Sensors for the optical detection of cyanide ion
Zhaochao Xu†, Xiaoqiang Chen†, Ha Na Kim and Juyoung Yoon*
Department of Chemistry and Nano Science and Department of Bioinspired Science, Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr; Fax: +82-2-3277-2384; Tel: +82-2-3277-2400
First published on 4th September 2009
Abstract
This tutorial review focuses on recent developments arising from studies of optical sensors for cyanide ions, which are categorized by approaches involving cyanide selective receptors, the utilization of metal coordinated complexes, and chemodosimeters.
 Zhaochao Xu | Zhaochao Xu received his PhD in 2006 from Dalian University of Technology under the supervision of Prof. Xuhong Qian. Subsequently he joined the group of Juyoung Yoon at Ewha Womans University as a postdoctoral fellow. Since October 2008 he has been a Herchel Smith Postdoctoral Research Fellow at the University of Cambridge in the group of David R. Spring. |
 Xiaoqiang Chen | Xiaoqiang Chen was born in 1980 in China. He obtained his PhD in 2007 from Dalian University of Technology (China) under the supervision of Prof. Xiaojun Peng. He is presently working as a postdoctoral fellow in the group of Prof. Juyoung Yoon at Ewha Womans University, Korea. |
 Ha Na Kim | Ha Na Kim received the BS degree in Ewha Womans University. She then was given the MS degree in medical science by Seoul National University in 2006. She is on a doctoral course in Prof. Juyoung Yoon’s research group in Ewha Womans University. |
 Juyoung Yoon | Juyoung Yoon received his PhD (1994) from The Ohio State University. After completing postdoctoral research at UCLA and at Scripps Research Institute, he joined the faculty at Silla University in 1998. In 2002, he moved to Ewha Womans University, where he is currently a Professor of the Department of Chemistry and Nano Science and the Department of Bioinspired Science. His research interests include investigations of fluorescent chemosensors, molecular recognition and organo EL materials. |
Introduction
Although substances containing cyanide have been used as poisons for centuries, it was not until 1782 that this anion was first isolated by the Swedish chemist Scheel.1 The extreme toxicity of cyanide in physiological systems, as well as the continuing environmental concern caused by its widespread industrial use, has led to considerable research into the development of methods for cyanide detection. Various methods used previously to analyze cyanide employ titrimetric,2 voltammetric,3 potentiometric,4 and electrochemical methods,5 as well as ion chromatography,6etc. However, these methods often require extensive, time consuming procedures that involve the use of sophisticated instrumentation with high detection limits. Optical sensors for cyanide, in which a change in color and/or fluorescence intensity (or emission wavelength) is monitored, have been studied actively over the past ten years due to their simple, inexpensive, and rapid implementation.Generally, three different approaches, which are shown schematically in Fig. 1, have been employed to design optical sensors for cyanide ions. The most popular strategy involves the use of sensors in which the binding sites and signaling subunits are linked covalently. In this case, interaction of cyanide with the binding site causes a change in color or fluorescence of the signaling subunit. A coordination complex-based displacement approach has also been used. In these sensors, the introduction of cyanide ions leads to regeneration of spectroscopic behavior of the noncoordinated indicator. A third method for the determination of cyanide is known as a chemodosimeter approach. These types of sensors rely on the occurrence of specific, most often irreversible chemical reactions, which take place upon an interaction with cyanide. Compared to other types of anion selective optical chemosensors,7–12 cyanide selective optical chemosensors take advantage of two significant and characteristic properties of cyanide, its strong nucleophilicity and high binding affinity towards copper ions. Thus far, cyanide sensing has been a part-topic in relatively few early review articles.8,10,13,14 To the best of our knowledge, cyanide sensors have not been reviewed thoroughly in recent years, even though some cyanide selective colorimetric sensors were recently reported.15 This review begins with a brief discussion of the source and toxicity of cyanide, and then moves to a discussion of cyanide sensors, which are classified according to the abovementioned approaches.
 | ||
| Fig. 1 Three approaches for chemosensors: (a) chemosensor bearing a signaling subunit as well as a binding site; (b) displacement approach; (c) chemodosimeter. | ||
Cyanide sources and toxic effects
Sources
Cyanide containing salts are widespread chemicals found in surface water originating not only from industrial waste but also from biological sources. Cyanide is used in many chemical processes, such as electroplating, plastics manufacturing, gold and silver extraction, tanning, and metallurgy.16,17 In addition, cyanide is a chemical warfare agent.1 Various industries, including petrochemical, gold mining, metal electroplating, photographic, steel manufacturing, are responsible for cyanide pollution. The production of nitrile, nylon and acrylic plastics is also associated with environmental concerns caused by cyanide. Biological sources of cyanide include bacteria, fungi and algae, which produce this ion as part of their nitrogen metabolic pathways. Vegetables containing cyanogenic glycosides are sources of cyanide ingestion in humans and animals. Dietary foodstuffs that contain moderate to high levels of cyanogenic glycosides include cassava, a dietary staple in several regions of Africa, as well as other common foods such as lima beans, sorghum, linseed, kernels of fruits, sweet potatoes and bamboo shoots.17 Tobacco smoke is also a common source of cyanide and can lead to high levels of this ion in the blood. Other potential sources of cyanide in humans and animals are sodium nitroprusside, succinonitrile and organic thiocyanates.17 The United States Environmental Protection Agency (EPA) has set the maximum contaminant level (MCL) for cyanide in drinking water at 0.2 ppm.Toxicity and metabolic effects
The LD50 (estimated dose that is lethal to 50% of the exposed population) of hydrogen cyanide and cyanogen chloride has been reported to be 2500–5000 mg.min/m3 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg.min/m3. The LD50 of hydrogen cyanide in humans is 1.0 mg/kg, and the estimated LD50 for cyanide solutions applied to the skin is approximately 100 mg/kg.1 Cyanide can affect many functions in the body, including the vascular, visual, central nervous, cardiac, endocrine, and metabolic systems. Perhaps the best known effect of cyanide is its inhibition of respiration, which is caused by the inhibition of the terminal oxidase (cytochrome oxidase) of the mitochondrial respiratory chain. Sublethal doses of cyanide cause a decrease in the rate of glycolysis and inhibit the operation of the TCA cycle. Cyanide also acts as an inhibitor of metallo-enzymes and of some non-metallo-enzymes that function through the intermediacy of Schiff bases.1,16 The toxicodynamic effects of cyanide can depend on the dose, route and speed of administration, the chemical form of cyanide and other factors including gender, age, weight, stress level, and general physical condition.
000 mg.min/m3. The LD50 of hydrogen cyanide in humans is 1.0 mg/kg, and the estimated LD50 for cyanide solutions applied to the skin is approximately 100 mg/kg.1 Cyanide can affect many functions in the body, including the vascular, visual, central nervous, cardiac, endocrine, and metabolic systems. Perhaps the best known effect of cyanide is its inhibition of respiration, which is caused by the inhibition of the terminal oxidase (cytochrome oxidase) of the mitochondrial respiratory chain. Sublethal doses of cyanide cause a decrease in the rate of glycolysis and inhibit the operation of the TCA cycle. Cyanide also acts as an inhibitor of metallo-enzymes and of some non-metallo-enzymes that function through the intermediacy of Schiff bases.1,16 The toxicodynamic effects of cyanide can depend on the dose, route and speed of administration, the chemical form of cyanide and other factors including gender, age, weight, stress level, and general physical condition.Sensors based on the covalently linked binding site and signaling subunit approach
Although cyanide is not a strong hydrogen bonding acceptor compared to other anions, several cyanide selective sensors that rely on hydrogen bonding interactions have been described. In addition, ditopic systems bearing two metal sites were examined as cyanide selective receptors, which function in organic solvents.Hydrogen bonding based receptors
Lees et al. described a luminescent rhenium(I) polypyridyl-based receptor 1 for the recognition of anions (Fig. 2).18 This artificial receptor shows high affinity for halides, cyanide and acetate anions with binding constants as high as 104–105 M−1 in CH2Cl2. The overall order of binding affinity was found to be CN− > F− > I− > Cl−≈ Br−≈ OAc−≫ H2PO4− > NO3− > ClO4−. Although this affinity trend was not clearly explained, a combination of interactions involving electrostatic forces, hydrogen bonding strengths and steric effects have been reported to affect the binding affinity of receptors toward anions. | ||
| Fig. 2 Structures of receptors 1, 2, 3a and 3b. | ||
Anzenbacher and Castellano et al. designed the novel cyanide sensor 2 based on the changes in the anion-induced luminescence lifetime (Fig. 2).19 The addition of fluoride and cyanide to this sensor caused significant changes in the UV-vis and steady-state emission properties of compound 2 in CH2Cl2–CH3CN (98 : 2, v/v). Quenching of the steady state photoluminescence was observed upon the addition of cyanide. In addition, cyanide causes a change in the luminescence lifetime of the sensor from 377 to 341 ns in the same solvent system.
Vilar et al. recently utilized azo-phenylthiourea compounds 3a and 3b as probes for cyanide (Fig. 2).20 In methanol, the dyes undergo color changes from pale orange to red in the presence of cyanide with a detection limit of 8 ppm. In DMSO, cyanide induces a color change (to dark purple) as does fluoride (to blue), while CH3CO2− and H2PO4− (both to violet/red) also promote color changes. The authors suggested that the origin of the color changes was not a simple change in hydrogen-bonding interaction, but rather a process that involves the deprotonation of thiourea NH groups. The optical properties of compound 3a incorporated in nanostructured Al2O3 films have been determined. An aqueous solution of cyanide induces a color change in these films, which can be used to detect cyanide at 2.6 ppm.
Metal complex based receptors
Hong et al. reported the results of a study on Zn-porphyrin/crown ether conjugates.21 These ditopic neutral receptors (4a and 4b) contain a Lewis-acidic binding site (zinc porphyrin moiety) and a Lewis-basic binding site (crown ether moiety) (Fig. 3a). The two receptors display a color change from the original red of Zn-porphyrin to green selectively in the presence of sodium cyanide. The origin of this unique color change was proposed to be associated with the binding of cyanide in a ditopic manner, in contrast to other sodium salts, which are bound to the receptors in a monotopic fashion. | ||
| Fig. 3 (a) Structures of ditopic receptors 4a and 4b. (b) Structures of receptors 5a and 5b and their proposed ditopic binding modes. | ||
Chen et al. described a similar approach to sensor design utilizing the ditopic characteristics of aza-crown ether-capped porphyrins 5a and 5b (Fig. 3b).22 These artificial receptors selectively recognize sodium cyanide and potassium cyanide as a result of ditopic binding in methanol. The binding of receptor 5a to sodium cyanide is 56 times stronger than that for potassium cyanide, whereas the selectivity of 5b for potassium cyanide is 12 times higher than that for sodium cyanide.
Displacement approach
Among the various systems designed to detect cyanide, sensors utilizing the affinity of this anion for copper have attracted special attention. Cyanide reacts with copper ions to form stable [Cu(CN)x]n− species. One of the most important advantages of sensors based on this chemistry is that they should be operable in aqueous solutions.Cu complex
Mareque-Rivas et al. designed a cyanide sensor system that considers the ability of copper ions to affect the kinetics of electron transfer across a copper binding, self assembled monolayer (SAM) to a negatively charged redox probe in solution and the ability of (H)CN to bind and remove copper ions (Fig. 4).23 The SAM-modified gold electrode exhibits a charge transfer resistance value (RCT) of 32 Ω cm2. An inspection of the impedance plots showed that the RCT values increase with increasing cyanide concentration. Nanomolar concentrations of cyanide (0.03 ppb detection limit), even at pH 7.3, could be detected using this method.Li et al. examined light-emitting polyacetylene bearing imidazole moieties in the context of a new type of cyanide sensor.24 The polymer sensor displays selective fluorescence quenching in the presence of Cu2+. Interestingly, the Cu2+-promoted luminescence quenching can be inhibited by the addition of cyanide, as shown in Fig. 5.
 | ||
| Fig. 5 Schematic representation of Cu2+ and cyanide sensors based on the fluorescence ‘‘turn-off’’ and ‘‘turn-on’’ of polyacetylenes. | ||
Li and coworkers also employed the same strategy in the design of a new imidazole-functionalized polyfluorene 6, which is a sensitive and selective cyanide chemosensor (Fig. 6).25 The fluorescence of compound 6 was quenched completely by Cu2+ at concentrations as low as 0.20 ppm. The quenched fluorescence of a solution of compound 6 and Cu2+ was recovered upon the addition of cyanide with a detection limit as low as 0.31 ppm.
 | ||
| Fig. 6 Schematic representation of cyanide sensor based on the fluorescence “turn-off” and “turn-on” of 6. | ||
The Mareque-Rivas group also used a combination of tri-n-octylphosphine oxide (TOPO)-coated CdSe quantum dots (QDs), 2,2′-bipyridine (bipy) and CuCl2 (Fig. 7) to fabricate a turn-on fluorescence cyanide probe.26 The ability of bipyridine-bound copper(II) ions to quench the photoluminescence of hydrophobic CdSe quantum dots was used advantageously in this cyanide selective, turn-on fluorescence sensor. This QD system detects cyanide at 20–100 mM concentrations at a physiological pH. TOPO-coated CdSe QDs placed on a polystyrene film displayed a similar “On–Off–On” type change upon the addition of cyanide.
 | ||
| Fig. 7 Cyanide QD (quantum dot) based probe. | ||
On the other hand, Dong et al. utilized CdTe quantum dots as turn-on fluorescent sensors for cyanide.27 Copper ion-modified CdTe quantum dots were prepared and the quenched fluorescence due to Cu2+ was revived in the presence of cyanide with a detection limit of 3.9 ppb at pH 7.0.
Qin and Li et al. recently reported that an old and inexpensive compound, zincon (2-carboxy-2′-hydroxy-5′-sulfoformazylbenzene (7)), can be used as a highly sensitive and selective chemosensor for cyanide in aqueous solutions with a detection limit of 0.13 ppm (Fig. 8).28 When the Cu2+ concentration increases, the peak intensity of the absorption maximum at 463 nm of this sensor decreases with the concurrent formation of a new peak at ca. 600 nm. The addition of cyanide to the resulting complex 7-I causes an increase in the absorption band at 463 nm, which can be observed with the naked eye.
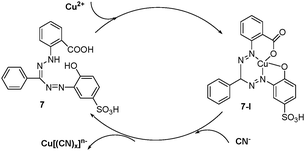 | ||
| Fig. 8 The speculated conversion cycle of zincon 7 in the presence of Cu2+ and cyanide. | ||
Yoon, Park and coworkers devised a simple method for detecting cyanide ions in aqueous solution at pH 7.4.29 The fluorescent probe 8 designed for this purpose displays a fluorescence quenching effect with Cu2+. Therefore, the addition of cyanide induces “Off–On” type fluorescence enhancement (Fig. 9). This sensing system has been incorporated into a microfluidic platform, in which the fluorescent sensor 8-Cu2+ displays green fluorescence upon the addition of cyanide. Finally, studies of biological applications using Caenorhabditis elegans demonstrated that this system can be employed for the in vivo imaging of cyanide.
 | ||
| Fig. 9 Proposed binding mechanism of 8 with Cu2+ and cyanide. | ||
Co complex
Recently, Zelder utilized the “base on”/“base off” coordination of the intramolecular bound benzimidazole nucleobase of vitamin B12 in the construction of a novel sensor for cyanide (Fig. 10).30 In its “base on” conformation, the sensor is red colored (λmax = 550, 520 and 361 nm) and the addition of cyanide induces a color change to violet (λmax = 579, 542 and 368 nm). As a result, this system can be used for the specific colorimetric detection of millimolar concentrations of cyanide in water. Zelder and coworkers also employed a similar strategy in the design of corrinoid derivatives 9a–9c, in which the substitution of CoIII-bound water by cyanide enables the rapid colorimetric detection of micromolar amounts of cyanide (Fig. 11).31 | ||
| Fig. 10 Structures of vitamin B12 and the binding mechanism with cyanide. | ||
 | ||
| Fig. 11 Structures of corrinoids 9a–9c and a schematic for the binding mode with cyanide. | ||
Chemodosimeter approach
The exceptional nucleophilicity of cyanide has served as a basis for the development of various chemodosimetric probes for cyanide, in most cases in aqueous solutions. As shown below, this approach has recently attracted the attention of many groups.C–C bond formation utilizing cyanohydrin reaction
Ahn et al. reported that the fluorescence signaling of anion binding can be modulated by intramolecular H-bonding stabilization of anion–ionophore adducts.32,33 In a recent report from this group,34 it was shown that a carbonyl addition intermediate, such as compound 10-I, which is stabilized by intramolecular H-bonding, is responsible for the fluorescence enhancement observed in acetonitrile (Fig. 12). In contrast, another possible intermediate, 10-II, is believed to show fluorescence quenching. Moreover, the formation of adduct 10-I should be favored over the alternative deprotonation process leading to compound 10-II, which was confirmed by NMR analysis. | ||
| Fig. 12 A plausible equilibrium pathway for interaction of 10 with cyanide. | ||
Ahn et al. extended this intramolecular H-bonding stabilization concept to the design of a heteroditopic receptor 11a containing both a crown ether and a trifluoroacetylcarboxanilide group (Fig. 13). In this system, cyanide was added to the trifluoroacetyl group to produce an alkoxide adduct that interacts with the potassium ion bound to the crown ether moiety. As a consequence of a highly cooperative ion-pair interaction, this sensor selectively recognizes both potassium and cyanide ions in acetonitrile solutions with an association constant as high as 1.9 × 107 M−1. This affinity is two orders of magnitude higher than that of 11b.35
 | ||
| Fig. 13 Structures of ferrocene derivatives 11a, 11b and 12. | ||
The Ahn group also described a new probe 12 that displays fluorescence quenching in the presence of cyanide in MeOH–water (9 : 1) solutions but not when other anions are present (Fig. 13).36
The intramolecular H-bonding stabilization concept serves as the basis for the boradiazaindacene 13 derivative described by Akkaya et al. (Fig. 14).37 This chemosensor displays a large decrease in fluorescence intensity and a reversible color change from red to blue in the presence of cyanide in CH3CN. Highly fluorescent polymeric films doped with this sensor were also prepared. The introduction of cyanide causes the color/emission of the film to change from an orange/fluorescent to a blue/nonfluorescent, and the addition of trifluoroacetic acid reverses this change.
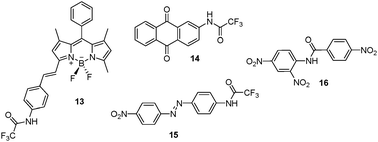 | ||
| Fig. 14 Structures of probes 13–16. | ||
This strategy was used by Cheng et al. to design a 2-(trifluoroacetylamino)anthraquinone sensor 14 (Fig. 14), which undergoes a “naked eye” observable, colorless to yellow transformation when low concentrations (13 ppb) of cyanide in CH3CN–H2O (95 : 5, v/v) are added.38 Cheng and coworkers also devised a colorimetric probe 15 (Fig. 14) that exhibits excellent selectivity for cyanide in CH3CN–H2O (95 : 5, v/v). A change from colorless to yellow takes place upon the addition of cyanide.39
Recently, Guo et al. reported a simple N-nitrophenyl benzamide derivative 16 (Fig. 14) for the ‘naked-eye’ detection of cyanide in DMSO–H2O (1 : 1, v/v).40 This sensor can detect cyanide at concentrations as low as 23 ppb in the above solvent system by utilizing the strong affinity of cyanide toward the acyl carbonyl carbon.
One general process operating in enzymatic reactions involves carbonyl activation by a properly located phenol hydroxyl group that leads to the general acid catalysis of nucleophilic addition. This strategy was adopted intelligently by Kim and Hong et al. in the design of a cyanide sensing system 17 (Fig. 15). As a consequence of activation by hydrogen bonding with the phenolic group in the salicylaldehyde moiety, the carbonyl group of compound 17 is expected to undergo nucleophilic addition with cyanide.41 Studies show that the 1H NMR spectrum of compound 17 in the presence of cyanide does not contain a resonance for the aldehyde proton (Ha, initially at 10.4 ppm) but instead shows a new resonance at 5.6 ppm (Hb). The chemical shift of this resonance is consistent with a cyanohydrin proton resulting from cyanide addition to the aldehyde moiety. Interestingly, the addition of cyanide to compound 17 in DMSO also causes a clear color change from light yellow to dark red.
Kim and Hong et al. extended this concept to the design of a coumarin-based fluorescent chemodosimeter 18 containing a salicylaldehyde group (Fig. 16).42 Rapid proton transfer of the phenol hydrogen in the excited state results in a strong fluorescence of an aqueous solution of compound 18 at pH 7.4. Cyanide at concentrations of 260 ppb in aqueous solution can be detected using this fluorescent chemodosimeter.
Yoon and Park et al. applied this strategy for the activation of a carbonyl group by an adjacent phenol in devising a fluorescein aldehyde-based cyanide sensor 19 (Fig. 16).43 In CH3CN–H2O (9 : 1, v/v), the “OFF–ON” type emission change can be monitored at wavelengths >500 nm. The practical use of this probe was demonstrated by its incorporation into a microfluidic platform for the selective detection of cyanide in living cells.
Ahn et al. constructed N-acyl-triazene derivatives that serve as simple and tunable chemodosimeters based on the strong affinity of cyanide toward an N-acyl carbonyl carbon (Fig. 17).44 Significant changes in the absorption spectrum (from colorless to deep purple) take place when acetonitrile solutions of N-acetyltriazene 20a are titrated with both cyanide and F−. In contrast, the N-isopropanoyl-triazene 20b in acetonitrile shows a significant response to cyanide and only a weak response to F−. The absorption properties of both triazenes 20a and 20b are altered only by the addition of cyanide (faint yellow to red–pink, λmax = 521 nm) when methanol–water (9 : 1, v/v) is used as a solvent. This observation is most likely due to the fact that only cyanide adds to the acyl group of these sensors.
Sessler et al. employed the well-known benzil–cyanide reaction (Fig. 18) in the design of a colorimetric method for the detection of cyanide.45 The π-extended analogue of benzil 21a was selected for this purpose. This substance is soluble in a 70 : 30 (v/v) mixture of methanol–water (Fig. 19). Dilute solutions of compound 21a in this medium are yellow and become colorless when low concentrations of cyanide but not other anions are added. Using a dilute solution of compound 21a (7.20 × 10−6 M) in 70 : 30 (v/v) MeOH–water, a limit of detection <44 ppb can be realized and visualized by simple naked eye analysis. Prior to this report, Sessler et al. also described a novel probe 21b (Fig. 19) that undergoes benzil rearrangement when treated with cyanide (Fig. 18) in ethyl acetate. In this organic solvent, a yellow to colorless change and large fluorescence enhancement are observed within 1 min.46
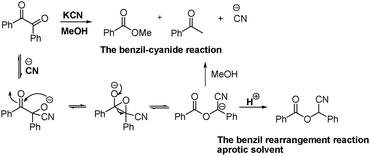 | ||
| Fig. 18 Proposed mechanisms of the benzil–cyanide reaction and benzil rearrangement reaction. | ||
 | ||
| Fig. 19 Structures of 21a and 21b. | ||
Sun et al. prepared two probes, 22a and 22b, that feature the use of the dipyrrole carboxamide moiety for anion recognition (Fig. 20a).47 These structurally simple anion probes exhibit high selectivity for cyanide over other common inorganic anions in partially aqueous environments [CH3CN–H2O (9 : 1, v/v)]. Both compounds 22a and 22b respond to cyanide only, because cyanohydrin derivatives (22b-I and 22b-II) are generated (Fig. 20b). This process is associated with a color change from colorless to yellow (Fig. 20b) and a fluorescence change for compound 22a from blue to green.
 | ||
| Fig. 20 (a) Structures of 22a and 22b. (b) Proposed cyanohydrins formation from reaction of 22b with cyanide. | ||
C–C bond formation utilizing chromogenic oxazines
Raymo et al. designed a chromogenic oxazine that can be used for the selective colorimetric detection of cyanide. In this system, the [1,3]oxazine ring of compound 23 opens to form a 4-nitrophenylazophenolate chromophore (23-I) in the reaction with cyanide (Fig. 21).48,49 In acetonitrile, the addition of cyanide causes a decrease in the original absorption band at 381 nm (pale yellow) with the concomitant appearance of a new absorption band at 581 nm (red). In aqueous solutions, a significantly higher amount of cyanide is required to elicit a UV response. In order to overcome this limitation, a two-phase system consisting of dichloroethane and phosphate buffer (pH 9) was used in conjunction with phase transfer catalysis. Micromolar concentrations of cyanide can be detected using this two-phase system.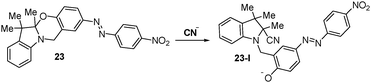 | ||
| Fig. 21 Reaction of chemodosimeter 23 with cyanide. | ||
Tian et al. designed a highly sensitive and selective cyanide chemosensor based upon oxazine derivatives (24a and 24b). In this system, the C–O bond of the oxazine moiety cleaves at the spiro center (24a-I and 24b-I) when nucleophilic cyanide anions are present (Fig. 22).50 The addition of the cyanide anion to the oxazines in MeCN–H2O solution [19 : 1, pH 7.6 phosphate buffer (7.6 mM)] results in a color change associated with the disappearance of the absorbance at 343 nm and the appearance of a new absorption band at 411 nm. These sensors show very rapid responses to cyanide (ca. 30 s) with a detection limit of 26 ppb.
 | ||
| Fig. 22 Reactions of chemodosimeters 24a and 24b with cyanide. | ||
C–C bond formation utilizing dicyano-vinyl group
Lee et al. recently reported that the new calix[4]pyrrole-based, dual functional, chemodosimetric sensor 25 serves as a cyanide selective indicator (Fig. 23).51 Complete bleaching of the color of compound 25 (yellow) was observed when cyanide was added even in the presence of an excess of another anion. A large bathochromic shift from λmax = 374 nm to λmax = 403 nm was observed for compound 25 upon cyanide anion complexation in CH3CN–DMSO (3%). | ||
| Fig. 23 Reaction of 25 with cyanide. | ||
C–C bond formation utilizing pyrylium or acridinium compounds
García, Martínez-Máñez et al. utilized pyrylium salt-containing polymers as colorimetric sensors for cyanide.52 The electrophilic character of the pyrylium ring in probe 26 and the nucleophilicity of cyanide combine to induce a remarkable color change from yellow to red as a consequence of the formation of the cyano-enone derivative 26-I in acetonitrile (Fig. 24a). Based on this observation, the authors fabricated methacrylic copolymer films containing the pyrylium probe (Fig. 24b). This copolymer showed a gradual increase in the 537 nm band when cyanide was added at pH 11. | ||
| Fig. 24 (a) Mechanism for reaction of the monomer 26 with cyanide. (b) Structure of the sensing film. | ||
The results of studies on a selective chemodosimeter based on the acridine moiety were recently reported by Tae et al. Among the various anions, only cyanide in DMSO–water (95 : 5, v/v) promotes the selective fluorescent quenching of the acridinium salt 27 with an accompanying concomitant color change from orange to pale blue (Fig. 25).53 As shown in Fig. 25, this strategy takes advantage of the nucleophilic addition of cyanide at the 9-position of the N-methylacridinium group. The resulting adduct 27-I was formed initially, which rapidly reacted with oxygen to produce acridinone 27-II.
 | ||
| Fig. 25 Mechanism of the reaction of 27 with cyanide. | ||
C–C bond formation utilizing squaraine, croconium or triarylmethane dyes
A new squaraine based chemodosimeter 28, which relies on the nucleophilicity of cyanide and the highly electron-deficient four-membered ring (Fig. 26), was described by Martínez-Máñez et al.54 Studies of this sensor led to the observation that a colorimetric change in compound 28 in water–acetonitrile (8 : 2, v/v, pH 9.5) takes place only in the presence of cyanide. The addition of cyanide to the squaraine ring in compound 28 results in both the loss of acceptor properties of the ring and a rupture of the electronic delocalization with the concurrent disappearance of the 641 nm charge transfer band. Although the reaction between probe 28 and cyanide does not occur instantaneously, this sensor system displays high selectivity toward cyanide and a detection limit of 2.5 ppm. | ||
| Fig. 26 Structures of probes 28 and 29. | ||
Cheng and Zhang et al.55 developed a near-infrared, colorimetric chemodosimeter, based on the dye 1,3-bis(4-N,N-diethylamino-2-hydroxyl-phenyl)croconine sensor 29 (Fig. 26) that can be used to detect the cyanide anion at pH 9.0 in ethanol–H2O (70 : 30) solution (pH 9.0, buffered with TRIS). The addition of cyanide causes a decrease in absorbance in the NIR region (823 nm) and an increase in absorbance in the range of 550–725 nm with an isosbestic point at 725 nm. A brown to dark green color change was observed with the naked eye immediately, and the color changed slowly to yellow after ca. 1 h.
Afkhami et al. designed an optical absorption based, one-shot cyanide sensor that is formed by the immobilization of methyl violet 30 on an acetylcellulose membrane.56 In this system, cyanide ion reacts with the immobilized methyl violet, resulting in a decrease in the absorbance of the film at 598 nm (Fig. 27). The response time of this sensor was ca. 8–12 min depending on the cyanide concentration, and the method has a detection limit of 62 ppm. The color of this one-shot sensor is readily and fully regenerated using a methyl violet solution.
 | ||
| Fig. 27 The reversible addition mechanism of 30 with cyanide. | ||
Kaur and Singh et al.57 recently employed triarylmethane–leuconitrile as a cyanide sensor. The triarylmethane dye 31 in CH3CN can be used to detect cyanide in water selectively by the occurrence of a dramatic color change from blue–green to colorless (Fig. 28). This change was attributed to the nucleophilic addition of cyanide. An instant “dip-in” sensor comprised of compound 31 dyed on wool was developed in this effort.
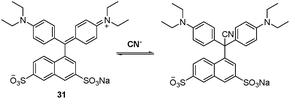 | ||
| Fig. 28 The reversible addition mechanism of 31 with cyanide. | ||
C–S bond formation
Wang et al. reported the results of an investigation of compounds 32a and 32b containing donor–acceptor type chromophores with well-tuned reactivity towards cyanide (Fig. 29a).58 These sensor systems employ compound 32a for the highly selective colorimetric detection of micromolar concentrations of cyanide in the presence of other anions. In addition, compound 32b has triple signaling properties that are advantageous for the highly sensitive, reliable and quantitative detection of cyanide. Cyanide can be detected at concentrations as low as 26 ppb in DMF–H2O (99 : 1, v/v) using probe 32b. Moreover, compound 32b offers the capability for multiple signaling, including visible absorption, a high contrast change in color, and absorption and fluorescence spectral changes in the visible and NIR wavelength regions. The mechanism of action for compound 32b with cyanide, explored by using a model system, involves cyanide attack on the benzothiadiazole ring sulfur, followed by a second addition of cyanide. The resulting imidosulfite adduct is readily oxidized to form a more stable sulfamide (Fig. 29b). | ||
| Fig. 29 (a) Structures of chromophores 32a and 32b. (b) Proposed mechanism for the chemical reaction with cyanide. | ||
C–B bond formation
Martínez-Máñez et al. described the use of subphthalocyanine 33 as a probe for the ‘‘naked eye’’ detection of cyanide (Fig. 30).59 In a 3% vol/vol aqueous solution, both fluoride and cyanide induce a pink to pale yellow color change, whereas the color change is remarkably selective to cyanide in 5% vol/vol aqueous acetonitrile solutions of compound 33. These results have been attributed to the solvent dependence of the relative nucleophilicities of fluoride and cyanide caused by hydrogen bonding and other solvation effects. The detection limit of compound 33 for cyanide is as low as 0.1 ppm at pH 9.6 [CH3CN–CHES (0.01 M)], and ca. 10 ppm at pH 7 [CH3CN–HEPES (0.01 M)]. | ||
| Fig. 30 Structures of probes 33, 34a and 34b. | ||
Selective colorimetric and fluorimetric molecular probes 34a and 34b (Fig. 30), which are based on a subphthalocyanine dye, have been developed for cyanide detection by Palomares and Torres et al.60 A distinct pink to colorless change was observed upon the addition of cyanide to aqueous solutions of these substances. The carboxysubphthalocyanine derivative 34b anchored covalently to a transparent, mesoporous nanocrystalline, high-surface-area metal oxide film can be used to detect low concentrations of cyanide ions in pure water with no interference from other anions or cations.
Do and Lee et al.61 recently described a strategy involving the coupling of borane as a donor and BODIPY as an acceptor which resulted in the fabrication of a boron-based sensor 35 (Fig. 31). This receptor showed a 3-fold enhancement in fluorescence intensity in response to cyanide ions as a consequence of an addition reaction (35-I) that blocks intramolecular electron transfer.
 | ||
| Fig. 31 Structures of receptor 35 and its adduct 35-I. | ||
Based on the hypothesis that cationic boranes may be particularly well-adapted for cyanide complexation due to the favorable Coulombic receptor–anion attractions, Gabbaïet al. designed cationic boranes, such as compound 36a, that serve as selective sensors for cyanide in aqueous solutions (Fig. 32).62 The unusual cyanide binding property of compound 36a was attributed to the favorable Coulombic effects that increase the Lewis acidity of boron and strengthen the receptor–cyanide interaction. Using the two receptors, 36a and 36b, they demonstrated that the anion binding selectivity of the cationic boranes can be tuned using both steric and electronic effects. For example, the Lewis acidity of the ammonium borane increases when the trimethylammonium moiety in compound 36b is positioned ortho to the boron center, thus making fluoride binding possible (Fig. 32). In this case, increased steric crowding of the boron center prevents the coordination of the larger cyanide anion. In H2O–DMSO (6 : 4, v/v, HEPES 6 mM, pH 7), the cyanide binding constant of compound 36a and the fluoride binding constant of compound 36b are 3.9 × 108 and 910 M−1, respectively.
Recently, Kawashima et al. reported new cationic triarylborane 37 (Fig. 33) as a selective optical sensor for cyanide in DMSO–HEPES (pH 7, 0.5 M) (4 : 6, v/v)].63 The complex formation constant was calculated to be 1.24 × 105 M−1, and selective UV absorption changes and fluorescence quenching effects were observed due to the formation of compound 37-I.
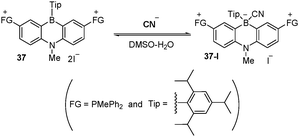 | ||
| Fig. 33 Structures of receptor 37 and its adduct 37-I. | ||
Boronic acids have been actively examined for the construction of cyanide sensing systems. In most cases, the change from electron deficient R–B(OH)2 to electron rich R–B−(CN)3 in aqueous solutions at physiological pH can alter the color or fluorescence properties of the probe. Geddes et al. reported new pyridinium boronic acid derivatives 38a–c that serve as fluorescent sensors for cyanide (Fig. 34).64 In the absence of cyanide, intramolecular charge transfer (ICT) in these systems occurs efficiently and the fluorophores in the probes are effectively quenched. On the other hand, the extent of ICT from the amino moiety to the pyridinium nitrogen is reduced in the presence of cyanide, which was attributed to enhanced electron donation from the cyanide-complexed boronic acid to the quaternary nitrogen. The decrease in ICT enhances the intensity of blue-shifted fluorescence. These three water-soluble fluorescent probes have been used to determine the free cyanide concentration at concentrations up to physiologically lethal levels of >0.5 ppm.
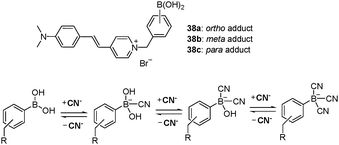 | ||
| Fig. 34 Structures of probes 38a–c and mechanism of cyanide addition to boronic acid adduct. | ||
Recently, Tomapatanaget et al. reported acceptor–donor–acceptor (A–D–A) systems (39a–c) composed of naphthoquinone, imidazole and boronic acid moieties (Fig. 35).65 The fluorescence band at 460 nm was switched on upon the substitution of cyanide on the sensors in a CTAB micelle. This was attributed to the ITC caused by the poor acceptability on the boron center after cyanide addition.
 | ||
| Fig. 35 Structures of receptors 39a–c and their adducts. | ||
Application
The various sensors described in this review have potential applications for the detection and quantification of cyanide in real samples due to their detection limits being below the EPA standard for drinking water. The capability of naked eye or fluorescence detection strongly suggests that these sensors can be used in kits for the detection of cyanide. A few reports have already described systems that apply sensors to nanostructured Al2O3 films,20 SAM,23 polymer backbones,24,25,52 QDs,26 and polymer films37/wool.57 These results suggest that in the near future, various types of solid systems will be configured to perform as sensitive and practical “dip-in” naked eye cyanide sensors.Chemical analysis using portable microfluidic devices enables environmental testing outside of the laboratory in a low cost in situ manner.66 The nematode, which inhabits the interstitial water between soil particles, is considered to be an ideal organism for testing the cyanide toxicity of aquatic media, such as municipal and industrial wastewater.67,68 Yoon and Park et al. recently applied a microfluidic sensor system they devised (Fig. 36a and b)29,43 to cell-imaging43 and in vivo imaging of cyanide in Caenorhabditis elegans (Fig. 36c–e).29
 | ||
| Fig. 36 Fluorescence images of 19 in the absence (a) and presence of cyanide (b). Fluorescence images of the nematode C. elegans exposed to sensor 8 only (c), with Cu2+ (d), with Cu2+ and cyanide (e). | ||
The selective sensing of cyanide anions in water by using a hybrid biomaterial composed of a mesoporous TiO2 film of crystalline nanoparticles and the protein hemoglobin has also been reported.69 Low levels of cyanide (<0.2 ppm) can be detected by monitoring the absorption changes of the hybrid biomolecular films upon cyanide binding to the heme groups.
Concluding remarks
This review covers recent reports describing cyanide sensing. Attention has been given to approaches that involve hydrogen bonding, displacement and nucleophilic addition, and addition to boron. As described above, the nucleophilicity of cyanide and the strong affinity of cyanide for Cu2+ and boron are among the properties that have been used advantageously in the design of selective cyanide probes. Considerable attention has been focused on the development of cyanide sensing systems in recent years. Therefore, it is likely that various types of practical kits for monitoring drinking water and industrial waste, or for detecting alarming biological terror/warfare agents will soon be devised. In addition, the design of new cyanide probes and the discovery of new types of receptors for cyanide will contribute greatly to the intellectual foundation of the anion recognition field.Acknowledgements
This work was supported by the NRL program of KOSEF/MEST (R04-2007-000-2007-0), the SRC program of KOSEF/MEST (R11-2005-008-02001-0) and the WCU program (R31-2008-000-10010-0). H. N. Kim thanks BK 21.References
- S. I. Baskin and T. G. Brewer, Medical Aspects of Chemical and Biological Warfare, ed. F. Sidell, E. T. Takafuji and D. R. Franz, TMM Publication, Washington, DC, 1997, ch. 10, pp. 271–286 Search PubMed
.
- T. Suzuki, A. Hiolki and M. Kurahashi, Anal. Chim. Acta, 2003, 476, 159 CrossRef CAS
.
- A. Safavi, N. Maleki and H. R. Shahbaazi, Anal. Chim. Acta, 2004, 503, 213 CrossRef CAS
.
- D. Shan, C. Mousty and S. Cosnier, Anal. Chem., 2004, 76, 178 CrossRef CAS
.
- V. K. Rao, S. R. Suresh, N. B. S. N. Rao and P. Rajaram, Bull. Electrochem., 1997, 13, 327 Search PubMed
.
- T. T. Christison and J. S. Rohrer, J. Chromatogr., A, 2007, 1155, 31 CrossRef CAS
.
- J. Yoon, S. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355 RSC
.
- T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094 CrossRef CAS
.
- P. A. Gale, Acc. Chem. Res., 2006, 39, 465 CrossRef CAS
.
- R. Martínez-Máñez and F. Sancanón, Chem. Rev., 2003, 103, 4419 CrossRef CAS
.
- S. K. Kim, D. H. Lee, J.-I. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23 CrossRef CAS
.
- H. N. Lee, Z. Xu, S. K. Kim, K. M. K. Swamy, Y. Kim, S.-J. Kim and J. Yoon, J. Am. Chem. Soc., 2007, 129, 3828 CrossRef CAS
.
- D. G. Cho and J. L. Sessler, Chem. Soc. Rev., 2009, 38, 1647 RSC
.
- G. J. Mohr, Anal. Bioanal. Chem., 2006, 386, 1201–1214 CrossRef CAS
.
- F. H. Zelder and C. Mannel-Croise, Chimia, 2009, 63, 58 CrossRef CAS
.
- K. W. Kulig, Cyanide Toxicity, U.S. Department of Health and Human Services, Atlanta, GA, 1991 Search PubMed
.
- Guidelines for Drinking-Water Quality, World Health Organization, Geneva, 1996 Search PubMed
.
- S.-S. Sun and A. J. Lees, Chem. Commun., 2000, 1687 RSC
.
- P. Anzenbacher, Jr., D. S. Tyson, K. Jursíková and F. N. Castellano, J. Am. Chem. Soc., 2002, 124, 6232 CrossRef
.
- N. Gimeno, X. Li, J. R. Durrant and R. Vilar, Chem.–Eur. J., 2008, 14, 3006 CrossRef CAS
.
- Y.-H. Kim and J.-I. Hong, Chem. Commun., 2002, 512 RSC
.
- H. Liu, X.-B. Shao, M.-X. Jia, X.-K. Jiang, Z.-T. Lia and G.-J. Chen, Tetrahedron, 2005, 61, 8095 CrossRef CAS
.
- V. Ganesh, M. P. C. Sanz and J. C. Mareque-Rivas, Chem. Commun., 2007, 5010 RSC
.
- Q. Zeng, P. Cai, Z. Li, J. Qina and B. Z. Tang, Chem. Commun., 2008, 1094 RSC
.
- Z. Li, X. Lou, H. Yu, Z. Li and J. Qin, Macromolecules, 2008, 41, 7433 CrossRef CAS
.
- A. Touceda-Varela, E. I. Stevenson, J. A. Galve-Gasión, D. T. F. Dryden and J. C. Mareque-Rivas, Chem. Commun., 2008, 1998 RSC
.
- L. Shang, L. Zhang and S. Dong, Analyst, 2009, 134, 107 RSC
.
- X. Lou, L. Zhang, J. Qin and Z. Li, Chem. Commun., 2008, 5848 RSC
.
- S.-Y. Chung, S.-W. Nam, J. Lim, S. Park and J. Yoon, Chem. Commun., 2009, 2866 RSC
.
- F. H. Zelder, Inorg. Chem., 2008, 47, 1264 CrossRef CAS
.
- C. Männel-Croisé and F. Zelder, Inorg. Chem., 2009, 48, 1272 CrossRef CAS
.
- Y. K. Kim, Y.-H. Lee, H.-Y. Lee, M.-K. Kim, G. S. Cha and K. H. Ahn, Org. Lett., 2003, 5, 4003 CrossRef CAS
.
- D.-S. Kim, H. Miyaji, B.-Y. Chang, S.-M. Park and K. H. Ahn, Chem. Commun., 2006, 3314 RSC
.
- Y. M. Chung, B. Raman, D.-S. Kim and K. H. Ahn, Chem. Commun., 2006, 186 RSC
.
- H. Miyaji, D.-S. Kim, B.-Y. Chang, E. Park, S.-M. Park and K. H. Ahn, Chem. Commun., 2008, 753 RSC
.
- H. Lee, Y. M. Chung and K. H. Ahn, Tetrahedron Lett., 2008, 49, 5544 CrossRef CAS
.
- Z. Ekmekci, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2008, 10, 461 CrossRef CAS
.
- H.-T. Niu, D. Su, X. Jiang, W. Yang, Z. Yin, J. He and J.-P. Cheng, Org. Biomol. Chem., 2008, 6, 3038 RSC
.
- H.-T. Niu, X. Jiang, J. He and J.-P. Cheng, Tetrahedron Lett., 2008, 49, 6521 CrossRef CAS
.
- Y. Sun, G. Wang and W. Guo, Tetrahedron, 2009, 65, 3480 CrossRef CAS
.
- K.-S. Lee, J. T. Lee, J.-I. Hong and H.-J. Kim, Chem. Lett., 2007, 36, 816 CrossRef CAS
.
- K.-S. Lee, H.-J. Kim, G.-H. Kim, I. Shin and J.-I. Hong, Org. Lett., 2008, 10, 49 CrossRef CAS
.
- S. K. Kwon, S. Kou, H. N. Kim, X. Chen, H. Hwang, S.-W. Nam, S. H. Kim, K. M. K. Swamy, S. Park and J. Yoon, Tetrahedron Lett., 2008, 49, 4102 CrossRef CAS
.
- Y. Chung, H. Lee and K. H. Ahn, J. Org. Chem., 2006, 71, 9470 CrossRef CAS
.
- D.-G. Cho, J. H. Kim and J. L. Sessler, J. Am. Chem. Soc., 2008, 130, 12163 CrossRef CAS
.
- J. L. Sessler and D.-G. Cho, Org. Lett., 2008, 10, 73 CrossRef CAS
.
- C.-L. Chen, Y.-H. Chen, C.-Y. Chen and S.-S. Sun, Org. Lett., 2006, 8, 5053 CrossRef CAS
.
- M. Tomasulo and F. M. Raymo, Org. Lett., 2005, 7, 4633 CrossRef CAS
.
- M. Tomasulo, S. Sortino, A. J. P. White and F. M. Raymo, J. Org. Chem., 2006, 71, 744 CrossRef CAS
.
- J. Ren, W. Zhu and H. Tian, Talanta, 2008, 75, 760 CrossRef CAS
.
- S.-J. Hong, J. Yoo, S.-H. Kim, J. S. Kim, J. Yoon and C.-H. Lee, Chem. Commun., 2009, 189 RSC
.
- F. García, J. M. García, B. García-Acosta, R. Martínez-Máñez, F. Sancenón and J. Soto, Chem. Commun., 2005, 2790 RSC
.
- Y.-K. Yang and J. Tae, Org. Lett., 2006, 8, 5721 CrossRef CAS
.
- J. V. Ros-Lis, R. Martínez-Máñez and J. Soto, Chem. Commun., 2002, 2248 RSC
.
- X. Zhang, C. Li, X. Cheng, X. Wang and B. Zhang, Sens. Actuators, B, 2008, 129, 152 CrossRef
.
- A. Afkhami and N. Sarlak, Sens. Actuators, B, 2007, 122, 437 CrossRef
.
- P. Kaur, D. Sareen, S. Kaur and K. Singh, Inorg. Chem. Commun., 2009, 12, 272 CrossRef CAS
.
- G. Qian, X. Li and Z. Y. Wang, J. Mater. Chem., 2009, 19, 522 RSC
.
- J. V. Ros-Lis, R. Martínez-Máñez and J. Soto, Chem. Commun., 2005, 5260 RSC
.
- E. Palomares, M. V. Martínez-Díaz, T. Torres and E. Coronado, Adv. Funct. Mater., 2006, 16, 1166 CrossRef CAS
.
- J. O. Huh, Y. Do and M. H. Lee, Organometallics, 2008, 27, 1022 CrossRef CAS
.
- T. W. Hudnall and F. P. Gabbaï, J. Am. Chem. Soc., 2007, 129, 11978 CrossRef CAS
.
- T. Agou, M. Sekine, J. Kobayashi and T. Kawashima, Chem.–Eur. J., 2009, 15, 5056 CrossRef CAS
.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, J. Am. Chem. Soc., 2005, 127, 3635 CrossRef CAS
.
- M. Jamkratoke, V. Ruangpornvisuti, G. Tumcharen, T. Tuntulani and B. Tomapatanaget, J. Org. Chem., 2009, 74, 3919 CrossRef CAS
.
- S. Kou, H. N. Lee, D. v. Noort, K. M. K. Swamy, S. H. Kim, J. H. Soh, K. Lee, S. Nam, J. Yoon and S. Park, Angew. Chem., Int. Ed., 2008, 47, 872 CrossRef CAS
.
- N. Tominaga, S. Kohra, T. Iguchi and K. Arizono, J. Health Sci., 2004, 50, 545 CrossRef CAS
.
- X. Chen, S.-W. Nam, M. J. Jou, Y. Kim, S.-J. Kim, S. Park and J. Yoon, Org. Lett., 2008, 10, 5235 CrossRef CAS
.
- K. Poland, E. Topoglidis, J. R. Durrant and E. Palomares, Inorg. Chem. Commun., 2006, 9, 1239 CrossRef CAS
.
Footnote |
| † Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |





