Hierarchical structures made of proteins. The complex architecture of spider webs and their constituent silk proteins
Markus
Heim†
,
Lin
Römer†‡
and
Thomas
Scheibel
*
Lehrstuhl für Biomaterialien, Fakultät für Angewandte Naturwissenschaften, Universität Bayreuth, Universitätsstr. 30, 95440 Bayreuth, Germany. E-mail: thomas.scheibel@bm.uni-bayreuth.de; Fax: +49 921 55 7346; Tel: +49 921 55 7360
First published on 16th October 2009
Abstract
Biopolymers fulfil a variety of different functions in nature. They conduct various processes inside and outside cells and organisms, with a functionality ranging from storage of information to stabilization, protection, shaping, transport, cellular division, or movement of whole organisms. Within the plethora of biopolymers, the most sophisticated group is of proteinaceous origin: the cytoskeleton of a cell is made of protein filaments that aid in pivotal processes like intracellular transport, movement, and cell division; geckos use a distinct arrangement of keratin-like filaments on their toes which enable them to walk up smooth surfaces, such as walls, and even upside down across ceilings; and spiders spin silks that are extra-corporally used for protection of offspring and construction of complex prey traps. The following tutorial review describes the hierarchical organization of protein fibers, using spider dragline silk as an example. The properties of a dragline silk thread originate from the strictly controlled assembly of the underlying protein chains. The assembly procedure leads to protein fibers showing a complex hierarchical organization comprising three different structural phases. This structural organization is responsible for the outstanding mechanical properties of individual fibers, which out-compete even those of high-performance artificial fibers like Kevlar. Web-weaving spiders produce, in addition to dragline silk, other silks with distinct properties, based on slightly variant constituent proteins—a feature that allows construction of highly sophisticated spider webs with well designed architectures and with optimal mechanical properties for catching prey.
 Markus Heim | Markus Heim studied biochemistry at the Technische Universität München where he received his MSc in 2006. He was a fellow of the Graduiertenförderung Universität Bayern e.V. from 2007 to 2009, and is currently working on his PhD thesis under the supervision of Thomas Scheibel at the Lehrstuhl für Biomaterialien, University of Bayreuth. His research focuses on the structure–function relationships of spider silks and silk-like proteins. |
 Lin Römer | Dr. Lin Römer has been leading the application-driven development of spider silk technology as a postdoctoral fellow in the lab of Prof. Scheibel at the Technische Universität München and the University of Bayreuth. He received his diploma of chemistry with focus on biochemistry from the University of Marburg and his PhD from the Technische Universität München. In 2008 he joined AMSilk GmbH, a spin-off from the Technische Universität München, as Head of Research and Development. AMSilk develops tailor-made materials using spider silk technology, thus enabling new and superior products with previously unobtainable features for various industries and applications. |
 Thomas Scheibel | Professor Thomas Scheibel is chair of biomaterials at the Universität Bayreuth in Germany. He received both his Diploma of biochemistry and a Dr rer. nat. from the Universität Regensburg in Germany, and his habilitation from the Technische Universität München in Germany. He was a Kemper Foundation postdoctoral fellow and a DFG postdoctoral fellow at the University of Chicago. He gained the Biomimetics award of the German Bundesministerium für Bildung and Forschung (BMBF) in 2006, and the “Innovation by nature award” of the BMBF in 2007. He is one of 10 recipients of the 2006 innovation tribute of the Bavarian prime minister, received the Heinz–Maier–Leibnitz Medal in 2007, and the Karl-Heinz Beckurts award in 2008. |
Introduction: classes of biopolymers
Organisms provide several building blocks (monomers) as a basis of biopolymers. Monomers occurring in nature include nucleotides, sugars, and amino acids, which polymerize into DNA, polysaccharides like cellulose, and proteins like collagen, hair or spider silk (Fig. 1). | ||
| Fig. 1 Overview of common biopolymers, their appearance in nature, and selected fibrous proteins. | ||
An especially well defined biopolymer is DNA, which is built of four individual nucleotides. Human DNA molecules, which are located in almost every cell of the human body, have a length of several metres. DNA is a linear biopolymer, often adopting a twisted, coiled conformation and its primary function is the storage, duplication and transcription of hereditary information. But beyond that, considering its linear character, DNA can also serve as a “track” used by certain proteins to move from one part of the cell to another.1
Other prominent examples of biopolymers are polysaccharides. The polysaccharide cellulose, for example, is the main structural component of the primary cell wall of most plants. Cellulose is an organic compound with the formula (C6H10O5)n which often consists of thousands of linked glucose units.2 About 30% of all plant matter is cellulose, and it is thus the most abundant organic compound on earth. Cellulose can laterally assemble, thereby gaining mechanical properties useful for the stabilization of plant cell walls.
Proteins are the most versatile representatives of biopolymers. Proteins are commonly composed of 20 different naturally occurring amino acids. The specific arrangement of these amino acids allows the construction of a myriad of different proteins with unique properties.3 Proteins are poly(amino acid) chains, whose amino acid sequence is known as the primary structure (Fig. 2). The secondary structure is the local spatial arrangement of the polypeptide chain. The most frequently occurring secondary structure motifs are α-helices and β-sheets. The three-dimensional arrangement of those secondary structure motifs is called the tertiary structure. Finally, the assembly of individual proteins in supramolecular protein complexes leads to the formation of quaternary structure. On one hand, such large complexes fulfil pivotal functional roles like transcription or nucleo–cytoplasmic transport. On the other hand, highly complex quaternary structures with a vast number of individual building blocks are also found in extracellular structural proteins including, e.g., collagen, keratin, and silk.4
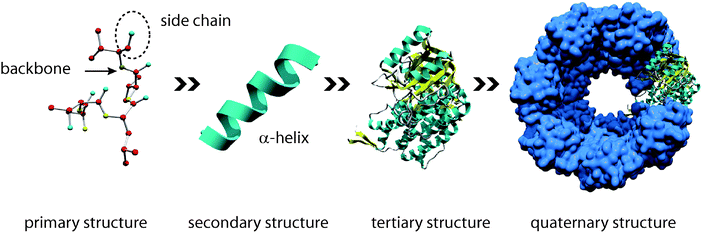 | ||
| Fig. 2 The hierarchical structure of proteins. The primary structure is defined by the sequence of amino acid residues and is responsible for subsequent folding processes. The secondary structure describes local conformations, e.g. α-helices or β-sheets. The spatial three-dimensional arrangement of an individual protein is its tertiary structure. Several proteins can interact to form quaternary structures (functional multimers). | ||
Structural proteins: intra- and extracellular protein fibers
Almost all eukaryotic cells possess an intracellular scaffold. This cytoskeleton is a dynamic structure that maintains cell shape, protects the cell, enables cellular motion, and plays an important role in both intracellular transport (the movement of vesicles and organelles) and cellular division.5–7 An intact and viable cytoskeleton is crucial for cell survival.One main component of the cytoskeleton is the protein actin. G-actin is a conserved globular protein and the monomeric subunit of small microfilaments (F-actin). The monomeric protein polymerizes under ATP hydrolysis in a fully reversible manner, and assembly and disassembly continuously occur during the lifetime of an organism.8 An actin filament is composed of two intertwined helices. These filaments are a major part of the cytoskeleton and act to sustain the outer cell shape. Additionally, actin filaments play an important role in intracellular transport processes. Several filaments can further assemble into larger fibers, which have a crucial role in the function of muscles.
Collagens are protein fibers which can be found in the extracellular matrix of tissue. Most collagens assemble into elongated fibrils and fibers with a defined structure.9 However, it is important to mention that not all members of the collagen family do form fibers. Collagens are a major component of skin, tendon and bone. The mechanical properties of these materials, tendons in particular, are a direct consequence of collagen-content and the arrangement of collagen molecules within the scaffold.10 Most collagens are secreted as procollagen and after secretion, several enzyme-mediated modifications are necessary for collagen structure formation. The primary feature of a matured (fibrous) collagen structure is a triple-stranded helix, in which three collagen polypeptide chains are coiled around each other to form a rigid superhelix, strengthening the overall structure. In contrast to most other proteins, collagens show an extraordinary high proline and glycine content. In addition, selected prolines and lysines of collagen are post-translationally hydroxylated to yield hydroxyproline and hydroxylysine residues. These modified amino acids allow tight intermolecular contacts, which enable the formation of the collagen triple helix. However, once assembled, collagens are thermodynamically stable and do not disassemble (due to covalent cross-linking of lysine residues of individual collagen chains). Interestingly, collagen triple helices can further assemble in a highly ordered process resulting in collagen fibrils with specific patterns (Fig. 3), which can even be observed by light microscopy. The assembly of several collagen triple helices into fibrils is an entropy driven process,11 similar to actin filament formation (see above). This ability of collagen triple helices to assemble into covalently linked fibrils is a crucial prerequisite for the rigidity and strength of tendon and skin. Collagen fibrils can further align to form collagen fibers usually having diameters of 1 to 20 microns. Collagen fibers are the major building block of fascicles, and eventually, tendons. Therefore, tendon formation is a multi-unit hierarchical process that includes collagen triple helices, fibrils, fibers, fascicles and tendon units.10 In contrast to tendons, bones are not solely made of collagen, and typically more than 70% (w/w) is made of hydroxyapatite particles/crystallites, which are embedded in the flexible collagen matrix. This stiff inorganic component mechanically supports the collagen fibers.12 Upon stress, both mineral particles and fibers deform elastically, resulting in a high toughness and resilience, which could not be achieved by one component alone and without the hierarchical arrangement of the respective building blocks.
 | ||
| Fig. 3 Organization of a collagen fiber. Three collagen monomers form a left-handed triple helix. These triple helices assemble to yield collagen fibrils that in turn align to form a mature collagen fiber. | ||
Extra-corporal protein structures—Keratins
Though not as tough as bone or as ductile as tendon, hair is a hierarchically structured protein fiber that is important for the survival of many animals. Found almost exclusively in mammals, it affords protection against harsh environmental conditions like for example low temperatures or humidity. Hair is a proteinaceous fiber that grows through the epidermis from follicles deep within the dermis. The main component of hair fibers is the helical protein keratin. Although hair looks like a monofilament under the microscope, it is in fact a multicellular tissue of several morphological components, each with a specific chemical composition.13 Hair can be divided into two major layers, the cuticle and the cortex. The outermost surface, or cuticle, consists of several layers of plate-shaped cells that overlap with up to 1 μm thick edges of the scales pointing to the tip of the fiber. The inner cortex of the fiber accounts for about 80% of an individual hair. Its hierarchical structure is important for its mechanical properties. The cortex contains cortical cells and a cell membrane complex. A cortical cell is further composed of fibrous bundles, which themselves consist of a large number of fine keratin fibrils. Several forms of keratin can be found in mammals, as well as other organisms.14 Important for the formation of hair is α-keratin, forming a right handed α-helix. Every keratin filament is made of an equal mixture of two types of keratin which form heterodimers. Two of these heterodimers form the tetrameric subunits which are the building block for keratin fibrils.However, keratin or keratin-like structures are not only suitable for protection. A very special utilization of extra-corporal keratin filaments can be found in geckos. Geckos are small lizards which live in warm climates throughout the world. More than a thousand different species of gecko have been identified, many of them well known for their specialized toe pads enabling them to climb smooth vertical surfaces and even ceilings. This ability is enabled by a very efficient attachment mechanism in which patterned surface structures interact with the profile of the substrate.15 Gecko feet have an intricate hierarchical structure which is essential to achieve the desired adhesion forces. The structure consists of lamellae (400–600 μm in size), setae (low micrometre dimensions) and spatulae (about 200 nm in size).16 A single seta is approx. 5 μm in diameter and branches at the tip into 100 to 1000 spatulae made of the aforementioned keratinous fibers (Fig. 4). Setae and spatulae provide enough attachment force to cling to almost any substrate. Studies on the spatulae-tipped setae on gecko footpads demonstrate that the attractive forces are van der Waals interactions between the finely divided keratin structures and the surface.17 Due to this attachment strategy, geckos retain the ability to remove their feet at will from the attachment surface by a peeling action.18
 | ||
| Fig. 4 Gecko toes show a lamellar structure. The lamellae consist of keratinaceous filaments (setae) that split into finer, knob-like structures (spatulae) at their ends. This hierarchical organization enables extended van der Waals interactions between toes and substrate. | ||
Silk—a brief introduction
Another remarkable extra-corporal protein fiber is silk. The most prominent silk producers are the caterpillars of the Bombyx mori silk moth since their silk cocoons have been harvested by man for several thousand years, and the silk has found widespread industrial application as a raw material for exclusive fabrics. Similar to insects, spiders also use silk for the protection of their offspring against environmental hazards and, in addition, many spiders possess a supplementary repertoire of different silk types to construct variously shaped webs to capture prey. Among different shapes of spider webs, orb-webs are the best investigated. Orb-weaving spiders generally use four different kinds of silk for the construction of their webs (Fig. 5). The framework (frame and radii) of the web is built using the so-called dragline silk, originating from the major ampullate gland (MA gland). Before commencing the construction of the characteristic capture spiral, the spider uses minor ampullate spidroin (MIS) silk fibers (the proteins are produced in the minor ampullate gland, MI gland) to form a temporary auxiliary spiral. The role of the auxiliary spiral is to stabilize the emerging web structure and to serve as a template for the subsequently built capture spiral. The capture spiral itself is formed by flagelliform silk, and the involved protein is produced in the spider’s flagelliform gland. The interconnection of the different silks in an orb-web and the fixation of the web structure on a substrate is accomplished via a gluey, silk-like substance termed “attachment cement”, originating from the piriform gland.19,20 During evolution, each of the aforementioned silks was selected and optimized regarding their mechanical characteristics and functionalities, which in combination resulted in those fascinating, persistent and mechanically stable structures of spider webs (Table 1). It is important to mention that most female orb-weaving spiders can produce even more types of silk (glues and fibers) than the much smaller males, particularly to protect their offspring (summarized in ref. 19). | ||
| Fig. 5 Spider orb-web with dewdrops. At least four different types of silk are used to construct this complex structure. | ||
| Material | Toughness/MJ m−3 | Strength/GPa | Extensibility (%) |
|---|---|---|---|
| Dragline silk (from Araneus diadematus) | 160 | 1.1 | 27 |
| Kevlar 49TM (aromatic para-polyamide) | 50 | 3.6 | 2.7 |
| Nylon 6,6 (polyamide) | 80 | 0.95 | 18 |
| Carbon fiber | 25 | 4 | 1.3 |
Assembly of spider silk fibers
Among the different types of spider silks, dragline silk is the most extensively studied and characterized. Thus, this silk is chosen to explain how the hierarchical organization of its proteinaceous components leads to a solid fibrous structure that mechanically outperforms man-made high-performance materials such as high tensile steel and aramid fibers (such as Kevlar), when considering selected material characteristics (e.g. toughness and breaking strain) (Table 1). In general, dragline silk is a composite fiber whose main components are two types of spider silk proteins (spidroins). In the case of the golden orb weaver Nephila clavipes, these are the major ampullate spidroins (MAS), namely major ampullate spidroin 1 (MaSp1, with at least two genetic variations, each further comprising two or more allelic variants)21 and major ampullate spidroin 2 (MaSp2). Another intensely studied silk, the dragline silk of the garden cross spider Araneus diadematus, contains the A. diadematus fibroins 3 and 4 (ADF-3 and ADF-4). Interestingly, because of the presence of prolines in both ADF-3 and ADF-4,22 the two proteins both reflect N. clavipes MaSp2 analogues since MaSp1 only shows a negligible proline content within its primary structure.23 Nevertheless, when considering the relative hydrophobicity of an MaSp1/MaSp2 and an ADF-3/ADF-4 pair, one of the proteins is generally more hydrophobic than its respective partner (MaSp1 and ADF-4 are more hydrophobic than MaSp2 and ADF-3).24The assembly process of MAS leads to the formation of distinguishable substructures or structural patches, finally resulting in a hierarchically built proteinaceous fiber. Further coating of the core fiber with glycoproteins and lipids then yields the high-performance dragline silk used as the orb-web stabilizing scaffold, and as the spider’s lifeline. The following sections will shed light on how the hierarchical structuring of two proteins can yield a complex proteinaceous thread with outstanding mechanical properties such as those of spider dragline silk.
The primary structure—layout for early folding and oligomerization events
Since the overall structure of a protein (secondary, tertiary, and quaternary structure) and therefore its ability to self-assemble or to integrate into macromolecular assemblies is intrinsically manifested in its primary structure, it should be regarded as the first hierarchical level of a fibrillar protein structure such as spider silk.MaSp1 and MaSp2, which are the main constituents of N. clavipes dragline silk, show a quite similar primary structure: the underlying proteins comprise a highly repetitive core domain with iterated repeats of alanine (A)-rich and glycine (G)-rich domains. MaSp2 further displays proline (P)-containing pentapeptide stretches.21 The core sequence of MAS is flanked by evolutionarily highly conserved and rather small (compared to the core region) terminal regions, which are non-repetitive. The primary structure determines how the proteins fold into a three-dimensional structure and thereby accounts for the ability of MAS to self-assemble into a mechanically stable protein thread (Fig. 6).
 | ||
| Fig. 6 Model of MAS primary structure. The protein core comprises iterated repeats of characteristic consensus motifs (X,Y). A consensus motif is typically built of 10–50 amino acid residues and is repeated up to 100 times. The repetitive core is flanked by amino- and carboxy-terminal domains with unique non-repetitive primary structures, each harboring a cysteine residue involved in intermolecular disulfide bridge formation. | ||
In vivo, freshly secreted MAS first adopt a random coil conformation with a certain amount of polyproline-II helical structure,25 mainly caused by the iterating sequence of the A- and G-rich consensus motifs of the repetitive core domain. This unique sequence, comprising iterations of hydrophobic and hydrophilic blocks, leads to an amphiphilic behavior of MAS. The amphiphilic character in concert with the lack of a defined secondary structure within the repetitive part of the solubilized MAS is an important pre-requisite for self-assembly and the formation of ascending levels of hierarchical order.
In contrast to the repetitive core, the non-repetitive primary structure of the carboxy-terminal domain of MAS favors folding into a defined secondary structure directly after secretion. A recent study demonstrated that a recombinantly produced protein mimicking the carboxy-terminal domain of the MAS of A. diadematusin vitro forms dimers in solution. The NMR solution structure at atomic resolution (mean square deviation 1.8 Å) shows that this domain adopts a mainly α-helical conformation consisting of 5 α-helices. The longest of these helices thereby interacts with its counterpart in another monomer via hydrophobic interactions. Further, the dimer is stabilized by an additional clamp-like mechanism between two different helices, each coming from one of the two involved monomers.26
The formation of dimers or oligomers via an intermolecular interaction of distinct α-helical secondary structure motifs seems to be a universal mechanism during silk fiber assembly, since the non-repetitive domain of the spider egg case silk protein (TuSp1) also adopts a mainly α-helical conformation consisting of 5 helices, presenting surface exposed and contiguous hydrophobic patches acting as interaction domains for oligomerization.27
Whether, under in vivo conditions, the MAS oligomers are heterogeneous or homogeneous multimers of MaSp1 and/or MaSp2 (or a combination of both) is still unclear and currently under investigation. Since oligomerization of spidroins is already detectable in the glandular sac of the spider,28 one could state that this process represents the second hierarchical organization level when considering distinct processes of transforming soluble proteins into a solid protein fiber. Strikingly, in the final dragline silk thread, MaSp1 is homogenously distributed within the silk’s core while MaSp2 clusters in the most interior part of the silk core (Fig. 7).29 However, it is so far unclear, how this distribution of MaSp1 and 2 is achieved and what role it plays concerning the mechanical properties of the resulting fibers.
 | ||
| Fig. 7 Core–skin structure of a silk thread. The proteinaceous core is subdivided into two distinct parts. A skin made of MIS surrounds a region where inhomogenously distributed MaSp2 clusters are embedded within the homogenous MaSp1 phase. The core is covered by a glycoprotein and a lipid-like layer. | ||
A molecular view: conformational transitions of MAS during assembly
Due to the amphiphilic character of MaSp1 and 2, the initially formed MAS oligomers are thought to adopt a micelle-like structure and accordingly small droplets are detectable in the MA gland quite early on.30 Simultaneously, the proteins undergo the first conformational transitions. During this process, a fold is created where 6–10 of the A-rich domains, connected via hairpins, start to form a secondary structure containing β-strands. This fold can be found approximately 7 times per MAS molecule and resembles a pearl necklace.31During the channeling of MAS from the glandular sac to the spigot, they encounter various changes in their chemical environment as well as distinct mechanical forces. Within the spinning duct, which connects the gland and the spigot, the pH is gradually decreased, the chaotropic salt sodium chloride is replaced by the more kosmotropic salt potassium phosphate, and water is resorbed by the epithelial cell lining of the duct.32 All these changes are accompanied by an increase in elongational flow and in shear force, caused by the tapering of the spinning duct.33 The combination of these processes results in secondary structure formation of MAS because potassium and phosphate are, as kosmotropic ions, able to alter the hydration pattern of the protein surface. This effect drastically increases hydrophobic interactions and promotes the formation of β-sheets. It could be shown with recombinantly produced MAS-analogues that elevated potassium phosphate concentrations induced assembly and the formation of β-sheet-rich silk structures via a liquid–liquid phase separation.20,34 As mentioned before, alignment of MAS is achieved by the elongational flow and high strain rate in the spinning duct, further favored by the dense β-sheet-structure of the MAS that allows tight intermolecular interactions and a liquid–solid phase transition.20 Summing up, the changing chemical conditions induce conformational transitions that reduce solubility and allow a tighter packing of the MAS molecules, whereas the driving force for the alignment of the proteins is elongational flow combined with shear stress. In vitro findings suggested that both chemical and mechanical triggers are necessary to elongate and align the aforementioned droplet-like protein structures to adopt a fibrillar morphology.35 The initiation of β-sheet propagation and MAS alignment can be considered to be the third hierarchical level during silk thread formation.
Formation of substructures within silk
A widely accepted theory suggested that stretches of 6–8 alanine residues align to form so-called β-crystallites (tightly packed β-sheets) which are embedded in an amorphous matrix primarily composed of the G-rich motifs of MaSp1 and the P-containing motifs of MaSp2. The amorphous matrix was assumed to maintain the extensibility of dragline silk since the G- as well as the P-rich motifs were thought to adopt a 31-helical conformation. The extensibility in concert with the β-crystallite imparted strength was proposed to be responsible for the outstanding toughness of dragline silk. However, the discovery that MaSp1 and MaSp2 are inhomogenously distributed within the thread indicated that this theory reflects a simplification.More recent findings indicate that the amorphous matrix is not fully disordered and shows, at least to a moderate degree, a three-dimensional hierarchical order. As seen in scanning transmission X-ray microscopy (STXM) studies on dragline silk of the black widow spider Latrodectus hesperus, only a small quantity of the fiber is composed of highly ordered β-crystallites accompanied by almost completely disordered regions. Both are dispersed in a matrix that shows a higher orientation than previously thought.36 Within the silk thread, those moderately ordered regions seem to comprise the main portion of MAS. The most highly ordered regions display dimensions that are too large to be solely formed by individual β-crystallites, even if they were surrounded by an additional interphase. An explanation for that finding was that the occurring alignment and the interchain interactions are more pronounced than originally assumed and that there is at least some longer-range order evident.36 This is the reason why these results can also affirm a more sophisticated, theoretical model describing the chain alignment and β-sheet stacking of MAS within the silk thread as a non-periodic lattice (NPL) crystal. The model considers the random occurrence of local compatibility or incompatibility of amino acid side chains between two adjacent β-sheets to describe how perfect and stable the respective crystalline regions are,37 and in which way they contribute to certain material characteristics of a spider silk thread.
Thus, the outstanding mechanical properties of dragline silk arise from a more complex distribution of distinct patches in which the MAS show different degrees of organization. Investigations on dragline silks of L. hesperus and the golden orb weaver N. clavipes using analytical transmission electron microscopy and X-ray diffraction revealed a clear bimodal distribution of crystalline areas within the spider silk, comprising two discrete size regimes. The smaller crystallites display a size of 2–3 nm, which identified them to be typical β-crystallites consisting of densely packed β-sheets made solely of poly-A stretches (Fig. 7).38 Their size is large enough to confer high strength to the fiber, since molecular dynamics simulations predict that β-sheets made of strands with 4–5 residues provide highest strength when loaded with uniform shear stress. This strength limit is an intrinsic feature of the protein building blocks and can only be overcome by combining β-crystallites with regions of lower (imperfect) order.39 In case of spider dragline silk, regions of reduced crystalline order, spanning a size range between approximately 70–500 nm, are detectable beside the 2–3 nm sized β-crystallites.40 The size of those imperfectly crystalline areas indicated that they are too large to be solely built of either poly-A or GGX-stretches. It seems more likely that these regions are formed by a mixture of different spider silk consensus motifs, since the large margin of 70–500 nm must result from variable intersheet spacings between the β-sheets, caused by incorporation of differently sized amino acids side chains.37
In summary, the hierarchical level of differently ordered substructures (from perfectly crystalline to completely unordered) seems to determine the structure–function relationship of spider dragline silk. Its influence on spider silk mechanics can be best described using a three-phase instead of the aforementioned two-phase model. As stated, crystalline domains assignable to one of two distinct size regimes are embedded in a matrix of varying order.36 A (mechanical) connection between the two crystalline regimes is most likely provided by an interphase comprising regions where the MAS molecules show a reduced degree of order. Further, the interphase itself is surrounded by the remaining portion of completely disordered MAS molecules (the “real” amorphous matrix). Using these findings as starting conditions for a model (Termonia’s model) that relates the distribution of differently ordered and unordered regions to the macroscopic mechanical properties of silk yields the following result:41 the small 2–3 nm sized β-crystallites confer a relatively low elastic modulus (10 GPa) but high tensile strength (1 GPa) to the silk, while the larger crystals are responsible for a higher elastic modulus (∼25 GPa) but lower tensile strength (∼0.27 GPa; all values were calculated according to Termonia’s model using the bimodal crystal distribution and crystal sizes experimentally obtained for L. hesperus dragline silk). Therefore, this model underlines that the bimodal crystal distribution and the mechanical connection maintained by the interphase is finally responsible for a biomaterial combining high elastic modulus and high elastic strength at the same time, a mechanical phenomenon called force-elongation behavior.40
Beyond the presence of differently sized crystalline areas and a small amount of completely unordered MAS, a longer-range organization of the distinct domains is still intensively discussed. Experimental data obtained during spectroscopic and microscopic investigations suggested that the highly ordered domains are organized in nanofibrils or fibrillar entities, respectively.29,36 On the other hand, there is also data available indicating the existence of small isotropic globular structures with no defined longer-range order along the fiber axis. In those studies no fibrillar structures, even on a micrometre scale, were detectable.38,42
Although, at first glance, these findings appear contradictory, they may just be the result of different elongational flow and shear conditions during silk fiber spinning and the observed longer-range structures (nanofibrils or nanoglobules) are intermediates created by a process during which micellar structures coalesce and elongate in an elongational flow field, as already shown by a microfluidic approach in vitro.35
Since the distribution of such fibrillar or globular structures, independently of their formation, will have an impact on the mechanical performance of a silk fiber, further investigations should clarify whether the occurrence and distribution of these longer-range structures is actively controlled by the spider in regard to the respective mechanical features needed at the moment of silk spinning.
Functional coatings on dragline silk
The aforementioned MAS structures are found in the core of a dragline silk thread. This core is coated with a proteinaceous layer (the skin) of a silk-like protein resembling an MIS43 that is typically used to create the auxiliary spiral during web construction. It is so far unknown what kind of interactions between the core and the skin layer exist, but this combination of proteinaceous components seems to be also important for the overall mechanical properties of dragline silk (Fig. 7). While the hierarchical organization of MAS and MIS is responsible for the mechanical properties of dragline silk, additional layers confer further features to the silk thread. The proteinaceous core is covered by a glycoprotein layer which is thought to control the humidity of the silk,44 thereby fine-tuning its mechanical properties. Further, this layer seems to have an antimicrobial function, protecting the silk thread from degradation by micro-organisms. An outermost, lipid-like coating also serves as an anti-microbial protection and is used as a means of sexual communication, mainly by incorporating pheromones.43It is important to mention that the hierarchical structure of dragline silks can be actively altered by a spider and is thus not static. Most importantly, the spinning speed alters the mechanical properties of the silk by influencing the time that MAS molecules have to align and therefore influences the silk’s hierarchical structure.20 Finally, various environmental parameters such as temperature, humidity, and nutrition of the spider also directly influence the hierarchical organization at the nano- and microscale.20
Construction of spider webs
As mentioned above, a web-weaving spider can facilitate more than one type of silk of varying mechanical (and other) properties. A well designed combination of these different silks is used to construct a web, representing the final level of the hierarchical organization of silk.Orb webs show a characteristic architecture mainly composed of four different types of silk. The spatial arrangement of the silks and their interconnectivity, besides the individual mechanical properties of the different silk threads involved, govern the mechanical behavior of the whole web during prey capturing. The webs are always built in the same temporal chronology: the creation of the hub and first radii made of MAS fibers is subsequently followed by completion of the frame and radii. Afterwards, the auxiliary spiral made of MIS is spun, serving as a template for the construction of the capture spiral made of flagelliform silk.45
The well-known orb web architecture (Fig. 5) seems to be beneficial in cases where high kinetic energies have to be dissipated. This is underlined by the fact that the energy absorption of intact, high energy absorbing webs (e.g. built by the spider Micrathena schreibersi) is two orders of magnitude higher than that of the dragline silk involved and even four orders of magnitude higher than that of the flagelliform silk. In contrast, the energy dissipation of intact low energy absorbing webs (e.g. built by the spider Leucauge globosa) is lower than that of the involved individual spider silk threads.46
These findings indicate a delicate relationship between architecture and mechanical performance. All high energy absorbing orb webs share a common feature: they are suspended under high pre-tension, display an elevated number of radii and spiral turns, and the ratio of radii to spiral turns is greater than one. The relatively high number of radii renders the web more rigid, at the expense of the web displacement capability. In contrast, low energy absorbing webs of medium and small sized orb weavers display radii to spiral turn ratios lower than one. In general, the number of radii and spiral turns divided by a web’s area gives a rather constant value in the case of low energy absorbing webs while that value can vary for high energy absorbing webs. The lower kinetic energies of prey intercepted by medium or small sized orb weavers are largely dissipated by web and fiber displacement as well as silk fiber extension. Therefore, it seems not to be astonishing that the energy absorbing capabilities of orb webs differ over 5 orders of magnitude.46
Spiders that are generally reliant on the interception of larger prey have to stick to the orb web architecture, while spiders hunting for small prey need not, because their silk fibers are tough enough to fulfill their role independently of the architecture. This offers the possibility to use alternative web constructs (e.g. funnel webs, sheet webs) and to populate completely new habitats, where these spiders may have an enhanced frequency of prey contact.
Summary and outlook
Spider dragline silk is well suited to study structure–function relationships of proteins. Numerous investigations concerning silk revealed a defined hierarchical organization of proteins in the final thread, comprising distinguishable structures on different length scales. The two proteins MaSp1 and MaSp2 encounter distinct processing conditions,32 involving self-assembly and alignment of the protein chains. First, the MAS molecules start to directly interact with each other, and the initial assemblies adopt a droplet-like structure.30 These covalently linked assemblies are thought to further align and interact in the spinning duct’s elongational flow field. Subsequently, the interplay of chemical and mechanical triggers initiates the formation of two distinct crystalline regimes, interconnected by an interphase and embedded in a hydrogel matrix. This type of organization resembles a particle enforced composite material based on proteins. Spinning conditions like reeling speed, nutrition of the spider and ambient factors such as humidity and temperature influence the ratio of crystallinity and the degree of orientation within the surrounding matrix, allowing the spider to actively tune the mechanical properties of the resulting silk thread.So far, it is not unambiguously clear whether sub-structures such as fibrils29,36 or micelles (or nanoglobules)38,42 are on the route towards silk threads or whether they just reflect by-products of the spinning process.
Understanding the processes of hierarchical organization of spider silk proteins is essential for at least two reasons: their outstanding mechanics render spider silk fibers an ideal material for technical and medical applications. Therefore, a deeper insight into the structure–function relationship of the underlying proteins is crucial for the potential biomimetic production of spider silk with tailored mechanical, chemical and physical properties. Such knowledge is also beneficial for further improvement of synthetic polymer-based materials, as spider silk organization can serve as a bio-inspired model for further strategies on novel sophisticated composite materials and for the mechanical tailoring of polymers.
Acknowledgements
This work was supported by funds of Universitäten Bayern e.V. (Graduiertenförderung nach dem Bayerischen Eliteförderungsgesetz) to MH and SCHE 603/4-3 to TS. We especially thank John G. Hardy and Andrew Smith for critical and fruitful comments on the manuscript, and Stanislav Gorb for kindly providing electron micrographs of gecko feet. We thank Fabio Falsone for the photo of a spider web.References
- J. Gorman and E. C. Greene, Nat. Struct. Mol. Biol., 2008, 15, 768 CrossRef CAS.
- M. Mutwil, S. Debolt and S. Persson, Curr. Opin. Plant Biol., 2008, 11, 252 CrossRef CAS.
- S. SenGupta and T. Scheibel, in Protein Folding-Misfolding: Some Current Concepts of Protein Chemistry, ed. J. P. Zbilut and T. Scheibel, Nova Publishers, New York, 2007, p. 1 Search PubMed.
- A. Lammel, D. Keerl, L. Römer and T. Scheibel, in Recent Advances in Biomaterials Research, ed. J. Hu, Transworld Research Network, Kerala India, 2008 Search PubMed.
- G. Woehlke and M. Schliwa, Nat. Rev. Mol. Cell Biol., 2000, 1, 50 CrossRef CAS.
- R. Basu and F. Chang, Curr. Opin. Cell Biol., 2007, 19, 88 CrossRef CAS.
- R. Li and G. G. Gundersen, Nat. Rev. Mol. Cell Biol., 2008, 9, 860 CrossRef CAS.
- J. Condeelis, Trends Cell Biol., 2001, 11, 288 CrossRef CAS.
- K. E. Kadler, D. F. Holmes, J. A. Trotter and J. A. Chapman, Biochem. J., 1996, 316, 1 CAS.
- F. H. Silver, J. W. Freeman and G. P. Seehra, J. Biomech., 2003, 36, 1529 CrossRef.
- K. E. Kadler, Y. Hojima and D. J. Prockop, J. Biol. Chem., 1987, 262, 15696 CAS.
- H. S. Gupta, J. Seto, W. Wagermaier, P. Zaslansky, P. Boesecke and P. Fratzl, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 17741 CrossRef CAS.
- C. Popescu and H. Hocker, Chem. Soc. Rev., 2007, 36, 1282 RSC.
- L. Langbein and J. Schweizer, International Review of Cytology—a Survey of Cell Biology, 2005, 243, 1 Search PubMed.
- E. Arzt, S. Gorb and R. Spolenak, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10603 CrossRef CAS.
- G. Huber, S. N. Gorb, R. Spolenak and E. Arzt, Biol. Lett., 2005, 1, 2 Search PubMed.
- W. R. Hansen and K. Autumn, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 385 CrossRef CAS.
- T. W. Kim and B. Bhushan, J. Adhes. Sci. Technol., 2007, 21, 1 CrossRef CAS.
- L. Römer and T. Scheibel, in Fibrous Proteins, ed. T. Scheibel, Landis Bioscience, Austin, Texas, USA, 2008, and references therein Search PubMed.
- M. Heim, D. Keerl and T. Scheibel, Angew. Chem., Int. Ed., 2009, 48, 3584 CrossRef , and references therein.
- W. A. Gaines and W. R. Marcotte, Insect Mol. Biol., 2008, 17, 465 CrossRef CAS.
- P. A. Guerette, D. G. Ginzinger, B. H. Weber and J. M. Gosline, Science, 1996, 272, 112 CAS.
- M. B. Hinman and R. V. Lewis, J. Biol. Chem., 1992, 267, 19320 CAS.
- D. Huemmerich, T. Scheibel, F. Vollrath, S. Cohen, U. Gat and S. Ittah, Curr. Biol., 2004, 14, 2070 CrossRef CAS.
- T. Lefèvre, J. Leclerc, J. F. Rioux-Dube, T. Buffeteau, M. C. Paquin, M. E. Rousseau, I. Cloutier, M. Auger, S. M. Gagne, S. Boudreault, C. Cloutier and M. Pezolet, Biomacromolecules, 2007, 8, 2342 CrossRef CAS.
- F. Hagn, L. Eisoldt, J. Hardy, C. Vendrely, M. Coles, T. Scheibel and H. Kessler, 2009, submitted for publication.
- Z. Lin, W. Huang, J. Zhang, J. S. Fan and D. Yang, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8906 CrossRef CAS.
- A. Sponner, W. Vater, W. Rommerskirch, F. Vollrath, E. Unger, F. Grosse and K. Weisshart, Biochem. Biophys. Res. Commun., 2005, 338, 897 CrossRef CAS.
- A. Sponner, E. Unger, F. Grosse and K. Weisshart, Nat. Mater., 2005, 4, 772–775 CrossRef CAS.
- F. Vollrath and D. P. Knight, Nature, 2001, 410, 541 CrossRef CAS.
- D. Porter and F. Vollrath, Adv. Mater., 2009, 21, 487 CrossRef CAS.
- D. P. Knight and F. Vollrath, Naturwissenschaften, 2001, 88, 179 CrossRef CAS.
- D. P. Knight and F. Vollrath, Proc. R. Soc. Lond. B, 1999, 266, 519 CrossRef.
- U. K. Slotta, S. Rammensee, S. Gorb and T. Scheibel, Angew. Chem., Int. Ed., 2008, 47, 4592 CrossRef CAS.
- S. Rammensee, U. Slotta, T. Scheibel and A. R. Bausch, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6590 CrossRef CAS.
- M. E. Rousseau, D. H. Cruz, M. M. West, A. P. Hitchcock and M. Pezolet, J. Am. Chem. Soc., 2007, 129, 3897 CrossRef CAS.
- B. L. Thiel, K. B. Guess and C. Viney, Biopolymers, 1997, 41, 703 CrossRef CAS.
- J. Pérez-Rigueiro, M. Elices, G. R. Plaza and G. V. Guinea, Macromolecules, 2007, 40, 5360 CrossRef CAS.
- S. Keten and M. J. Buehler, Nano Lett., 2008, 8, 743 CrossRef CAS.
- J. E. Trancik, J. T. Czernuszka, F. I. Bell and C. Viney, Polymer, 2006, 47, 5633 CrossRef CAS.
- Y. Termonia, Macromolecules, 1994, 27, 7378 CrossRef CAS.
- H. J. Jin and D. L. Kaplan, Nature, 2003, 424, 1057 CrossRef CAS.
- A. Sponner, W. Vater, S. Monajembashi, E. Unger, F. Grosse and K. Weisshart, PLoS One, 2007, 2, e998 CrossRef.
- Y. Liu, Z. Z. Shao and F. Vollrath, Nat. Mater., 2005, 4, 901 CrossRef CAS.
- R. F. Foelix, Biology of Spiders, Oxford University Press, Oxford, UK, 2nd edn, 1996 Search PubMed.
- C. L. Craig, Biol. J. Linn. Soc., 1987, 30, 135 Search PubMed.
Footnotes |
| † Contributed equally to this work. |
| ‡ Present address: AMSilk GmbH, Am Klopferspitz 19, 82152 Planegg München, Germany. |
| This journal is © The Royal Society of Chemistry 2010 |
