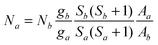Spectroscopic characterization of Fe-doped synthetic chrysotile by EPR, DRS and magnetic susceptibility measurements†
Elena
Borghi
*a,
Manlio
Occhiuzzi
*a,
Elisabetta
Foresti
b,
Isidoro Giorgio
Lesci
b and
Norberto
Roveri
b
aDipartimento di Chimica, Università di Roma “La Sapienza”, Piazzale Aldo Moro 5, 00185 Roma, Italy. E-mail: e.borghi@caspur.it; manlio.occhiuzzi@uniroma1.it; Fax: +39 06 490324; Tel: +39 06 49913678
bDipartimento di Chimica, Università di Bologna “Alma Mater Studiorum”, Via Selmi 2, 40126 Bologna, Italy. E-mail: elisabetta.foresti@unibo.it; isidorogiorgio.lesci@unibo.it; norberto.roveri@unibo.it; Fax: +39 051 2099523; Tel: +39 051 2099486
First published on 9th November 2009
Abstract
Fe-doped synthetic geomimetic chrysotile nanocrystals represent a reference standard to investigate the health hazard associated with asbestos fibers and constitute interesting inorganic nanotubes for specific technological applications in light harvesting systems, optoelectronics and photonics. As the fiber toxicity is catalyzed by iron ions in specific crystallographic sites and the mechanical behaviour of synthetic chrysotile nanotubes is strongly affected by the iron doping extent, the characterization of Fe substitution to Mg and/or Si sites in the chrysotile structure appears highly important. By EPR, DRS spectroscopic analyses and magnetic investigations, Mg and/or Si ion replacement by Fe3+ in a synthetic geomimetic chrysotile structure has been investigated. The results highlight that, as a function of the Fe doping extent and of the Fe doping process, iron can replace both Mg and Si sites. The contemporary iron substitution into the octahedral and tetrahedral sheets is associated with the presence of both of isolated Fe3+ centres in high-spin 3d5 configuration (S = ![[/]](https://www.rsc.org/images/entities/char_e11f.gif) , 6A1(6S)) in Oh and Td symmetry and of intra-lattice clustered species. Increasing the Fe doping extent increases the concentration of aggregated species, while magnetic susceptibility confirms a paramagnetic anisotropy. The results allow to define the opportunity of using or not metallic Fe during the synthesis to obtain doped chrysotile nanocrystals with tailored morphological and structural properties suitable as a reference to study asbestos toxicity and apt to prepare new inorganic nanotubes and quantum wires for innovative technological applications.
, 6A1(6S)) in Oh and Td symmetry and of intra-lattice clustered species. Increasing the Fe doping extent increases the concentration of aggregated species, while magnetic susceptibility confirms a paramagnetic anisotropy. The results allow to define the opportunity of using or not metallic Fe during the synthesis to obtain doped chrysotile nanocrystals with tailored morphological and structural properties suitable as a reference to study asbestos toxicity and apt to prepare new inorganic nanotubes and quantum wires for innovative technological applications.
Introduction
Chrysotile, Mg3Si2O5(OH)4, consists of sheets of tetrahedral silica in a pseudo-hexagonal network joined to an octahedral layer of Mg(OH)2 through the apical oxygen of the SiO4 layer and additional hydroxyl groups. Mineral chrysotile fibres form tetrahedral and octahedral layers curled concentrically or spirally and constitute a tubular structure of ∼22–27 nm in outer diameter with a hollow core of ∼5–8 nm in diameter.1–3Chrysotile accounts for the majority, over 95%, of all asbestos fibres manufactured and used in industrial applications.4 Health hazards associated with asbestos are well documented in the medical and general health literature and their deleterious effects on living beings have been widely investigated.5 Different factors, such as fibrous size, morphology, chemical composition and amount of metallic contaminants seem to be involved in the pathogenic and carcinogenic effects of asbestos.6 In mineral chrysotile the presence of impurities, ion substitutions, and structural disorder, affects morphology, chemical–physical properties and the biological–mineral system interaction. Iron, even if in limited amounts, is the most common di- and tri-valent metal substituent.7 The iron content of asbestos is important in generating HO˙, as well as redox active iron associated with, or mobilized from, the fibre surface.8 The location of the sites responsible for the catalytic and redox activity of the Fe-mediated asbestos is unknown. In evaluating asbestos health hazards, it is therefore important to know the chemical–physical characteristics of chrysotile fibres as a function of Fe doping extent. In fact the mechanical behaviour and bio persistence of chrysotile appear strongly affected by the iron doping extent, the characterization of Fe substitution to Mg and/or Si sites in the chrysotile structure appears highly important.
In serpentine minerals principal substitutions involving ferrous and ferric iron occur in the octahedral site, but according to Moessbauer studies of natural chrysotile Fe+3 substitution may occur at the tetrahedral Si4+ sites and at the octahedral Mg2+ positions.9,10
The morphological, structural and compositional heterogeneity in mineral chrysotile fibres constitutes an obstacle to investigate the Fe location in the chrysotile structure as a function of Fe doping extent.
The availability of synthetic geomimetic chrysotiles with constant stoichiometry, structure and uniform morphology allow investigations of chrysotile fibre interactions with biological model systems such as cells, mitochondria and serum proteins.11–14 In contrast to mineral chrysotile, geoinspired synthetic fibres revealed no cytotoxic effect and may thus be proposed as a reference standard (negative control) for asbestos toxicological studies.11,14 The synthetic geoinspired chrysotile fibres appear interesting in view of the possibilities for them to be suitably doped with foreign metallic ions, in order to investigate the toxicity induced by substitution.15 Geoinspired chrysotile nanotubes have been synthesized as a unique phase, and recently doped with Fe to different extents.16–18 Fe doped synthetic geoinspired chrysotile may constitute a reference model in toxicological studies19 to investigate the interaction of asbestos fibres with biological systems.
In fact genotoxic and cytotoxic evaluations carried out on Fe-doped geoinspired chrysotile have suggested that the generation of reactive oxygen species and other radicals catalyzed by iron ions in chrysotile fibres is potentiated when Fe ions are organized into specific chrysotile crystallographic sites, with a coordination state able to activate free radical generation.19 Therefore, it appears important to define the Fe substitution to Si and/or Mg in the geoinspired synthetic chrysotile structure as a function of Fe doping extent. The knowledge of the Fe-doped chrysotile structural modifications can help to obtain a clearer correlation between cytotoxicity and chemical–physical properties of chrysotile fibres.
Synthetic geoinspired chrysotile fibres have been investigated by morphological analysis (SEM, TEM and AFM), spectroscopic (FTIR and Raman), diffractometric (XRD), thermogravimetric analyses and bending tests by atomic force microscope as a function of the Fe doping extent.16,17 These characterizations evidenced that, in doped chrysotile, Fe replaced Mg and Si ions in both octahedral and tetrahedral sites respectively, inducing a flattening of the curved brucite-like layers at higher Fe doping extents.16 The nanotubes were found to exhibit elastic behaviour at small deformations (below ca. 20 nm). Young’s modulus values equal to (159–125) GPa for the undoped chrysotile nanotubes and (279–260) GPa for the Fe doped ones obtained by the force-deflection curves using the bending equation for a clamped beam under a concentrated load.17 The ability of a low Fe doping extent to highly modify the chrysotile nanotubes mechanical behaviour is relevant if we consider the geoinspired synthetic nanotubes chance to be utilized in preparing new quantum wires and inorganic nanofibrous materials with optical and electronic properties for innovative technological applications.15 In fact the ionic self assembly approach has been successfully used, in either an aqueous or organic phase, for the formation of new nanohybrids (Porphyrins H and J-Aggregates) consisting of the over described geomimetic chrysotile nanotubes as inorganic building blocks and of functional organic molecules such as porphyrin chromophores.18
Continuing this research we have investigated Fe3+ doped chrysotile nanotubes by Electron Paramagnetic Resonance (EPR), UV/Vis diffuse Reflectance Spectroscopy (DRS) and variable-temperature magnetic susceptibility measurements with the aim to clarify structure and nuclearity of the iron substituted sites.
Experimental section
Fe-doped synthetic geomimetic chrysotile fibres
Two series of Fe-doped chrysotile fibres have been synthesized as previously reported by E. Foresti et al.16,20 and listed as (X) (samples with 0.29; 0.67; 0.71; 0.94 %wt Fe) and as (*) (samples with 0.67; 0.81; 1.67; 1.78 %wt Fe). The (X) series synthesis was carried out in a Parr Stirred “Mini” reactor model 4564, constructed with a “T316 Stainless steel” metal alloy containing 65 %wt Fe. The use of this reactor vessel yielded the presence of metallic Fe during the synthesis process. On the contrary, the (*) series synthesis was carried out in a Parr Stirred “Mini” reactor model 4652, constructed with a “C-276” metal alloy containing only 6.5 %wt Fe, consistent with the absence of metallic Fe during the synthesis process.Electron paramagnetic resonance
EPR spectra were recorded at room temperature (RT) and at liquid nitrogen (LN) on a Varian E-9 spectrometer (X-band, ν ∼ 9.5 GHz), equipped with TE102 cavity and an on-line computer for data processing. The magnetic field was measured with respect to the standard 2,2-diphenyl-1-1-picrylhydrazyl hydrate (DPPH).The absolute concentration of the paramagnetic species was determined from the integrated area of the spectra, taking as standard the Varian strong pitch (5×1015 spin/cm). This secondary standard was accurately calibrated by a series of primary standards.21 The standard and the samples, as powder, were placed in the EPR silica tube (i.d. 3 mm), in a weighed amount to fill the resonant cavity completely in order to have “full length geometry”. In these conditions the numbers of spins/cm, N, of two samples a and b are related by the following equation:
UV-Vis-DRS-spectroscopy
UV-Vis-DRS spectra were measured in reflectance mode by an integrating sphere on a Varian Cary 5 and converted into the Schuster-Kubelka-Munk function, F(R).22The F(R) function of a layer of “infinite thickness”, which is generally obtained with a layer depth of 1–2 mm up to 5 mm (usual for some silica materials) is defined by the expression
| F(R) = F(R∞) = (1 − R∞)2/2R∞ = K(λ)/S |
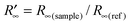 , hence
, hence 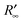 and R∞(sample) are identical only if R∞(ref) = 1. This is true only if the scattering coefficient S is independent of λ. It is worth noting that the F(R) function is proportional to the absorption coefficient for sufficiently low F(R) values. The reference substance used is a standard of polytetrafluoroethylene (PTFE).
and R∞(sample) are identical only if R∞(ref) = 1. This is true only if the scattering coefficient S is independent of λ. It is worth noting that the F(R) function is proportional to the absorption coefficient for sufficiently low F(R) values. The reference substance used is a standard of polytetrafluoroethylene (PTFE).
Due to broad and poorly structured spectra, the deconvolution by mathematical means was necessary. The deconvolution procedure could be arbitrary, but is considered acceptable when the lowest possible number of subbands that are necessary to obtain a satisfactory fit of the experimental spectrum is used. Conforming to recent literature data23,24 it can be considered correct to deconvolve the experimental DRS spectra of iron-doped chrysotile samples considering the four regions listed in Table 1 in the supporting information†. Deconvolution of DRS spectra was performed with the PeakFit software program (Jandel Scientific).
Magnetic susceptibility
Temperature-dependent magnetic susceptibility measurements were performed by a Quantum Design model MPSM SQUID magnetometer, in the range 5–300 K with a 3 K step, by an applied magnetic field of 50 Oe and 100 Oe. A cellulose capsule was filled with a powdered microcrystalline sample and placed inside a polyethylene straw to the end of the sample rod. The measured volume magnetic susceptibility, k, describes the magnetization M (emu) acquired by a material in a magnetic field H (Oe) according to: k = M/H (emu cm−3). Data were corrected for the magnetization of the sample holder and for atomic diamagnetism as calculated from Pascal’s constants, considering the total amount of Mg, Si, O and OH in the minimal unit formula, Mg3Si2O5(OH)4, of the chrysotile.Considering the gram magnetic susceptibility, χg = k/g (emu g−1), where g is the sample weight, for the analysis of the magnetic data we calculated the iron atomic magnetic susceptibility, χat (emu atom−1), according to:
| χat = χg/%wt Fe·atomic weight of Fe. |
Results and discussion
EPR analysis
The two series (X) and (*) of Fe(III) doped chrysotile samples showed both an intense signal at g = 4.5 with a small band g ≈ 6 and an intense symmetrical exchange narrowed line at g = 2.03 (Fig. 1a and b).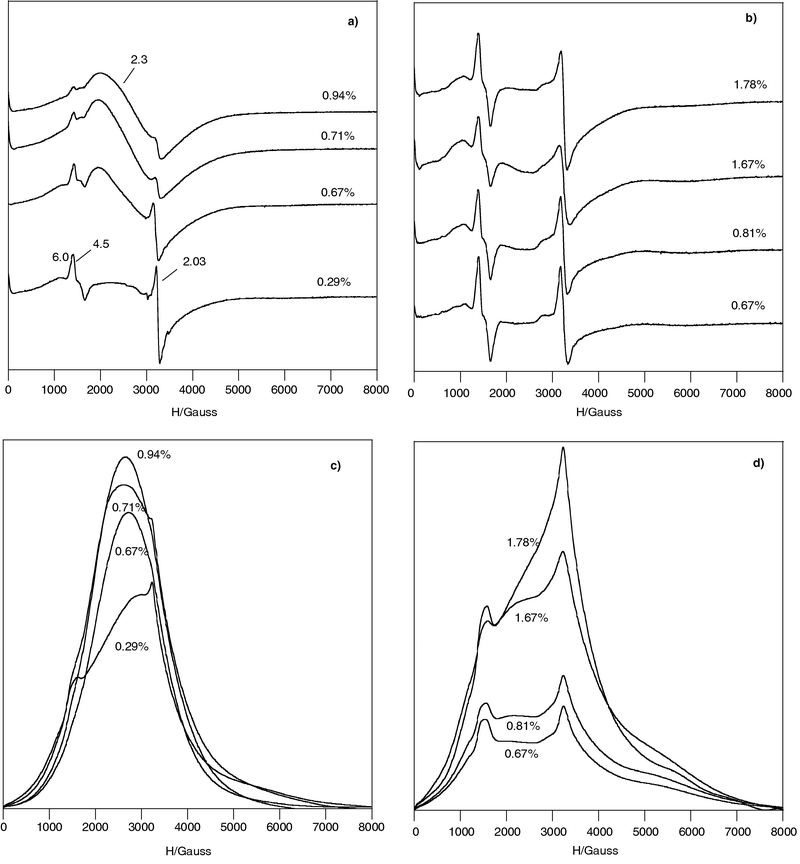 | ||
| Fig. 1 EPR spectra recorded at RT of Fe3+ doped chrysotile: (a) series (X); (b) series (*), the numbers list the Fe %wt and the g values; (c) integrated spectra of series (X); (d) integrated spectra of series (*). | ||
These signals are superimposed to a broad band centred at ≈3000 Gauss (g ≈ 2.3) and extending for several Kilogauss, clearly visible in the spectra of series (X) samples and also present in those of series (*). The integrated spectra evidenced the broad band in all samples (Fig. 1c and d) and in series (X) samples the broad band yielded the major contribution to the integrated area (Fig. 1c). This band is probably due to Fe3+ species located on surface with no defined coordination and/or to clusters.25
By DRX, FT-IR, TEM and RAMAN characterization Foresti et al.16,20 hypothesized that the presence of metallic Fe during the synthesis of (X) samples affected the chrysotile structure changing from curly to planar morphology, similar to the lizardite one, especially at higher Fe%wt content. A flattening of the curved layers could produce a negative charge excess on the tetrahedral sheet and a positive charge on the octahedral one. This structural modification allows to hypothesize electrostatic interactions between Td and Oh adjacent layers affecting the interlayer bonding and chrysotile fibre stability. On the contrary, the synthesis of series (*) yielded more crystalline material. In fact the comparison of the EPR spectra with similar Fe-content shows that series (X) spectra are less resolved than series (*) ones. Therefore we have investigated the more crystalline series (*), recording spectra at LN (Fig. 2).
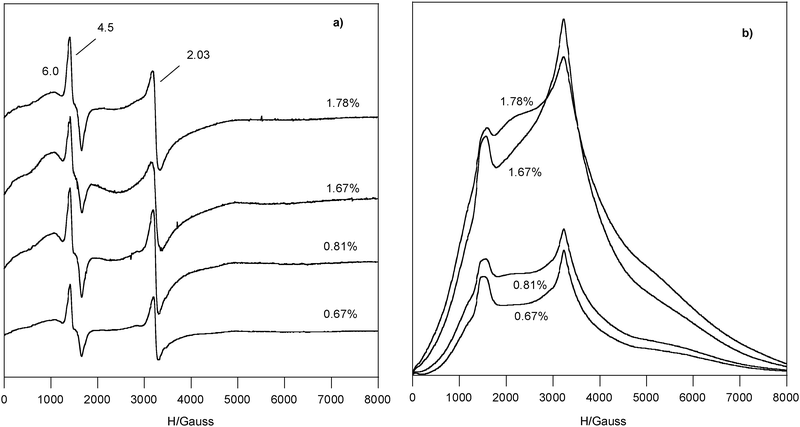 | ||
| Fig. 2 EPR spectra recorded at LN of Fe3+ doped chrysotile series (*) samples, (a) derivative, the numbers list the Fe %wt and the g values; (b) integrated spectra. | ||
Whereas the linewidth of g = 4.5 signal was independent from recording temperature (ΔHpp = 250–270 Gauss both at RT and LN), that of g = 2.03 signal varied (ΔHpp = 150–200 Gauss at RT and 110–150 Gauss at LN), showing a behaviour typical of exchange-coupled species (compare Fig. 2a with Fig. 1b).
The integrated spectra of series (*) Fe(III) doped chrysotile samples are reported in Fig. 2b. In all spectra the broad band yielded the main contribution to the integrated area, whereas the amount of the exchange-coupled species was higher than that of the one at g = 4.5 and the species at g = 6.0 was in the minority.
Irrespective on the Fe-content the concentration of Fe3+ detected by EPR, evaluated with the equation reported in the experimental part, was 39.1 ± 6.5 at LN and 19.4 ± 2.7 at RT (Fig. 3).
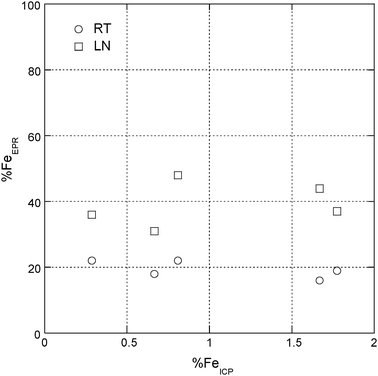 | ||
| Fig. 3 Percentage of Fe3+ detected by EPR in doped chrysotile series (*) samples at RT and at LN. | ||
In all samples the ratio between the integrated areas of the Fe3+ spectrum recorded at LN and at RT, R = ALN/ART, was about equal to 2. A quantitative estimate for the relative amount of the coexisting Fe3+ paramagnetic species, due to the broad band superimposed over all of the spectrum, was inaccurate.
We assigned the Fe3+ signals in doped chrysotile based on theoretical arguments and on literature data of this system and of similar Fe-doped materials.
The ferric ions observed in silica materials, in oxide matrices and in the comparable MCM41 systems are usually in high-spin 3d5 configuration (S = ![[/]](https://www.rsc.org/images/entities/char_e11f.gif) , 6A1(6S) ground state, t2g3eg2 (Oh) and e2t23 (Td) configurations).26 The number and position of EPR transitions for isolated Fe3+ ions in a site of well defined symmetry observable in a powder spectrum depends sensitively on the local ligand-field symmetry of the sites (reflected by the magnitude of the zero-field splitting (zfs) parameters D and E) and possible magnetic interactions between them. (see electronic supporting information, ESI, for theoretical aspects †). E/D parameter shows the degree of rhombic distortion in the electronic environment and has a limited range, which in this case is 0 − ⅓.
, 6A1(6S) ground state, t2g3eg2 (Oh) and e2t23 (Td) configurations).26 The number and position of EPR transitions for isolated Fe3+ ions in a site of well defined symmetry observable in a powder spectrum depends sensitively on the local ligand-field symmetry of the sites (reflected by the magnitude of the zero-field splitting (zfs) parameters D and E) and possible magnetic interactions between them. (see electronic supporting information, ESI, for theoretical aspects †). E/D parameter shows the degree of rhombic distortion in the electronic environment and has a limited range, which in this case is 0 − ⅓.
The signals at g ≈ 4.5 and at g ≈ 6 arise from the |−½〉 ↔ 〈½| transition of isolated Fe3+ sites in strong rhombic (D ≥ hν, E/D = ⅓, g ≈ 4.3) or axial distortion (D ≥ hν, E/D = 0, g ≈ 6) when the zero field splitting is large in comparison to the microwave energy, hν. This implies that a Fe3+ species giving rise to a line at g ≈ 4.3 is more strongly distorted than a Fe3+ site represented by a signal at g ≈ 6 due to the difference in the magnitude of the parameter E, which characterize the additional asymmetry.
The remarkable distortion of the unit cell with respect to the conventional crystal structures, observed by the X-ray diffraction studies on mineral chrysotile allowed us to develop the theory specially formulated for cylindrical lattices.3 By the structural refinement of the powder X-ray diffraction pattern, a more accurate disorder evaluation in staking of both sites has been recently obtained for the synthetic geoinspired undoped stoichiometric chrysotile nanocrystals, showing that the Si site has a distorted tetrahedral configuration and the Mg site an octahedral configuration, with a strong axial distortion.26,27
Taking into account the known structure of chrysotile, we can argue that for the octahedral site the D term is much higher than that for the tetrahedral one therefore E/D ≈ ⅓, geff ≈ 4.3 for isolated Fe3+ ions in the Si site and E/D ≈ 0, geff ≈ 6 for those in the Mg sites is the more logical choice. This assignment is in agreement with literature data on zeolites.28 In zeolites, the first group of signals at g above 4.3 was assigned to different isolated Fe(III) ions and the second group at g = 2.0 corresponds to isolated and oligomeric Fe species. In particular, the line at g ≈ 4.3 is frequently assigned to isolated Fe3+ sites incorporated in tetrahedral framework positions while the line at g ≈ 6 is assigned to isolated Fe3+ species in higher coordination numbers.23,29
The symmetrical signal at g = 2.03, due to its sharpness, yielded a quite small contribution to the integrated area, representing a small amount of the Fe3+ detected by EPR. It is assigned to exchange coupled Fe3+ ions present in the chrysotile lattice. Due to the short distance (≈3 Å) between some Si and Mg sites,24,25 magnetic coupling of Fe3+ ions in Oh and/or Td site is possible.
Extra-lattice Fe3+ ions might be present as isolated and/or clustered species adsorbed on the surface of the chrysotile nanotubes. Due to the variability of local site symmetry, the isolated Fe3+ ions, if adsorbed on the surface, are EPR silent. Moreover the presence of extra-lattice-iron(III) seems excluded by DRS (see next section) and FT-IR characterisation20. On the contrary, because the magnetic interactions yield and exchange-narrowing Fe3+ signal, intra-lattice clusters are detectable, so the broad band detected in all the spectra is assigned to the clustered species. This broad band gave the main contribution to the integrated area, and as the ratio between the integrated areas measured at LN and RT (R = ALN/ART) is lower than the expected value in the Curie law (295/77 = 3.8), we conclude that in these oligomeric Fe species antiferromagnetic interactions are operative.
UV/Visible diffuse reflectance spectroscopy
Three types of electronic transitions can occur30 in the optical absorption spectra of Fe(III) doped chrysotile samples: (i) the ligand-field transitions or “single excitation processes”; (ii) the pair excitations or “double excitation processes” resulting from the simultaneous excitation of two neighbouring Fe3+ cations that are magnetically coupled; (iii) the ligand to metal charge-transfer transitions or LMCT transitions. (see ESI for theoretical aspects †).In the ferric-doped synthetic chrysotile the d–d transitions, forbidden for both spin and Laporte selection rules, become allowed or assisted through the magnetic coupling of electronic spins of next-nearest neighbour Fe3+ cation, in the Mg site and/or in the Si site positions.22 In the presence of magnetic coupling, i.e., in the presence of Fe3+–Fe3+ pairs, spin allowed (ΔS = 0) but Laporte forbidden transitions can occur among S states in the pair in ground field state [i.e., Fea3+(6A1)–Feb3+(6A1)] and in the pair in single excited field state [i.e., Fea3+(6A1)–Feb3+(4T1)]. In Fe-doped chrysotile, as in doped silica materials and in iron oxides, the Laporte selection rule can be relaxed by covalent bonding with oxygen through the 3d orbitals of the magnetically coupled Fe pair and O 2p orbitals.24 Additionally in a Fea3+(6A1)–Feb3+(6A1) pair magnetically coupled the two adjacent Fe3+ cations can be simultaneously excited to a quartet ligand field state. Spin allowed transitions can occur among S state in the pair in ground field state [i.e., Fea3+(6A1)–Feb3+(6A1)] and in the pair in double excited field state [i.e., Fea3+(4T1)–Feb3+(4T1)] at energies that are approximately the sum of two single-ion Fe3+ ligand field transitions.24,30
According to molecular orbital theory, for an Oh complex the LMCT transitions are t1g(π) → t2g(π*) and t1g(π) → eg(σ*), from the highest occupied molecular orbital with pure ligand character and t1g(π) symmetry to the orbitals with higher energy having eg(σ*) and t2g(π*) symmetry. For a Td Fe(III) complex the t1(π) → t2(π*) and the t1(π) → e(σ*) ligand-to-metal transitions are, usually, expected.
Therefore, in principle, two strong LMCT transitions, in about the same energy range,30 characterize octahedral and tetrahedral centres of high-spin Fe3+, so the presence of two bands in the CT region alone cannot be taken as proof of the coordination type.
The literature data for both iron-doped silica materials and for iron oxides clearly show that CT bands of Fe3+ are red-shifted with increasing number of coordinating oxygen ligands.23,31 CT bands for isolated [FeO4] tetrahedral group have been observed in the 215–240 nm range, while bands between 270–290 nm have been detected for isolated octahedral [FeO6] group and CT bands between 300 and 400 nm are assigned to octahedral Fe3+ in cluster-like FexOy species, which can be small oligomeric clusters and larger species. Reference materials of Fe3+ in different environments, like FePO4 (Td symmetry) and Fe2O3 (Oh symmetry), show significant absorptions, respectively, in the wavelength range of 200–330 nm for isolated Td coordination of Fe3+ ions and in the range of 320–640 nm for Fe3+ cluster-like species having an Oh coordination.31
According to the literature data, in Fe-doped chrysotile we expected the following bands: (i) two theoretical CT transitions, below 300 nm, red-shifted with increasing number of coordinating oxygen ligands, for each type of mononuclear isolated Fe(III) sites in Td (Si site position) and Oh (Mg site position) symmetry; (ii) in general two CT bands, between 300 and 400 nm, for octahedral Fe(III) in small FexOy clusters; (iii) above 400 nm CT bands for larger FexOy particles and weak peaks related with d–d transitions (see supporting information†).
DRS spectra of the series (*) samples have been recorded, according to literature data, in the range 200–1000 nm under the same conditions of light and sample thickness, so they can be compared to understand the influence of the Fe content on the Fe incorporation. Fe-doped chrysotile samples do not show bands in the region above 750 nm and the whole region from 200 to 750 nm is shown (seeFig. 1 in ESI †). Contrarily to iron-doped silica materials, the shape of Fe-doped chrysotile spectra below 300 and between 300–400 nm showed no well-differentiated and intense CT bands. Bands above 400 nm were observed in an exploded view. The deconvolution procedure has been performed in the different regions of interest (i.e., 200–400 nm, 400–600 nm, 600–750 nm, Fig. 4–6).
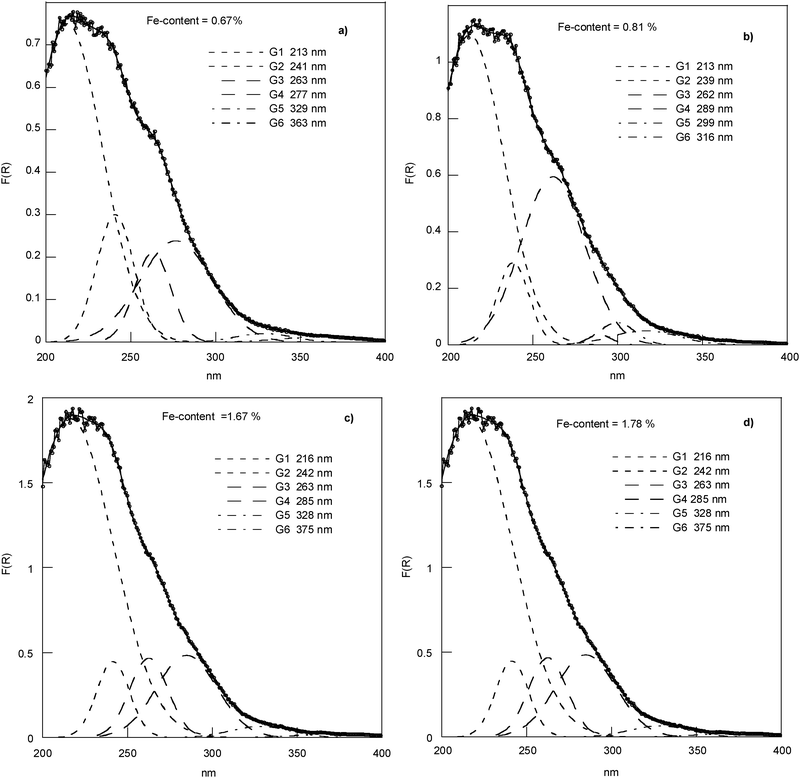 | ||
| Fig. 4 Experimental DRS spectra and deconvoluted subbands in region 200–400 nm. | ||
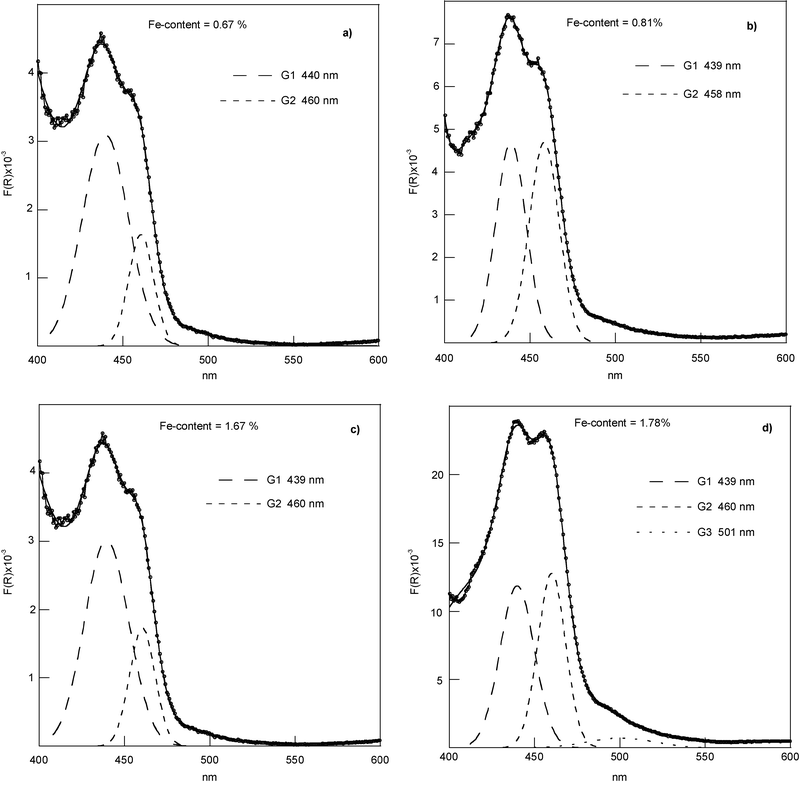 | ||
| Fig. 5 Experimental DRS spectra and deconvoluted subbands in region 400–600 nm. | ||
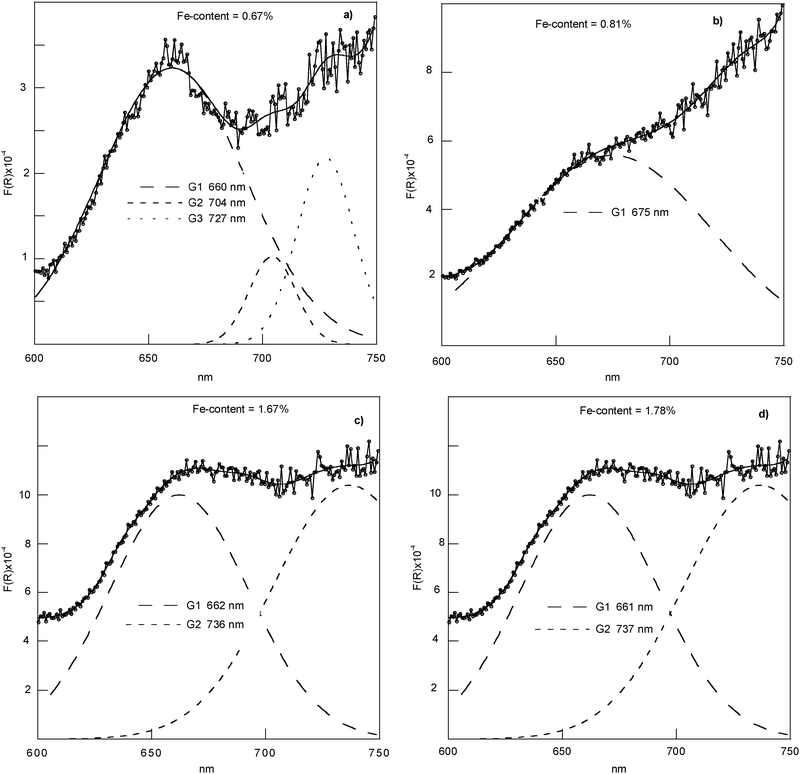 | ||
| Fig. 6 Exploded view of the DRS results and deconvoluted subbands in region 600–750 nm. | ||
The region in the range of 200–400 nm (Fig. 4) mainly reflects the LMCT transitions, which are expected red-shifted with a change in the coordination environment of the metal from Td to Oh. The CT subbands below 280 nm clearly show the presence of isolated framework Fe3+ sites in Td (Si site) and Oh (Mg site) coordination. The absence of significant absorption beyond 320 nm is indicative of the absence of extra-framework Fe.29 The minimal trioctahedral unit in the unit cell of the crystal structure of the chrysotile25 indicates three different Mg sites and two different Si sites. The presence in the Mg3Si2O5(OH)4 units of short distances Mg–Mg, Si–Si, Si–Mg, which are ≈3.0 Å, suggests the possibility of magnetic coupling in Fe-doped chrysotile with iron in the Mg site and/or in the Si site positions. CT bands between 300 and 400 nm could be assigned to Oh and/or Td FexOy clusters. As expected, the percentage of all bands of this region of the DRS spectrum changes with the Fe content.
The region 400–600 nm (Fig. 5) is the region reflecting the pair excitation processes resulting from the Fe3+–Fe3+ pairs (see ESI Table 1†). The UV-Vis characteristics of DRS spectra in this wavelength range evidence the presence of magnetic coupling. Fig. 5 shows subbands at 439 nm and 460 nm, the percentage of which depends on the Fe content, and that arises from larger FexOy oligomeric (probably Oh/Oh, and/or Td/Td) or eteromeric (in Oh/Td symmetries) clusters.
The presence of an expected weak d–d transition, again strictly dependent on the Fe content, in the region 600–750 nm (seeFig. 6) is suggesting the presence of Fe3+ clusters in an octahedral environment.31
The subbands obtained by the deconvolution procedure can be understood in terms of reflecting both the presence of isolated framework tetrahedral [FeO4] and octahedral [FeO6] species as the presence of neighbouring Fe3+ cations magnetically coupled.
A semi quantitative analysis of UV-VIS spectra of Fe-doped chrysotile samples is reported (seeTable 1).
| Region 200–750 nm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe content 0.67 %wt | Fe content 0.81 %wt | Fe content 1.67 %wt | Fe content 1.78 %wt | ||||||||||
| A = 56.9 | A = 82.5 | A = 138.0 | A = 139.2 | ||||||||||
| Region 200–400 nm: F(R) vs. nm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe content 0.67 %wt | Fe content 0.81 %wt | Fe content 1.67 %wt | Fe content 1.78 %wt | ||||||||||
| A | I% | Fe %wt | A | I% | Fe %wt | A | I% | Fe %wt | A | I% | Fe %wt | ||
| G1 | 28.4 | 49.9 | 0.33 | 40.9 | 49.5 | 0.40 | 85.2 | 61.7 | 1.03 | 88.2 | 63.3 | 1.13 |

|
| G2 | 8.25 | 14.5 | 0.10 | 6.85 | 8.30 | 0.07 | 10.8 | 7.84 | 0.13 | 10.8 | 7.77 | 0.14 | |
| G3 | 5.61 | 9.86 | 0.07 | 28.0 | 34.0 | 0.28 | 13.2 | 9.56 | 0.16 | 13.2 | 9.47 | 0.17 |

|
| G4 | 12.8 | 22.5 | 0.15 | 0.65 | 0.79 | 0.01 | 21.6 | 15.6 | 0.26 | 21.6 | 15.5 | 0.28 | |
| G5 | 0.82 | 1.44 | 0.01 | 2.08 | 2.52 | 0.02 | 2.83 | 2.05 | 0.03 | 2.83 | 2.03 | 0.04 |

|
| G6 | 0.72 | 1.26 | 0.01 | 2.46 | 2.98 | 0.02 | 1.02 | 0.74 | 0.01 | 1.02 | 0.73 | 0.01 | |
| Total | 56.6 | 99.5 | 0.67 | 81.0 | 98.1 | 0.81 | 134.7 | 97.5 | 1.62 | 137.7 | 98.8 | 1.77 | |
| Region 400–600 nm: F(R) × 10−3vs. nm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe content 0.67 %wt | Fe content 0.81 %wt | Fe content 1.67 %wt | Fe content 1.78 %wt | ||||||||||
| A | I% | Fe %wt 102 | A | I% | Fe %wt 102 | A | I% | Fe %wt 102 | A | I% | Fe %wt 102 | ||
| G1 | 0.11 | 0.19 | 0.13 | 0.11 | 0.13 | 0.11 | 0.10 | 0.07 | 0.12 | 0.30 | 0.22 | 0.38 |

|
| G2 | 0.03 | 0.05 | 0.04 | 0.10 | 0.12 | 0.10 | 0.03 | 0.02 | 0.04 | 0.26 | 0.19 | 0.33 | |
| Total | 0.13 | 0.24 | 0.17 | 0.21 | 0.25 | 0.21 | 0.13 | 0.09 | 0.16 | 0.56 | 0.41 | 0.71 | |
| Region 600–750 nm: F(R) × 10−4vs. nm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe content 0.67 %wt | Fe content 0.81 %wt | Fe content 1.67 %wt | Fe content 1.78 %wt | ||||||||||
| A | I% | Fe %wt 102 | A | I% | Fe %wt 102 | A | I% | Fe %wt 102 | A | I% | Fe %wt 102 | ||
| G1 | 0.03 | 0.05 | 0.03 | 0.06 | 0.07 | 0.06 | 0.07 | 0.05 | 0.09 | 0.08 | 0.06 | 0.10 |
|
| G2 | 0.06 | 0.04 | 0.07 | 0.06 | 0.04 | 0.08 | |||||||
| Total | 0.03 | 0.05 | 0.03 | 0.06 | 0.07 | 0.06 | 0.13 | 0.07 | 0.16 | 0.14 | 0.10 | 0.18 | |
The intensity percentage with respect to the experimental spectrum (I%) of each gaussian subband has been considered from the respective integrated area and multiplied with the overall Fe content (determined by ICP) to obtain an estimate of the percentage of the different Fe species in the samples (Fe %wt). The obtained values in Table 1 are inaccurate to a certain degree due to the deconvolution procedure, but they are nevertheless regarded to be helpful for a comparison of different Fe-chrysotile samples and for elucidating the relative amount of coexisting Fe species.
With increasing Fe content, the absolute amount of mononuclear isolated species (in Td and Oh symmetry) increases and the amount of Td is always higher than Oh ones (in agreement with EPR). The amount of small and larger Fe3+ clusters, oligo- and/or eteromeric, increases with increasing iron loading in the doped chrysotile samples.
Magnetic susceptibility
EPR and DRS evidenced Fe3+ distribution in both Si4+ and Mg2+ sites, thus magnetic studies could consider and estimate a separate magnetization for the two sites, according to the minimal Mg3Si2O5(OH)4 trioctahedral unit of chrysotile (see ESI †). However, the cation distribution at the Si (Td) and Mg (Oh) sites was not quantitatively determinable, the minimal chemical formula might be insufficient to consider the magnetic properties, given that the chrysotile is a poly-nuclear material with tetrahedral and octahedral layers, thus for the analysis of the magnetic data we have calculated the iron atomic magnetic susceptibility, χat, (see Experimental).The χatvs. T and χat−1vs. T plots for the two samples of ferric-doped chrysotile are considered in order to check the temperature dependence of magnetic susceptibility, which is indicative of magnetic properties and magnetic phase transitions (Fig. 7).32
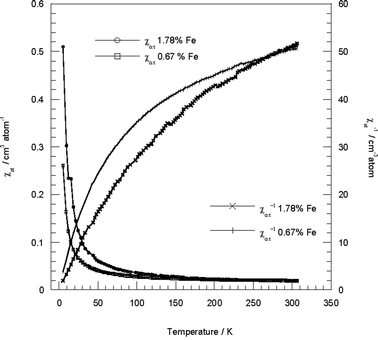 | ||
| Fig. 7 χ at vs. T and χat−1vs. T experimental data for iron-doped synthetic chrysotile in the range 5–300 K at a magnetic field of 50 Oe (0.67%wt Fe) and of 100 Oe (1.78%wt Fe). | ||
The χat−1vs. T plots show that: (i) the Curie-Weiss law, χ = C/(T − ϑ), is followed at temperatures higher than 300 K, analogously to iron-doped silica materials;33 (ii) depending on the iron content different curve lines are detected, evidencing a dissimilar magnetic behaviour; (iii) the non-linearity is indicative of magnetic correlations between the iron ions.
The behaviour of complex nature found in both samples of iron-substituted chrysotile seems to indicate the presence of spin-crossover systems (see ESI †). The main features of the magnetic behaviour of a crossover d5 system have been discussed with a simple model,34 which accounts for this unusual feature of the χ−1 line, suggesting that it can curve and develop maxima and minima. The occurrence of a phase change in the solid state should cause discontinuities in the physical properties of a crossover system.
For both samples decreasing temperature yielded χat−1 decrease, suggesting antiferromagnetic coupling. The curvature in the χat−1vs. T plot decreases deeply on cooling for the higher iron loading and at low iron content shows an increase at ∼80–100 K. This result indicates a total magnetism with an order higher than the first-one, correlated to the coexistence of a mixture of magnetic phases with different long-range order. Although χat−1 decreases on cooling, χat continuously increases. This implies the presence of magnetic species with different local spin in the ground state otherwise the χvs. T plot should exhibit the characteristic maximum.
The shapes of the χat−1 and χat lines are an adequate proof that a phase transition has occurred, but they cannot distinguish between states of different spin multiplicity. The presence of antiferromagnetic or ferromagnetic coupling interactions can be identified with plots similar to those used to represent the Curie behaviour (see ESI†).35
For these Fe(III)-chrysotile samples, antiferromagnetic coupling derives from the oxo bridge between two high spin iron(III) ions (i.e., the Fe–O–Fe moiety with Fe(III) in Oh and/or Td symmetries that are EPR silent).35 In fact, in this material there are μ-oxo-di-iron(III) centres containing metal ions in similar and different symmetry environments, owing to sub lattice in mixed octahedral and tetrahedral layers, as well as a mixture of different spin forms. Additionally in the iron-doped chrysotile system the crystalline lattice can modify the MOh and the MTd micro-symmetry of the central ion and may even lead to compression or expansion of the metal ligand interatomic distances. The possible super exchange interactions may induce different magnetic coupling and the existence of temperature dependent equilibrium between states of different spin multiplicity and so crossover points have to be expected.
Temperature-dependent reciprocal magnetic susceptibility indicates, with respect to the straight line relative to the Curie law (Fig. 8a), shift to higher values: this means presence of an antiferromagnetic coupling, which seem to be stronger at low rather than at high loading. The opposite shift indicates a ferromagnetic coupling. From a χatTvs. T plot (Fig. 8b), where the ordinate gives the Curie constant C (as a line parallel to the T axis for the Curie law), may be better identified the character of the interactions. As the temperature approaches zero, χatT decreases for an antiferromagnetic coupling, while for a ferromagnetic coupling χatT increases and must approach the Curie constant associated with the resulting spin state of the material, which at the high-temperature limit (i.e., T > 300 K) must follow the Curie-Weiss law. At the marked temperatures (K) (seeFig. 8a and b), there is evidence of the presence of crossover points into the coupled spin systems and of ferromagnetic behaviour in both samples.
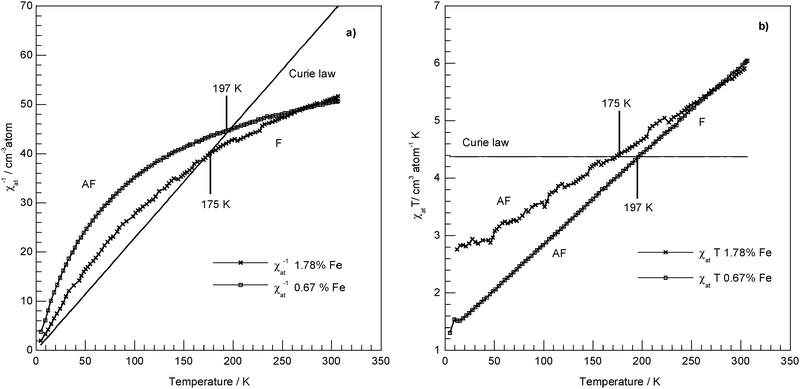 | ||
Fig. 8 Representation of the magnetic behaviour of iron-doped synthetic chrysotile against the Curie law for the Fe3+ high spin ion (S = ![[/]](https://www.rsc.org/images/entities/char_e11f.gif) , Curie Constant χMT = 4.377 emu mol−1 K): (a) χat−1vs. T experimental data and representation of the Curie law as χM−1 = f(T); (b) χatT vs. T experimental data and representation of the Curie law as χMT = f(T); AF = antiferromagnetic coupling; F = ferromagnetic coupling. , Curie Constant χMT = 4.377 emu mol−1 K): (a) χat−1vs. T experimental data and representation of the Curie law as χM−1 = f(T); (b) χatT vs. T experimental data and representation of the Curie law as χMT = f(T); AF = antiferromagnetic coupling; F = ferromagnetic coupling. | ||
These results are compatible with the presence of a spin-crossover system with at least two different magnetic states (i.e., anti- and ferromagnetic) and a configuration interaction, even if a further complexity could be present in the magnetic behaviour at such iron loading owing to the possibility of a ferrimagnetic sub lattice (see ESI †).35,36
The effective magnetic moment, μeff in Bohr magneton (μB) (see ESI for definition †), can be estimated at 300 K and 5 K from the χatT vs. T plot in Fig. 8b.
At room temperature, the magnetic moments are 6.91 μB at 0.67 Fe %wt and 6.85 μB at 1.78 %wt. These values are lower than the theoretically expected values for five uncorrelated spins, but higher than that for only one free Fe3+ ion. These data could validate two cases. The former one indicates that, just at room temperature, due to the particular crystal structure of chrysotile, the presence of metal–metal interactions between the dissolved ferric ions can be revealed and the values of μeff are consequently lowered. So the values could be significantly indicative of strong interactions and consistent with strongly coupled iron(III) centres. In the latter case, considering that at temperature ≥300 K the χat−1vs. T plot should be linear, the values of μeff are close to those expected for isolated paramagnetic species and they are suggesting a quite different contribution from temperature-independent paramagnetism for the two loading. The valence and the spin state seem to be confirmed as Fe3+ high spin.
At low temperature the magnetism arises only from isolated centres. The no zero values at 5 K of the μeff, 3.24 μB for low loading and 4.52 μB for high loading could indicate the presence of paramagnetic Fe(III) centres in different symmetry or in different ground states.34 However deviation from the Curie law at low temperature could be an indication of the presence of zero-field splitting,32 which is one of the most important sources of paramagnetic anisotropy, as was established by EPR experiments.
Conclusions
Previous characterization of Fe-doped chrysotile14,16 showed that Fe3+ ions enter in both octahedral and tetrahedral sites and suggest the possibility of magnetic coupling with Fe3+ ions in Oh and/or Td site considering the presence of short distances (≈3 Å). This study has been performed to further investigate Mg and/or Si centre replacement by Fe(III) ions in chrysotile structures as a function of the Fe doping extent and of the Fe doping process, with the aim to both distinguish the coexistence of different isolated Fe3+ species and inspect the magnetic properties of Fe-doped chrysotile.The characterization by combined EPR and UV-Vis-DRS allowed us to identify: (i) two isolated Fe3+ sites, in tetrahedral and octahedral coordination, as indicated by EPR (signals at g = 4.5 and g = 6.0, respectively) and by UV-Vis spectroscopy (CT bands below 300 nm); (ii) exchange-coupled Fe3+ paramagnetic ions in FexOy clusters, which amount increased with increasing Fe content, as indicated by EPR. Both the dependence of EPR signal intensity to the recording temperature and the characteristic UV-Vis spectra in the 400–600 nm region evidenced the presence of magnetic coupling. Our results indicating the contemporary Fe3+ substitution at the octahedral and tetrahedral sites and the occurrence of a major Fe3+ replacement to Si4+ at low and high Fe doping extent are strongly coherent with the Moessbauer studies carried out on mineral chrysotile fibres.9,10
The temperature-dependent magnetic susceptibility indicated magnetic correlations between the iron ions that are correlated to the presence and the coexistence of a mixture of magnetic phases with different long-range order. The deviation from the Curie law and the shape of the magnetic curves evidenced magnetic species with different local spin in the ground state, showing that a phase transition has occurred.
The present characterization indicates that the contemporary iron substitution into the octahedral and tetrahedral sheets is always associated with the presence of both isolated Fe3+ centres in Oh and Td symmetry and intra-lattice clustered species. The presence of the aggregate species with Fe cation at the Oh (Mg site) and/or at the Td (Si site) positions could be responsible for the different modification of the chrysotile structure observed by the previous characterization performed with Infrared and Raman.14,16
The results of this study highlight the approach that must be performed to obtain Fe-doped chrysotile, suitable as a reference model in asbestos health hazard: (i) the presence of metallic Fe must be excluded during the synthesis, to conserve the crystallinity and natural morphology of the sample; (ii) the extent (%wt) of Fe-doping must be low. Furthermore, the results of this study are fundamental in preparing, especially by magnetic fields, synthetic inorganic nanotubes opportunely Fe doped in order to obtain new quantum wires and nanofibrous materials with optical and electronic properties for innovative technological applications.
Acknowledgements
The authors thank Dr Carlo Bellitto and Dr Elvira Bauer of Istituto di Struttura della Materia, CNR, Roma, for the magnetic susceptibility measurements. In particular C.B. is thanked for the helpful discussion. Authors are grateful to CIRCMSB, University of Bologna (Funds for Selected Research Topics), MIUR (PRIN 2007 prot. 2007498XRF_005) and Chemical Center S.r.l.References
- K. Yada, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1971, 27, 659–664 CrossRef CAS
.
- A. Cressey, E. J. W. Whittaker and J. W. Eric, Mineral. Mag., 1993, 57, 729–732 CrossRef
.
- M. Mellini and P. F. Zanazzi, Amer. Mineral., 1987, 72, 943–948 Search PubMed
.
-
B. T. Mossman, in Health Effects of Mineral Dust, Reviews in Mineralogy, ed. G. D. Guthrie, Jr and B. T. Mossman, Mineralogical Society of America, Washington DC, 1993, vol. 28, p. 513 Search PubMed
.
- D. W. Kamp and S. A. Weitzman, Thorax, 1999, 54, 638–652 CrossRef CAS
.
- C. B. Manning, V. Vallyathan and B. T. Mossman, Int. Immunopharmacol., 2002, 2, 191–200 CrossRef CAS
.
- D. W. Kamp, P. Graceffa, W. A. Prior and S. A. Weitzman, Free Radical Biol. Med., 1992, 12, 293–315 CrossRef CAS
.
- J. A. Hardy and A. E. Aust, Chem. Rev., 1995, 95, 97–118 CrossRef CAS
.
- G. Stroink, D. Hutt, D. Lim and R. A. Dunlap, IEEE Trans. Magn., 1985, 21(5), 2074–2076 CrossRef
.
- G. Gonzalez-Mancera, F. Ortega-Gutierrez, N. E. Nava and H. S. Arriola, Hyperfine Interact., 2003, 148–149, 61–71 CrossRef
.
- E. Gazzano, E. Foresti, I. G. Lesci, M. Tomatis, C. Riganti, B. Fubini, N. Roveri and D. Ghigo, Toxicol. Appl. Pharmacol., 2005, 206, 356–364 CrossRef CAS
.
- C. Bergamini, R. Fato, G. Biagini, A. Pugnaloni, F. Giantomassi, E. Foresti, I. G. Lesci, N. Roveri and G. Lenaz, Cell. Mol. Biol., 2004, 50, 691–700 Search PubMed
.
- G. Falini, E. Foresti, I. G. Lesci, B. Lunelli, P. Sabatino and N. Roveri, Chem.–Eur. J., 2006, 12, 1968–1974 CrossRef CAS
.
- P. Sabatino, L. Casella, A. Granata, M. Iafisco, I. G. Lesci, E. Monzani and N. Roveri, J. Colloid Interface Sci., 2007, 314, 389–397 CrossRef CAS
.
- N. Roveri, G. Falini, E. Foresti, G. Fracasso, I. G. Lesci and P. Sabatino, J. Mater. Res., 2006, 21, 2711–2725 CrossRef CAS
.
- E. Foresti, M. F. Hochella, H. Kornishi, I. G. Lesci, A. S. Madden, N. Roveri and H. Xu, Adv. Funct. Mater., 2005, 15, 1009–1016 CrossRef CAS
.
- S. Piperno, I. Kaplan-Ashiri, S. R. Cohen, R. Popovitz-Biro, H. D. Wagner, R. Tenne, E. Foresti, I. G. Lesci and N. Roveri, Adv. Funct. Mater., 2007, 17, 3332–3338 CrossRef CAS
.
- G. De Luca, A. Romeo, V. Villari, N. Micali, I. Foltran, E. Foresti, I. G. Lesci, N. Roveri, T. Zuccheri and L. Monsu Scolaro, J. Am. Chem. Soc., 2009, 131, 6920, DOI:10.1021/ja901273h
.
- E. Gazzano, F. Turci, E. Foresti, M. G. Putzu, E. Aldieri, F. Silvagno, I. G. Lesci, M. Tomasi, C. Riganti, C. Romano, B. Fubini, N. Roveri and D. Ghigo, Chem. Res. Toxicol., 2007, 20, 380–387 CrossRef CAS
.
- E. Foresti, E. Fornero, I. G. Lesci, C. Rinaudo, T. Zuccheri and N. Roveri, J. Hazard. Mater., 2009, 162, 1494–1506 CrossRef
.
- D. Cordischi, M. Occhiuzzi and R. Dragone, Appl. Magn. Reson., 1999, 16, 427–445 CAS
.
-
G. Kortüm, Reflectance Spectroscopy, Springer, Berlin, 1969 Search PubMed
.
- M. Santhosh Kumar, M. Schwidder, W. Grünert and A. Brückner, J. Catal., 2004, 227, 384–397 CrossRef CAS
.
- Y. P. He, Y. M. Miao, C. R. Li, S. Q. Wang, L. Cao, S. S. Xie, G. Z. Yang, B. S. Zou and C. Burda, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 125411–125419 CrossRef
, and references therein.
- E. A Zhilinskaya, G. Delahay, M. Mauvezin, B. Coq and A. Aboukaïs, Langmuir, 2003, 19, 3596–3602 CrossRef CAS
.
- M. Leoni, A. F. Gualtieri and N. Roveri, J. Appl. Crystallogr., 2004, 37, 166–173 CrossRef CAS
.
- G. Falini, E. Foresti, M. Gazzano, A. F. Gualtieri, M. Leoni, I. G. Lesci and N. Roveri, Chem.–Eur. J., 2004, 10, 3043–3049 CrossRef CAS
.
- S. Dzwigaj, L. Stievano, F. E. Wagner and M. Che, J. Phys. Chem. Solids, 2007, 68, 1885–1891 CrossRef CAS
, and references therein.
- M. Schwidder, M. Santhosh Kumar, K. Klementiev, M. M. Pohl, A. Brückner and W. Grünert, J. Catal., 2005, 231, 314–330 CrossRef CAS
.
-
A. B. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 2nd edn, 1984 Search PubMed
.
- P. B. Amama, S. Lim, D. Ciuparu, Y. Yang, L. Pfefferle and G. L. Haller, J. Phys. Chem. B, 2005, 109, 2645–2656 CrossRef CAS
, and references therein.
-
R. L. Carlin, Magnetochemistry, Springer-Verlag, Berlin, 1986 Search PubMed
.
- T. Yamanaka and M. Okita, Phys. Chem. Miner., 2001, 28, 102–109 CrossRef CAS
.
- R. L. Martin and A. H. White, Trans. Met. Chem., 1968, 4, 113–198 Search PubMed
.
-
O. Kahn, Molecular Magnetism, VCH Publishers, Inc., New York, 1993 Search PubMed
.
- A. Kontny, A. B. Woodland and M. Koch, Phys. Chem. Miner., 2004, 31, 28–40 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Some theoretical aspects on: (i) Metal–Ligand bonding; (ii) UV/Visible Diffuse Reflectance Spectroscopy; (iii) EPR Spectroscopy; (iv) Magnetic susceptibility. See DOI: 10.1039/b915182f |
| This journal is © the Owner Societies 2010 |