Rearrangement of β-amino alcohols via aziridiniums: a review
Thomas-Xavier Métro, Béranger Duthion, Domingo Gomez Pardo* and Janine Cossy*
Laboratoire de Chimie Organique, Ecole Supérieure de Physique et de Chimie Industrielles de la Ville de Paris (ESPCI ParisTech), CNRS, 10 rue Vauquelin, 75231 Paris Cedex 05, France. E-mail: domingo.gomez-pardo@espci.fr; janine.cossy@espci.fr; Fax: (+33) (0)1 40 79 46 60; Tel: (+33) (0)1 40 79 44 29
First published on 8th September 2009
Abstract
This tutorial review focuses on the rearrangement of β-amino alcohols via aziridinium intermediates. It covers the literature from 1947 to January 2009 (55 references). The rearrangement of β-amino alcohols can be performed by activation of the hydroxy group followed by the addition of nucleophiles (Nu). In most examples, an aziridinium intermediate is involved in the rearrangement. The ratio of amines resulting from the attack of nucleophiles at either the C-1 or C-2 position of the aziridinium intermediate, depends on the nature of the nucleophiles and the R2 substituent. In some cases, solvent as well as temperature can influence the ratio of amines.
 Thomas-Xavier Métro | Thomas-Xavier Métro graduated with a Master’s degree in medicinal biochemistry from the Université de Pharmacie de Montpellier in 2004. He achieved his PhD in organic chemistry in 2007 from the Université Pierre et Marie Curie (Paris) under the supervision of Dr Domingo Gomez Pardo and Professor Janine Cossy. His research work was dedicated to the rearrangement of amino alcohols and its application to the synthesis of bioactive molecules. After having spent one year in Process R&D at Sanofi-Aventis Sisteron, he is currently working on a post-doctoral project in medicinal chemistry at Sanofi-Aventis R&D Montpellier. |
 Béranger Duthion | Béranger Duthion studied chemistry at the Ecole Supérieure de Physique et de Chimie Industrielles de la Ville de Paris (ESPCI ParisTech). As a graduate student, he joined the Laboratory of Organic Chemistry of the ESPCI ParisTech and obtained a Master’s degree in bioorganic and organic chemistry at the Université Pierre et Marie Curie (Paris) in 2007. He is currently doing his PhD under the supervision of Dr Domingo Gomez Pardo and Professor Janine Cossy at ESPCI ParisTech. |
 Domingo Gomez Pardo | Domingo Gomez Pardo is currently Maître de Conférences at the ESPCI ParisTech. He received his PhD in organic chemistry in 1992 from the Université Pierre et Marie Curie (Paris) working with Professor J. d’Angelo. He is interested in synthetic methodologies (rearrangement of amino alcohols, ring expansion reactions, synthesis of nitrogen heterocycles, stereoselective reactions) and in their application to the synthesis of natural products and biologically active molecules. |
 Janine Cossy | Janine Cossy studied at the University of Reims with Professor J. P. Pète. After a postdoctoral stay with Barry Trost, at the University of Wisconsin, she returned to Reims to become Director of Research of the CNRS in 1990. She then moved to Paris to become Professor of Organic Chemistry at the ESPCI ParisTech. She is Director of the CNRS Unit UMR 7084. From 2003 to 2007, she was President of the Organic Division of the French Chemical Society and since 2005, she has been Associate Editor for Organic Letters. |
Aziridines have been extensively used as useful building blocks.1,2 The control of the regio- and stereoselectivity in the opening of aziridines provides convenient access to chiral nitrogen-containing compounds.3–6 Although N,N-dialkyl aziridiniums are stronger electrophiles than neutral aziridines, their use as key intermediates in organic synthesis is rare.7–9 Aziridiniums, generally obtained through activation of β-amino alcohols, can be opened by a wide range of nucleophiles with or without rearrangement depending on the regioselectivity of the nucleophilic attack.
This review covers only the rearrangement of β-amino alcohols of type A, B, and C (Fig. 1) as the rearrangement of prolinols of type D has been recently covered.10
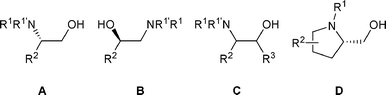 | ||
| Fig. 1 | ||
1. General overview
When β-amino alcohols A are converted into a derivative bearing a good leaving group (LG), the latter can be displaced through an SNi mechanism to generate aziridiniums F (Scheme 1). In a similar fashion, β-amino alcohols B can give F. Subsequently, the liberated anion LG− can then attack F either on the less and/or more substituted carbon atom (C-1 and/or C-2) to produce amines E and/or G. In the case of β-amino alcohols A, a rearrangement will be observed if anion LG− attacks aziridiniums F at C-2. On the other hand, the rearrangement of β-amino alcohols B into E will take place if anion LG− attacks aziridiniums F at C-1. However, even if the attack is regioselective, a mixture of E and G could be obtained as these compounds are in a thermodynamic equilibrium with F. The proportion of amines E and G is the result of thermodynamic control. Complete rearrangement of amino alcohols A (or B) will be observed only if G (or E) are the thermodynamically more stable products (Scheme 1).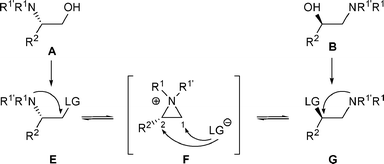 | ||
| Scheme 1 | ||
In some cases, nucleophiles other than LG− are present in the reaction medium and this “external nucleophile” (Nu) can compete with LG−, attacking aziridiniums F at C-1 to produce amines I or at C-2 to produce amines H (Scheme 2).
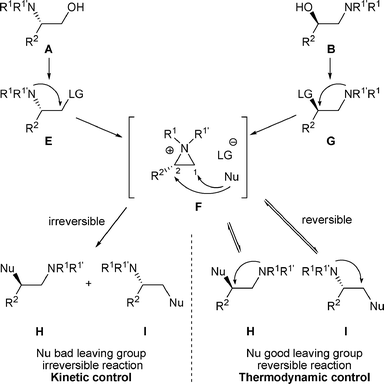 | ||
| Scheme 2 | ||
The ratio of amines I and H depends mainly on the “external nucleophile” (Nu) present in the reaction medium. If Nu, present now in I and H is a good leaving group, the reaction is reversible and the proportion of amines I and H will correspond to a thermodynamic equilibrium (thermodynamic control). On the other hand if Nu, present in I and H is not a good leaving group, the opening of F is irreversible and the reaction will be under kinetic control. In this case, the proportions of amines I and H will correspond to the regioselectivity of the nucleophilic attack on the aziridinium intermediate F (Scheme 2).
The major factors that determine the proportion of amines I and H are the nature of the nucleophile as well as the substituents R1, R1′, and R2 (as well as R3 for β-amino alcohols C). To a lesser extent, the solvent and the temperature may also affect the product distribution.
2. Influence of the R2 substituent on the rearrangement of β-amino alcohols A and B
2.1 R2 = Alkyl
When the R2 substituent of β-amino alcohols A or B is an alkyl group, the obtained ratio of amines H and I depends strongly on the nucleophile (LG− or Nu) (cf.Schemes 1 and 2).2.1.1.1 By halide (Cl−, Br− or F−). The first rearrangement of β-amino alcohols of type A was described in 1947 by Brode and Hill.11 The authors observed that the treatment of β-amino alcohols 1 and 2 with SOCl2 led to the ammonium chlorides 3 and 4, which after treatment with NaOH led to the same β-chloro amine (Scheme 3). Even though the authors were not able to determine if the obtained product was β-chloro amine 6 or 7, they supposed that 3 and 4 were transformed into 6 or 7via the aziridinium intermediate 5 after treatment with NaOH.
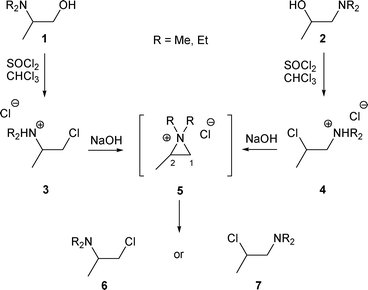 | ||
| Scheme 3 | ||
This hypothesis was confirmed by Zirkle et al.,12 who showed that under similar conditions, the chloro amine 7 was produced (attack of 5 at C-2 by the chloride anion). As the chloride atom is a good leaving group, the reaction is reversible, implying that 7 is the thermodynamic product (Scheme 3).
The formation of an aziridinium intermediate was also proven by treating amines 6 and 7 with an “external nucleophile”. By using the diphenyl acetonitrile anion, 8 and 9 were obtained in a 50/50 ratio, no matter which β-chloro amine 6 or 7 was reacting (Scheme 4).13
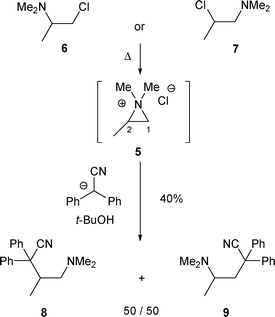 | ||
| Scheme 4 | ||
We have to point out that the treatment of 10 with SOCl2 led to 11 which was rearranged to 12 at 140 °C without any base (Scheme 5).12 Another example of β-amino alcohol rearrangement without any base was described by Achini.14
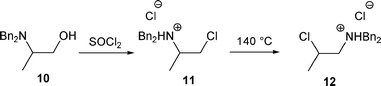 | ||
| Scheme 5 | ||
The rearrangement of β-amino alcohols of type A induced by SOCl2 can tolerate a diversity of functionalities present on the N-alkyl moiety, such as a nitrile,14 an allyl, an imidazole15 or a sulfonyl group.16
However, when β-amino alcohols 13 and 16, possessing an electron-withdrawing group (CN or CO2t-Bu) in a position α to the nitrogen atom, were treated with SOCl2 then with a base, either the expected rearranged product was not observed (Scheme 6, eqn (1)) or a mixture of rearranged and non-rearranged products was obtained (Scheme 6, eqn (2)).17 The formation of the non-rearranged product can be explained by the low nucleophilicity of the nitrogen due to the orbital overlap nN →σ*C–EWG which prevents the total or partial formation of the aziridinium intermediate. Thus a direct substitution of the chlorosulfite intermediate by the chloride anion occurred.18
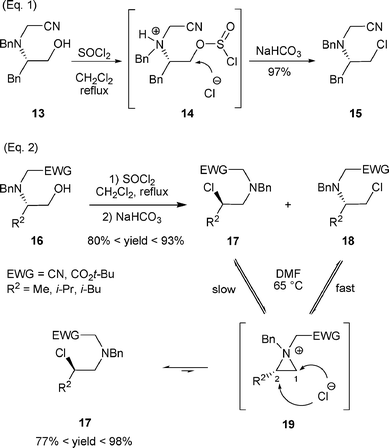 | ||
| Scheme 6 | ||
Interestingly, the proportion of β-chloro amines 17 and 18 could be modified by applying conditions facilitating the formation of an aziridinium intermediate. Thus, after heating 17 and 18 at 65 °C in DMF, only the secondary chloride 17 was isolated. The authors explained this result by postulating that the aziridinium intermediate 19 is formed faster from 18 than from 17 (Scheme 6, eqn (2)).
Similar to the formation of β-chloro amines from β-amino alcohols when treated with SOCl2, β-bromo amines were obtained from N,N-dialkyl β-amino alcohols 20 or from N-alkyl β-amino alcohol 22 when treated with SOBr2 (Scheme 7).19
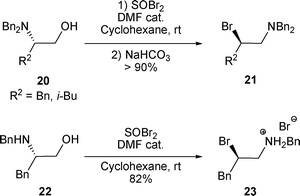 | ||
| Scheme 7 | ||
Reagents other than SOCl2 and SOBr2 can promote the rearrangement of β-amino alcohols of type A to the corresponding β-amino halides of type H. Among those, MsCl/Et3N, TsCl/Et3N, Ms2O/LiCl, DAST (F3S–NEt2) and Deoxo-Fluor® [F3S–N(CH2CH2OCH3)2] are worthy of note.
For example, when β-amino alcohol 24 was treated with MsCl/Et3N, mesylate 25 was first observed by NMR spectroscopy, and this compound was rapidly transformed to β-chloro amine 27via aziridinium 26 (Scheme 8).20
 | ||
| Scheme 8 | ||
The use of tosyl chloride also allowed the rearrangement of β-amino alcohols A to β-chloro amines H. When N,N-dibenzyl β-amino alcohols 28 were activated with TsCl in the presence of pyridine (Py) and DMAP, β-chloro amines 29 were obtained in 38% to 48% yields (Scheme 9).21
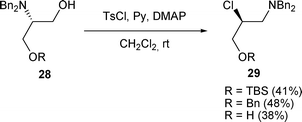 | ||
| Scheme 9 | ||
The rearrangement of β-amino alcohols A to products H can also be performed by treatment with Ms2O/Et3N followed by the addition of LiCl. When 24 was treated with Ms2O in the presence of Et3N, the rearranged mesylate 31 was formed and corresponded to the nucleophilic attack of a mesylate anion at the C-2 position of the aziridinium 30. As the mesylate was a good leaving group, the reaction was reversible implying that 31 was the thermodynamic product. When mesylate 31 was treated with LiCl, β-chloro amine 27 was obtained with retention of configuration of the stereogenic center which can be explained by the formation of the aziridinium intermediate 30 followed by the attack of Cl− (Scheme 10). The replacement of LiCl by LiBr led to the corresponding β-bromo amine.20
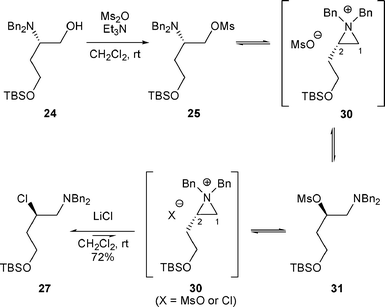 | ||
| Scheme 10 | ||
Other conditions, such as the combination of CBr4 with PPh3, were able to convert β-amino alcohols A to β-bromo amines H. Thus, treatment of β-amino alcohol 32 with CBr4/PPh3 in CH2Cl2 at room temperature furnished β-bromo amine 34 in 71% yield. Treatment of this latter with AgOTf led to the formation of aziridinium 35 which was attacked at C-1 by the pyrrole to produce, after deprotection of the acetal with TFA, the bicyclic compounds 36 and 36′ (Scheme 11).22
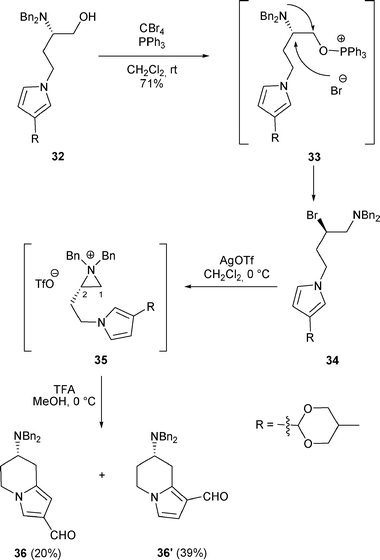 | ||
| Scheme 11 | ||
β-Fluoro amines H can also be obtained by rearrangement of β-amino alcohols A. When 37 was reacted with Deoxo-Fluor®, β-fluoro amines 38 and 39 were isolated in 71% and 15% yield respectively. In a similar manner, (R)-N,N-dibenzyl-2-amino-propan-1-ol 40 was transformed to 41 and 42 in 82% and 7% yield respectively (Scheme 12).
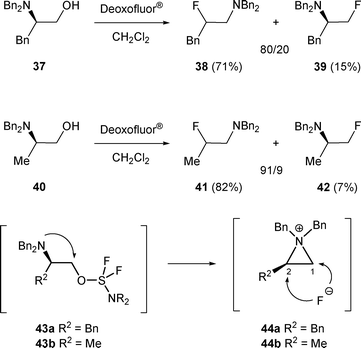 | ||
| Scheme 12 | ||
The major β-fluoro amines 38 and 41 are the result of the attack of the fluorine anion on the C-2 position of the aziridinium intermediate 44 formed from 43.23 Contrary to bromide and chloride, fluoride is not a good leaving group. Consequently, the opening of aziridinium 44 by a fluorine anion is irreversible, and the obtained β-fluoro amines were the result of kinetic control.
2.1.1.2 By nucleophilic sulfur. The use of sulfides as external nucleophiles also leads predominantly to a nucleophilic attack on aziridinium intermediates at C-2. These intermediates were obtained by activation of β-amino alcohols A.
When β-amino alcohol 45 was treated with MsCl/Et3N and then with KSCN, thiocyanate 47 was formed in 81% yield via aziridinium intermediate 46 (Scheme 13).24
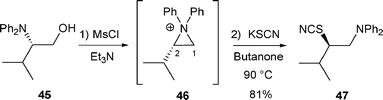 | ||
| Scheme 13 | ||
β-Thioamine 5025 was isolated in 80% yield, via aziridinium 49 formed from β-amino alcohol 48 when this latter was reacted with MsCl/Et3N followed by the addition of n-propanethiol (Scheme 14).26 As the thio group is not a good leaving group, the reaction is under kinetic control.
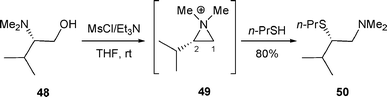 | ||
| Scheme 14 | ||
2.1.1.3 By nucleophilic oxygen. β-Amino formates (compounds of type H) can also be formed from β-amino alcohols A. When 51 reacted with SOBr2 in DMF, β-amino formates 53 and 54 were isolated in 92% and 2% yield respectively (Scheme 15).
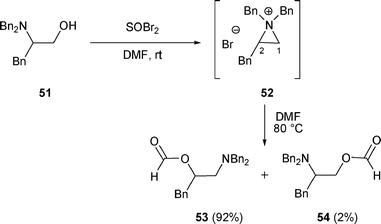 | ||
| Scheme 15 | ||
The rearrangement of β-amino alcohol 51 can be explained by the nucleophilic attack of DMF at the more substituted carbon (C-2) of the aziridinium intermediate 52, which led to a Vilsmeier-type intermediate that was further hydrolyzed to the corresponding formate esters 53 and 54.27
Recently, β-amino alcohols A have been converted to β-amino alcohols H by treatment with a stoichiometric amount of trifluoroacetic anhydride (TFAA) and Et3N followed by the addition of NaOH.28 Later on, it was demonstrated that a catalytic amount of trifluoroacetic anhydride can be used to induce the rearrangement of β-amino alcohols A to β-amino alcohols H. Under stoichiometric or catalytic conditions, 20 was transformed to 55 regioselectively via aziridinium intermediate 56. This enantioselective transformation was achieved in good yield (Scheme 16).28
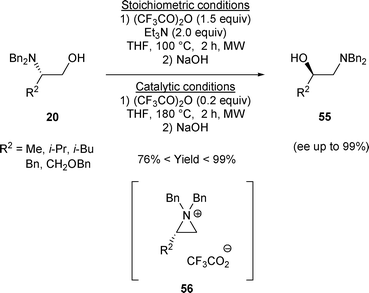 | ||
| Scheme 16 | ||
It is worth noting that when N-benzylamino alcohol 22 was treated with trifluoroacetic anhydride (1 equiv.) and Et3N (1 equiv.) followed by the addition of NaOH, N-benzylamino alcohol 57 was isolated in 70% yield and with an excellent enantiomeric excess (Scheme 17).29
 | ||
| Scheme 17 | ||
The rearrangement of β-amino alcohols was also realized using a catalytic amount of H2SO4 (5 mol%) in THF at 180 °C under microwave irradiation. Under these conditions, 20 was transformed into 55 regioselectively in good yield and with an excellent enantiomeric excess (Scheme 18).28
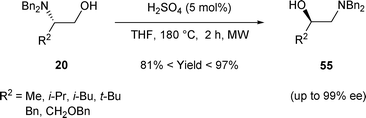 | ||
| Scheme 18 | ||
2.1.2.1 By nucleophilic oxygen. Depending on the oxygenated nucleophiles used, a predominant attack of the aziridinium at C-1 by the nucleophiles can also be observed. For example, after treatment of 58 with DCC and p-nitrophenol at 95–98 °C for 60 h, compounds 60 and 61 were obtained in a ratio of 10/90 in favour of amino ether 61 (attack of 59 at C-1 by the phenate ion) (Scheme 19).30
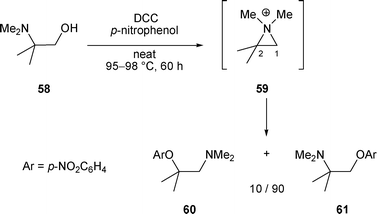 | ||
| Scheme 19 | ||
We have to point out that the nucleophilic attack of a diphosphate anion [(n-Bu4N)3HPO7] on an aziridinium intermediate is similar to the attack of a phenate ion.31
2.1.2.2 By nucleophilic carbon. When nucleophilic carbons are used to open an aziridinium, the C-1 position of the aziridinium intermediate is the favoured site of attack. In the case of β-chloro amine 27, obtained by treatment of β-amino alcohol 24 with MsCl/Et3N, the addition of NaCN led to 62a and 63a in a 1/10 ratio in favour of 63a. It is worth noting that whereas 27 did not react with cuprates, the addition of Me2CuLi to β-mesylamine 31, obtained by treatment of 24 with Ms2O, led to 63b in moderate yield (46%) (Scheme 20).20
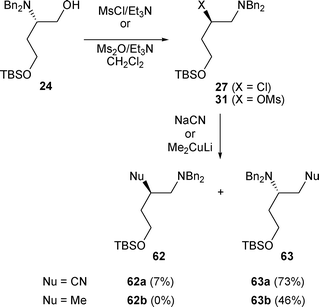 | ||
| Scheme 20 | ||
2.1.2.3 By nucleophilic nitrogen. Contrary to nucleophilic sulfur, oxygen and carbon, the regioselectivity of the nucleophilic nitrogen attack promoting the opening of aziridinium intermediates, derived from amino alcohols A and B, is difficult to rationalize. Treatment of secondary alcohol 64 with Tf2O followed by the addition of the O-silylated hydroxylamine led to the rearranged amine 66 in 56% yield resulting from the attack of the amine on aziridinium 65 at C-1 (Scheme 21).32
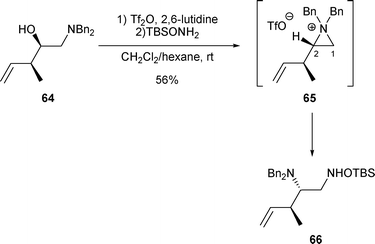 | ||
| Scheme 21 | ||
Similarly, the treatment of β-bromo amine 68 with the primary amino group present in 69 led to 70 (46% yield) (Scheme 22).19
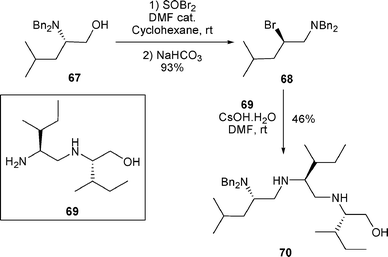 | ||
| Scheme 22 | ||
However, other reactions were not as clean. The regioselectivity of the nucleophilic attack of the phthalimide (PhtNH) on aziridinium intermediate 71 was moderate, as compounds 72 and 73 were obtained in a 29/71 ratio (Scheme 23).20
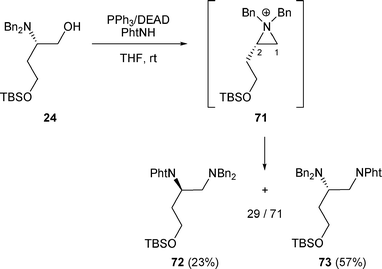 | ||
| Scheme 23 | ||
Poor regioselectivity is also observed in the opening of aziridinium 74 with azides, as the treatment of β-chloro amine 15 with NaN3 at 150 °C in DMSO led to 77 and 78 in a 47/53 ratio. These tetrazoles resulted from an intramolecular [2 + 3]-cycloaddition of intermediates 75 and 76 issued from the nucleophilic attack on the aziridinium 74 by azide anions at C-2 or C-1 respectively (Scheme 24). Although the orbital overlap nN →σ*C–CN may lower the nucleophilicity of the nitrogen atom in 15, conditions facilitating the formation of an aziridinium intermediate (DMSO, 150 °C) allowed the rearrangement of β-chloro amine 15.18
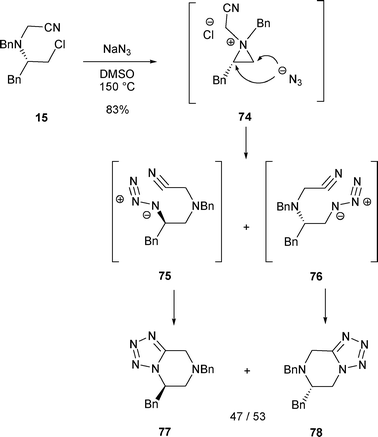 | ||
| Scheme 24 | ||
The regioselectivity of the nucleophilic attack on an aziridinium intermediate depends on the amino compound as well as on the nature of the substituents present in the aziridinium.26
The influence of the N-alkyl groups (R1) as well as the R2 group on the regioselectivity of the attack of the aziridinium intermediates by amines has been the subject of a few studies. Thus, when β-amino alcohol 79 (R1 = allyl) was treated with Tf2O/Et3N in the presence of morpholine, a mixture of regioisomers 81 and 82 was obtained in a ratio of 12/88 in favour of 82 (attack of the aziridinium 80 at C-1). The selectivity was increased when the two N,N-allyl substituents were replaced with two N,N-benzyl groups (Scheme 25).33
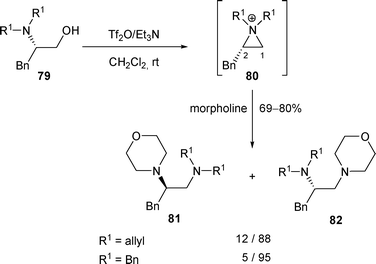 | ||
| Scheme 25 | ||
In the case of the nucleophilic attack of aziridiniums 84 with bulky R2 groups (Bn > Me), by methylamine, a better regioselectivity was observed. The major products were diamines 85, which corresponded to the attack of methylamine at the less sterically hindered position of aziridinium 84 (position C-1) (Scheme 26).34 In this latter case, the mechanism of the rearrangement has been studied and two processes can take place, either the attack of the aziridinium at C-1 by methylamine (predominant process) and a direct substitution of the mesylate 83 by methylamine (minor process).34
 | ||
| Scheme 26 | ||
Furthermore, it has been shown that aziridinium intermediates are not always formed, particularly when β-amino alcohols are treated with sulfonyl reagents in a non-polar solvent. A cyclic intermediate 87 can be formed instead of an aziridinium intermediate (Fig. 2).35
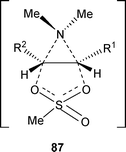 | ||
| Fig. 2 | ||
2.2 R2 = an electron-withdrawing group (EWG): CF3 or CO2R
Aziridiniums F′, issued from β-amino alcohols A′, where R2 is an electron-withdrawing substituent such as CF3 or CO2R, are in general, attacked by nucleophiles at C-2 as this carbon atom is more electropositive than C-1. If the Nu group present in amines H′ and I′ is not a good leaving group, the reaction will be under kinetic control and compounds H′ will be favoured (attack at C-2) (Scheme 27). | ||
| Scheme 27 | ||
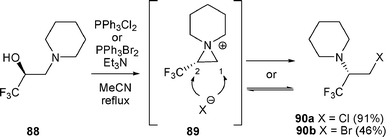 | ||
| Scheme 28 | ||
The formation of 90a and 90b could be explained by the presence of the electron-withdrawing CF3 group which could decrease the nucleophilicity of the nitrogen in compound 90 enough to prevent reversion to aziridinium 89. In this case, β-amino halide 90 would be the kinetic product. On the other hand, if the nitrogen atom in 90 was nucleophilic enough to form aziridinium 89, anion X− would have attacked the aziridinium intermediate 89 at C-1 and/or C-2, but β-amino halide 90 would have been the thermodynamic product.
2.2.2.1 By halide. Similar to 88, β-amino alcohol 91, containing an ester group, led to β-mesyl amine 92 when treated with MsCl/Et3N at room temperature (Scheme 29).37 In this case, it was not possible to know if an aziridinium intermediate was formed during the transformation or not. The mesylate 92 was described as stable and this stability was assigned to the steric hindrance of the two N,N-benzyl groups which could prevent 92 from the formation of an aziridinium intermediate from the β-elimination of the mesylate.
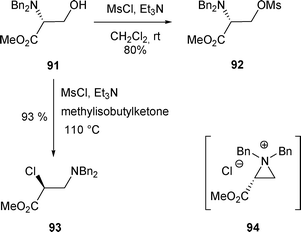 | ||
| Scheme 29 | ||
However, it was later proven that when harsher conditions were used, mesylate 92 could be transformed into an aziridinium species. Thus, when β-amino alcohol 91 was treated with MsCl/Et3N at 110 °C in methylisobutylketone, the rearranged halide 93 was obtained in 93% yield. This halide is the result of the nucleophilic attack of the aziridinium 94 at C-2 by the chloride anion (Scheme 29).38
When a fluoride anion was used as the nucleophile, the products obtained were also the result of an attack at the C-2 position of the aziridinium intermediate. β-Fluoro amine 96 was obtained after treatment of β-amino alcohol 95 with DAST (Scheme 30).39–41
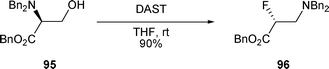 | ||
| Scheme 30 | ||
The presence of an alkyl group R3 such as in β-amino alcohols 97 can modify the regioselectivity of the attack of the aziridinium intermediate by the fluoride anion. When 97a (R3 = Me) was treated with DAST, a mixture of regioisomers 99a (attack at C-2) and 100a (attack at C-1) was obtained. It is worth noting that when R3 was an isopropyl group, only β-amino fluoride 99b was isolated (Scheme 31). 39
 | ||
| Scheme 31 | ||
2.2.2.2 By nucleophilic nitrogen and sulfur. In general, the attack of an aziridinium substituted by an ester group at C-2 by nucleophilic nitrogen or nucleophilic sulfur takes place at C-2. When mesylate 92 was heated at 80 °C in MeCN in the presence of a nucleophilic nitrogen, the major compound 102 generally resulted from the nucleophilic attack of the aziridinium 101 at C-2 (Scheme 32). However, the rearranged compounds 102 were obtained with yields strongly depending on the nucleophilic nitrogen. The best yields were obtained with morpholine (92%).37
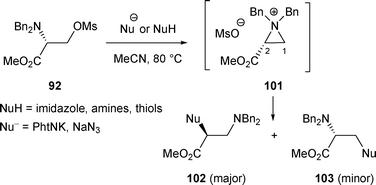 | ||
| Scheme 32 | ||
When thiols were used as nucleophiles, aziridinium 101 was attacked at C-2 and the rearranged products 102 were isolated in moderate yields (42–50%) (Scheme 32).37
2.3 R2 = Aryl
When R2 is an aryl group, the C-2 position of the aziridinium F′′, being an electrophilic benzylic position, is prone to nucleophilic attack. No matter which nucleophile is used, the attack of the aziridinium always occurs at C-2, thus producing amines H′′ (Scheme 33).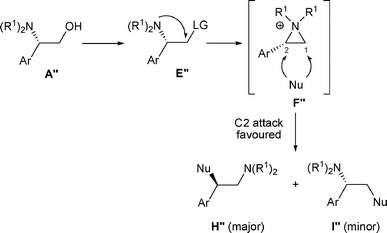 | ||
| Scheme 33 | ||
2.3.1.1 By halide. The first rearrangement of a β-amino alcohol possessing an R2 aryl group was realized with β-amino alcohol 104 using SOCl2 in Et2O without any base. Under these conditions, the rearranged β-chloro ammonium 105 was obtained in 72% yield (Scheme 34).42 The first explanation for the rearrangement of 104 to 105 was the direct transformation of the chlorosulfite intermediate 107 to the rearranged β-chloro amine 108 (Scheme 34, path 1). A second explanation could be the formation of the aziridinium intermediate 109 issued from an intramolecular nucleophilic attack of the pyrrolidine ring at the carbon bearing the chlorosulfite in 107 (Scheme 34, path 2). This aziridinium 109 could be attacked by the chloride anion at the benzylic position (C-2) to produce the ammonium salt 105via108 (Scheme 34).
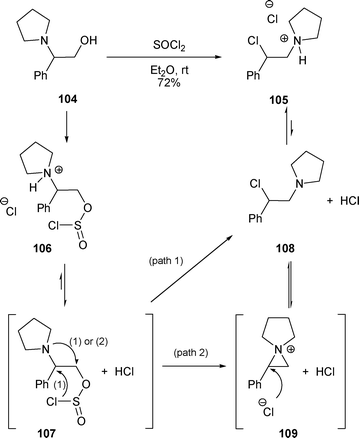 | ||
| Scheme 34 | ||
The rearrangement of β-amino alcohols induced by SOCl2 is general, as compounds 110 were respectively transformed to the corresponding β-chloro amines 111 in good to excellent yields (58% < yield < 100%) (Scheme 35).14,17
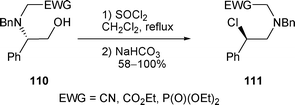 | ||
| Scheme 35 | ||
It is worth noting that, β-chloro amine 115 was the only isolated product when a mixture of β-amino alcohols 112 and 113 was treated with MsCl/Et3N in dichloromethane (nucleophilic attack on aziridinium intermediate 114 at C-2 by the chloride anion) (Scheme 36).43
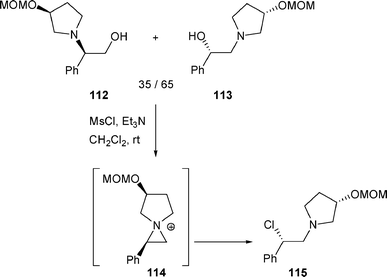 | ||
| Scheme 36 | ||
2.3.1.2 By nucleophilic nitrogen, oxygen and sulfur. When β-amino alcohol 116 was treated with MsCl/Et3N in the presence of n-butylamine, diamine 11844 was the result of the nucleophilic attack on intermediate 117 (Scheme 37).26
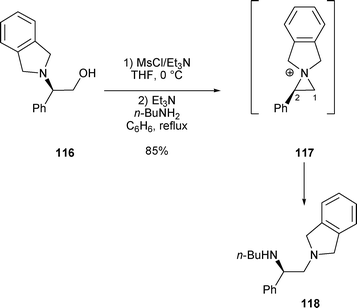 | ||
| Scheme 37 | ||
Similar results were obtained when amines,45–48 alcohols49 or thiols45,49 were used as nucleophiles. Good regioselectivity for the nucleophilic attack on the aziridinium intermediate at C-2 was observed.
Mitsunobu conditions have also been used to induce the rearrangement of β-amino alcohols in which R2 is an aryl group. Treatment of β-amino alcohol 119 with PPh3/1,1′-(azodicarbonyl)dipiperidine (ADDP) in the presence of 2-methoxyphenol led to ethers 120 and 121 in a 96/4 ratio in favour of 120 (92% yield) (nucleophilic attack on the aziridinium intermediate at C-2) (Scheme 38).50
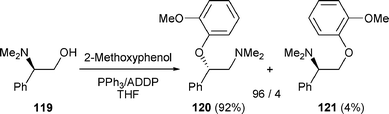 | ||
| Scheme 38 | ||
The use of acetyl chloride also promoted the rearrangement of β-amino alcohol 104. Whereas ester 123 was obtained as the major product (58% yield), the rearranged ester 122 was isolated in moderate yield (15%).42 A concerted mechanism was proposed to explain the transformation of 104 to 122via intermediate 124 (Scheme 39).
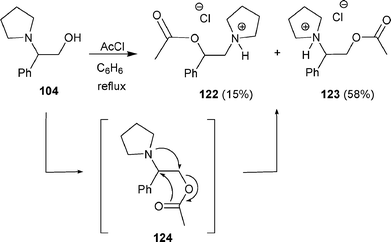 | ||
| Scheme 39 | ||
β-Amino alcohol 125 has been transformed to β-amino alcohol 126 by treatment with a stoichiometric amount of trifluoroacetic anhydride (TFAA) and Et3N followed by the addition of NaOH or by treatment with a catalytic amount of trifluoroacetic anhydride. Under these conditions β-amino alcohol 125 was transformed, via an aziridinium intermediate, to β-amino alcohol 126 in good yield and with excellent enantioselectivity (Scheme 40).28
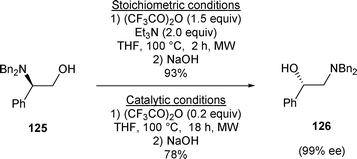 | ||
| Scheme 40 | ||
β-Amino alcohols possessing a quaternary center can also be rearranged under these conditions with almost no loss of chirality. (R)-N,N-Dibenzyl-2-allyl-2-phenylglycinol 127 was transformed to 128 in 63% yield when subjected to TFAA (2.0 equiv.) and Et3N (3.0 equiv.) (Scheme 41).28
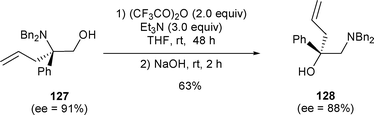 | ||
| Scheme 41 | ||
It is worth noting that when N-benzylphenylglycinol 129 was treated with TFAA (1.0 equiv.) and Et3N (1.0 equiv.) followed by the addition of NaOH, the rearranged N-benzylamino alcohol 130 was isolated in 72% yield with an enantiomeric excess of 94% (Scheme 42).29
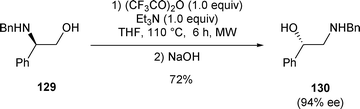 | ||
| Scheme 42 | ||
The rearrangement of β-amino alcohol 125 was also induced by a catalytic amount of H2SO4 (5 mol%) in THF at 180 °C under microwave irradiation (MW) for 2 h. Under these conditions 125 was converted to 126 (65% yield, 95% ee) (Scheme 43).28
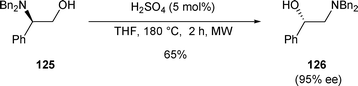 | ||
| Scheme 43 | ||
3. Influence of the R3 substituent on the rearrangement of β-amino alcohols C (R2 = aryl)
In general, for β-amino alcohols A where R2 is an aryl group, the rearranged products are the result of the nucleophilic attack at the benzylic position of the aziridinium intermediate. However, for β-amino alcohols C where R2 is an aryl group, the ratio of compounds L and M can be influenced by the presence of the R3 substituent (Scheme 44).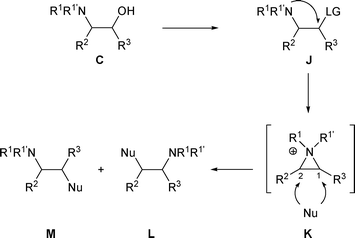 | ||
| Scheme 44 | ||
3.1 R3 = Alkyl
In general, if the R3 substituent of β-amino alcohols C is an alkyl group, the rearranged products are the result of a nucleophilic attack on the benzylic position of the aziridinium intermediate. Thus, when β-amino alcohols 131 were treated with MsCl/Et3N in the presence of potassium thioacetate (AcSK), the only products isolated were compounds 133, which resulted from the attack of the nucleophile on the benzylic position of the aziridinium intermediate 132 (Scheme 45).51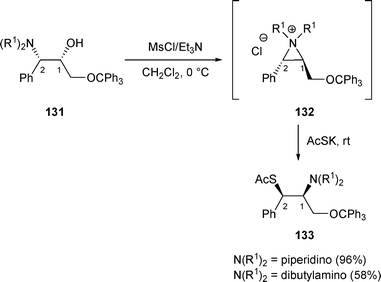 | ||
| Scheme 45 | ||
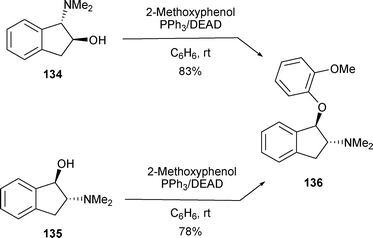
This benzylic attack was also observed when β-amino alcohols 134 and 135 were reacted under Mitsunobu conditions (DEAD/PPh3) in the presence of 2-methoxyphenol to furnish 136 (Scheme 46).52
 | ||
| Scheme 46 | ||
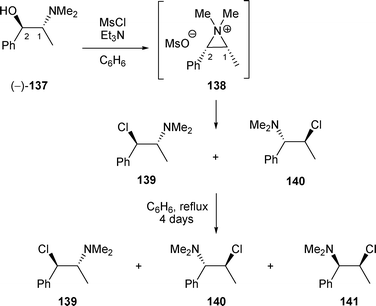
However, when N-methylpseudoephedrine (−)-137 was treated with MsCl/Et3N, a mixture of regioisomers 139 and 140 was obtained.52β-Amino chloride 140 may not be the result of a kinetic control as after four days in refluxing benzene, conditions that should have brought about a thermodynamic equilibrium, a mixture of β-amino chlorides 139 and 140 was still observed.26 The formation of β-amino chloride 141 was probably the result of an epimerisation at the benzylic position (Scheme 47).
 | ||
| Scheme 47 | ||
Similarly, when N-methylpseudoephedrine (+)-137 was reacted under Mitsunobu conditions in the presence of 4-nitrobenzoic acid, a mixture of regioisomers 142 and 143 was obtained in a 33/67 ratio in favour of nitrobenzoate 143, (attack of the nucleophile at the C-2 position of the aziridinium) (Scheme 48).53
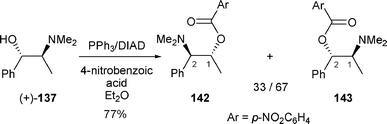 | ||
| Scheme 48 | ||
When the same conditions were applied to N-methylephedrine 144, this latter was not transformed to the rearranged product 146, but rather to β-amino ester 145, which corresponds to the nucleophilic attack on an aziridinium intermediate at the benzylic position with retention of configuration (Scheme 49).53
 | ||
| Scheme 49 | ||
The use of β-amino alcohol 147 as the starting material for the synthesis of ecopipam is an interesting example where the rearrangement of a β-amino alcohol C was realized on an industrial scale.54 The ecopipam precursor 150 was obtained by nucleophilic attack of the arylmagnesium bromide 149 on the benzylic position of the aziridinium 148. The rearrangement of β-amino alcohol 147 was achieved in a wide range of yields (0–84%) by activation of the hydroxy group with phosphorus-based reagents. A good yield (80–82%) of 150 was obtained when pentavalent phosphorus-based reagents were used. Among them the best reagent to induce the rearrangement was revealed to be ClP(O)(OPh)2 with which 150 was formed in 84% yield (Scheme 50).
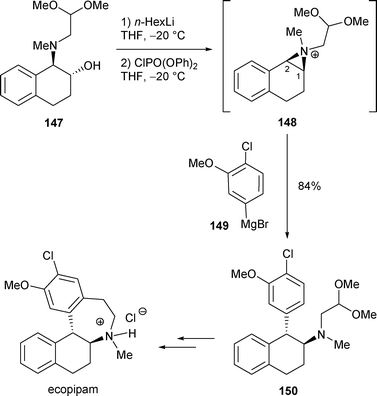 | ||
| Scheme 50 | ||
3.2 R3 = Electron-withdrawing group
When β-amino alcohols C′ possess an R2 aryl group and an R3 electron-withdrawing group (R3 = EWG), both the C-1 and C-2 positions of aziridinium K′ are activated, resulting in competitive formation of amines L′ and/or M′ (Scheme 51).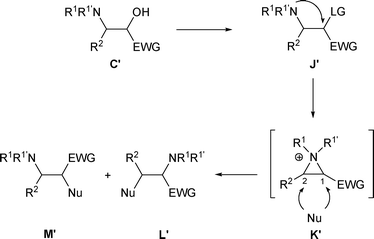 | ||
| Scheme 51 | ||
When a mixture of β-amino alcohols 152 and 153 was treated with MsCl/Et3N, benzylic chlorides of type 155 were obtained exclusively (Scheme 52).55,56 Even when R3 was an ester group, no isomer from the nucleophilic attack on aziridinium 154 at C-1 was detected.
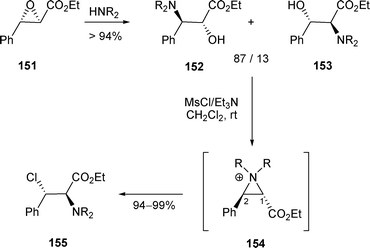 | ||
| Scheme 52 | ||
When β-chloro amine 156 was treated with K2CO3 in the presence of amines, diamines 159 were obtained in good yields (68–93%) accompanied by traces of the regioisomers of type 158 (0–8%). Diamines 159 correspond to the attack of external amines on the aziridinium intermediate 157 at C-2 (Scheme 53). On the other hand, when thiols were used as nucleophiles, the other regioisomers, compounds 158, were formed as the major products (ratio 158/159 > 82/18) (Scheme 53).55,56 The regioselective attack on aziridinium intermediate 157 by nucleophiles was probably related to HSAB theory.
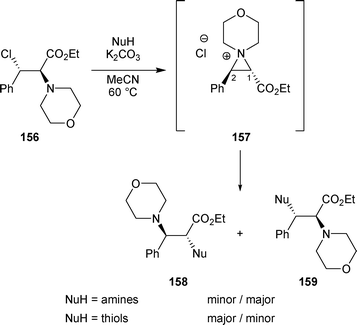 | ||
| Scheme 53 | ||
Finally, when β-amino hydroxy phosphonate 160 was treated with MsCl/Et3N in the presence of nucleophiles (amines, chloride and H2O), only rearranged products 161, resulting from the attack of nucleophiles at the benzylic position of the aziridinium were observed (Scheme 54).57 The presence of the electron-withdrawing phosphonate group did not affect the regioselectivity of the nucleophilic attack on the aziridinium intermediate.
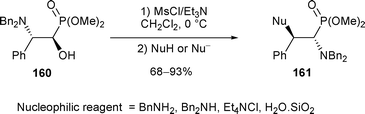 | ||
| Scheme 54 | ||
Conclusion
The rearrangement of β-amino alcohols of type A, B, or C can be performed by activation of the hydroxy group followed by the addition of nucleophiles. In most examples, an aziridinium intermediate is involved in the rearrangement. The ratio of amines H and I (L and M), resulting from the attack on aziridinium intermediates at either C-1 or C-2 by nucleophiles, depends on the nature of the nucleophiles and R2 and R3 substituents. In some cases, the solvent as well as the temperature can influence the ratio of amines H and I (L and M). A summary of the rearranged products H and/or I issued from A and B (Scheme 55) is reported in Table 1.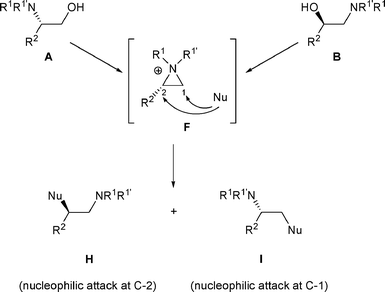 | ||
| Scheme 55 | ||
Notes and references
- A. Padwa, in Comprehensive Heterocyclic Chemistry III, ed. A. Padwa, Elsevier, Oxford, 2008, vol. 1, pp. 1–82 Search PubMed.
- J. B. Sweeney, Chem. Soc. Rev., 2002, 31, 247–258 RSC.
- X. E. Hu, Tetrahedron, 2004, 60, 2701–2743 CrossRef.
- W. McCoull and F. A. Davis, Synthesis, 2000, 1347–1365 CrossRef CAS.
- H. M. I. Osborn and J. Sweeney, Tetrahedron: Asymmetry, 1997, 8, 1693–1715 CrossRef CAS.
- Y. Kim, H.-J. Ha, S. Y. Yun and W. K. Lee, Chem. Commun., 2008, 4363–4365 RSC.
- G. L. Hamilton, T. Kanai and F. D. Toste, J. Am. Chem. Soc., 2008, 130, 14984–14986 CrossRef CAS and references cited therein.
- M. D’hooghe, V. Van Speybroeck, M. Waroquier and N. De Kimpe, Chem. Commun., 2006, 1554–1556 RSC.
- F. Couty, G. Evano and D. Prim, Tetrahedron Lett., 2005, 46, 2253–2257 CrossRef CAS and references cited therein.
- J. Cossy and D. Gomez Pardo, Chemtracts, 2002, 15, 579–605 CAS.
- W. R. Brode and M. W. Hill, J. Am. Chem. Soc., 1947, 69, 724 CrossRef CAS.
- J. F. Kerwin, G. E. Ullyot, R. C. Fuson and C. L. Zirkle, J. Am. Chem. Soc., 1947, 69, 2961–2965 CrossRef CAS.
- P. Ofner, J. Chem. Soc., 1951, 1800–1803 RSC and references cited therein.
- R. Achini, Helv. Chim. Acta, 1981, 64, 2203–2218 CrossRef CAS.
- Y. L. Janin, A.-M. Aubertin, A. Chiaroni, C. Riche, C. Monneret, E. Bisagni and D. S. Grierson, Tetrahedron, 1996, 52, 15157–15170 CrossRef CAS.
- T. G. Back, M. Parvez and H. Zhai, J. Org. Chem., 2003, 68, 9389–9393 CrossRef CAS.
- M. Sivaprakasam, F. Couty, G. Evano, B. Srinivas, R. Sridhar and K. R. Rao, Synlett, 2006, 781–785 CAS and references cited therein.
- F. Couty, F. Durrat and D. Prim, Tetrahedron Lett., 2004, 45, 3725–3728 CrossRef CAS.
- R. N. Salvatore, A. S. Nagle and K. W. Jung, J. Org. Chem., 2002, 67, 674–683 CrossRef CAS and references cited therein.
- C. Thomas, F. Orecher and P. Gmeiner, Synthesis, 1998, 1491–1496 CrossRef CAS and references cited therein.
- K. Weber, S. Kuklinski and P. Gmeiner, Org. Lett., 2000, 2, 647–649 CrossRef CAS.
- T. Lehmann, H. Hubner and P. Gmeiner, Bioorg. Med. Chem. Lett., 2001, 11, 2863–2866 CrossRef CAS.
- C. Ye and J. N. M. Shreeve, J. Fluorine Chem., 2004, 125, 1869–1872 CrossRef CAS and references cited therein.
- J. C. Anderson, R. Cubbon, M. Harding and D. S. James, Tetrahedron: Asymmetry, 1998, 9, 3461–3490 CrossRef CAS.
- Authors describe the formation of the rearranged β-thio amine 50 with the (S) configuration. Yet, rearrangement of (S)-valinol 48 and the subsequent formation of the aziridinium intermediate 49 should normally lead to the formation of the rearranged amine with the (R) configuration.
- R. K. Dieter, N. Deo, B. Lagu and J. W. Dieter, J. Org. Chem., 1992, 57, 1663–1671 CrossRef CAS.
- M. Dakanali, G. K. Tsikalas, H. Krautscheid and H. E. Katerinopoulos, Tetrahedron Lett., 2008, 49, 1648–1651 CrossRef CAS.
- For a review, see: T.-X. Métro, D. Gomez Pardo and J. Cossy, Chem.–Eur. J., 2008, 15, 1064–1070 Search PubMed and references cited therein.
- B. Duthion, T.-X. Métro, D. Gomez Pardo and J. Cossy, Tetrahedron, 2009, 65, 6696–6706 CrossRef CAS.
- F. L. Bach and E. Cohen, Chem. Commun., 1968, 415–416 RSC.
- A. Steiger, H. J. Pyun and R. M. Coates, J. Org. Chem., 1992, 57, 3444–3449 CrossRef CAS.
- J. D. White and J. D. Hansen, J. Org. Chem., 2005, 70, 1963–1977 CrossRef CAS.
- C. McKay, R. J. Wilson and C. M. Rayner, Chem. Commun., 2004, 1080–1081 RSC.
- P. O’Brien and T. D. Towers, J. Org. Chem., 2002, 67, 304–307 CrossRef CAS.
- D. Picq, M. Cottin, D. Anker and H. Pacheco, Tetrahedron, 1983, 39, 1797–1801 CrossRef CAS.
- T. Katagiri, M. Takahashi, Y. Fujiwara, H. Ihara and K. Uneyama, J. Org. Chem., 1999, 64, 7323–7329 CrossRef CAS.
- C. Couturier, J. Blanchet, T. Schlama and J. Zhu, Org. Lett., 2006, 8, 2183–2186 CrossRef CAS.
- P. Ioannidis and M. Nilsson, WO 2006/038872.
- D. F. Hook, F. Gessier, C. Noti, P. Kast and D. Seebach, ChemBioChem, 2004, 5, 691–706 CrossRef CAS and references cited therein.
- D. Gani, P. B. Hitchcock and D. W. Young, J. Chem. Soc., Chem. Commun., 1983, 898–900 RSC.
- P. E. Floreancig, S. E. Swalley, J. W. Trauger and P. B. Derwan, J. Am. Chem. Soc., 2000, 122, 6342–6350 CrossRef CAS.
- S. L. Shapiro, H. Soloway and L. Freedman, J. Am. Chem. Soc., 1958, 80, 6060–6064 CrossRef CAS.
- M. Couturier, J. L. Tucker, B. M. Andresen, K. M. DeVries, B. C. Vanderplas and F. Ito, Tetrahedron: Asymmetry, 2003, 14, 3517–3523 CrossRef CAS.
- Authors describe the formation of the rearranged amine 118 with the (R) configuration. Yet, rearrangement of (R)-β-amino alcohol 116 and the subsequent formation of the aziridinium intermediate 117 should normally lead to the formation of the rearranged amine with the (S) configuration.
- S. E. De Sousa, P. O'Brien and P. Poumellec, J. Chem. Soc., Perkin Trans. 1, 1998, 1483–1492 RSC and references cited therein.
- N. M. Swingle, K. V. Reddy and B. E. Rossiter, Tetrahedron, 1994, 50, 4455–4466 CrossRef CAS.
- E. Curthbertson, P. O’Brien and T. D. Towers, Synthesis, 2001, 693–695 CrossRef CAS.
- M. J. Frizzle, S. Caille, T. L. Marshall, K. McRae, K. Nadeau, G. Guo, S. Wu, M. J. Martinelli and G. A. Moniz, Org. Process Res. Dev., 2007, 11, 215–222 Search PubMed.
- S. R. Anderson, J. T. Ayers, K. M. DeVries, F. Ito, D. Mendenhall and B. C. Vanderplas, Tetrahedron: Asymmetry, 1999, 10, 2655–2663 CrossRef CAS.
- M. Okuda and K. Tomioka, Tetrahedron Lett., 1994, 35, 4585–4586 CrossRef CAS.
- C. Jimeno, A. Moyano, M. A. Pericas and A. Riera, Synlett, 2001, 1155–1157 CrossRef CAS.
- J. Freedman, M. J. Vaal and E. W. Huber, J. Org. Chem., 1991, 56, 670–672 CrossRef CAS.
- M. A. Poelert, L. A. Hulshof and R. M. Kellogg, Recl. Trav. Chim. Pays-Bas, 1994, 113, 355–364 CAS.
- D. Gala, V. H. Dahanukar, J. M. Eckert, B. S. Lucas, D. P. Schumacher, I. A. Zavialov, P. Buholzer, P. Kubisch, I. Mergelsberg and D. Scherer, Org. Process Res. Dev., 2004, 8, 754–768 Search PubMed and references cited therein.
- T.-H. Chuang and K. B. Sharpless, Org. Lett., 2000, 2, 3555–3557 CrossRef CAS and references cited therein.
- M.-J. Tranchant and V. Dalla, Tetrahedron, 2006, 62, 10255–10270 CrossRef CAS.
- D. G. Piotrowska and A. E. Wroblewski, Tetrahedron, 2003, 59, 8405–8410 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
