, A183 CrossRef CAS.
- C. Bailly, D. J. Williams, F. E. Karasz and W. J. McKnight, Polymer, 1987, 28, 1009 CrossRef CAS.
- Y. Luan, H. Zhang, Y. Zhang, L. Li, H. Li and Y. Liu, J. Membr. Sci., 2008, 319, 91 CrossRef CAS.
- B. S. Pivovar, Y. X. Wang and E. L. Cussler, J. Membr. Sci., 1999, 154, 155 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |