Cobalt and nickel with various morphologies: mineralizer-assisted synthesis, formation mechanism, and magnetic properties†
Feng
Cao
ab,
Ruiping
Deng
ab,
Jinkui
Tang
ab,
Shuyan
Song
ab,
Yongqian
Lei
ab and
Hongjie
Zhang
*a
aState Key Laboratory of Rare Earth Resource Utilizations, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, Jilin, China. E-mail: hongjie@ciac.jl.cn; Fax: +86-431-85698041; Tel: +86-431-85262127
bGraduate School of the Chinese Academy of Sciences, Beijing 100039, P. R. China
First published on 3rd September 2010
Abstract
Uniform Ni microflowers and Co microspheres have been synthesized by a simple one-pot hydrothermal method with high yield. The products were characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), selected area electron diffraction (SAED) and superconducting quantum interference device (SQUID). The synthesis of various shaped nanomaterials was achieved using different alkali metal salts. This is the first report on synthesizing Ni and Co nanostructures using different mineralization to modulate the morphology of the final products. In addition, these synthesized Ni and Co nanocrystals exhibit room-temperature enhanced magnetic properties. Such a facile one-pot approach may also be applied for the synthesis of other metal materials.
Introduction
Over the past decade, magnetic nanomaterials have been of great interest for researchers from a wide range of disciplines, including ferrofluids, magnetic resonance imaging, data storage, magnet sensors, catalysis, environmental remediation and biotechnology/biomedicine.1 Numerous efforts have been employed in controlling the sizes, shapes, and phases of well-defined nanomaterials because these parameters represented key elements that determined their performances.2 Although great progress has been made in preparing well-defined nanomaterials, it remains a challenge to explore a simple and general strategy to synthesize uniform nanomaterials with controlled sizes, shapes, and phases for different systems.3Nickel and cobalt as well-known semiconductor metal materials have been extensively studied in diverse fields including catalysis, environmental protection, magnetic storage media, and clinical diagnosis and treatment.4 In recent years, considerable effort has been made to design effective methods to synthesize Ni and Co nanostructures with unique morphologies. Until now, well-defined Ni and Co nanostructures with different morphologies, such as belts, fibers, rods, flowers and spheres have been synthesized successfully using a variety of methods including lasing ablation, solvothermal and hydrothermal treatments.5 However, the development of a general and simple method for fabricating pure Ni and Co nanomaterials is strongly desirable.6
Herein, we report for the first time a facile and quick (2 h) synthesis of various morphologies of pure Ni and Co nanostructures by a simple one-pot hydrothermal treatment. We demonstrate that the control of nanocrystal shape can be influenced by the addition of mineralizers while keeping the same conditions. Furthermore, the effects of reaction parameters on the formation of nanostructures and their formation mechanism were also discussed. The magnetic properties of the as-synthesized Ni and Co nanostructures were studied in detail to explore the morphological effect on the magnetic characteristics. A comparative study of these two systems, namely, Ni microflowers and Co microspheres, should shed more light on the magnetic properties of these two nanostructured systems.
Experimental
Preparation of sample
All of the chemical reagents used in this experiment were of analytical grade and used without further purification. In a typical synthesis of nickel microflowers, 1 mmol Ni(NO3)2·4H2O and 0.5 g alkali salts (Na2SO4) were added successively into 20 mL deionized water. The resulting mixture was sonicated until a clear solution was obtained. Afterward, 2 mL of 1 M NaOH and 10 ml of hydrazine hydrate N2H4·H2O (80%) were sequentially added into the solution. After the mixture was magnetic stirred for 10 min, the final solution was then transferred into a Teflon-lined autoclave of 30 mL capacity. After heating at 180 °C for 2 h, the tank was cooled down to room temperature naturally. The final products were collected by centrifugation, washed with distilled water and absolute ethanol, and finally dried under vacuum at 60 °C for 2 h.In a typical synthesis of cobalt microspheres, 1 mmol Co(NO3)2·4H2O and 1.42 g alkali salts (Na2SO4) were added successively into 20 mL deionized water. The resulting mixture was sonicated until a clear solution was obtained. Afterward, 2 mL of 1 M NaOH and 10 mL of hydrazine hydrate N2H4·H2O (80%) were sequentially added into the solution. After the mixture was magnetic stirred for 10 min, the final solution was then transferred into a Teflon-lined autoclave of 30 mL capacity and heated at 140 °C for 2 h. The final sample was obtained by centrifugation and washed with water and ethanol.
Characterization and measurements
The X-ray diffraction pattern of the products was collected on a Rigaku-D/max 2500 V X-ray diffractometer with Cu-Kα radiation (λ = 1.5418 Å), with an operation voltage and current maintained at 40 kV and 40 mA. Field-emission scanning electron microscopy (FESEM) images were obtained with a XL30 ESEM FEI microscope. Transmission electron microscopic (TEM) images, and selected area electron diffraction (SAED) patterns were obtained on a JEOL JEM-2010 microscope.Magnetic measurement
Magnetic properties of the samples were measured by a Magnetic Property Measurment System (MPMS XL-7). The measurements for all samples were done on the pure and dried powders. The zero-field-cooling (ZFC) and field-cooling (FC) magnetizations of the as-prepared powder were measured from 5 to 350 K in an applied field of 100 Oe. Hysteresis loops of the samples were collected at 300 and 5 K, respectively.Results and discussion
Controlled synthesis and formation mechanism of nickel microflowers
The phase and purity of the as-prepared sample was investigated by the XRD analysis. As shown in Fig. 1, all the peaks can be identified as fcc-Ni metal (JCPDS no. 04-0850, space groupFm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (225); a = b = c = 3.524 Å). No additional peaks are observed in the XRD pattern, which confirms the purity of the sample. The morphology of the product was characterized by FESEM, TEM and HRTEM. The low-magnification FESEM image (Fig. 2a) reveals that the product is composed of uniform ordered microflowers in high yield. The high-magnification FESEM images (Fig. 2b) clearly show that the flower-like shape have a size of 2–3 μm with various nanopetals with sharp tips protruding from the core. It is very interesting that, the nanopetals of the microflowers are sawtooth-like. Hence, the microflowers with rough surface can be expected that the plate structure of Ni microflowers would be providing more reaction sites. The chemical composition of these microflowers was further characterized by using EDS (Fig. 2a, inset). Peaks of the elements Ni, Si and Pt were observed in the EDS pattern (the Si and Pt signals came from the substrate). Fig. 2c and d shows the low-magnification TEM image of a single Ni microflower. It can be clearly seen that the nanopetals of the microflowers consisted of various sharp tips protruding from the core, which was consistent with the results of FESEM. The HRTEM image (Fig. 2d) of the selected area in Fig. 2c shows a crystalline character with a lattice spacing of 0.2 nm, which can be indexed to the (111) plane of fcc Ni. A SAED pattern in inset of Fig. 2d taken from the edge of microflower reveals highly single crystalline nature of the Ni microflowers.
m (225); a = b = c = 3.524 Å). No additional peaks are observed in the XRD pattern, which confirms the purity of the sample. The morphology of the product was characterized by FESEM, TEM and HRTEM. The low-magnification FESEM image (Fig. 2a) reveals that the product is composed of uniform ordered microflowers in high yield. The high-magnification FESEM images (Fig. 2b) clearly show that the flower-like shape have a size of 2–3 μm with various nanopetals with sharp tips protruding from the core. It is very interesting that, the nanopetals of the microflowers are sawtooth-like. Hence, the microflowers with rough surface can be expected that the plate structure of Ni microflowers would be providing more reaction sites. The chemical composition of these microflowers was further characterized by using EDS (Fig. 2a, inset). Peaks of the elements Ni, Si and Pt were observed in the EDS pattern (the Si and Pt signals came from the substrate). Fig. 2c and d shows the low-magnification TEM image of a single Ni microflower. It can be clearly seen that the nanopetals of the microflowers consisted of various sharp tips protruding from the core, which was consistent with the results of FESEM. The HRTEM image (Fig. 2d) of the selected area in Fig. 2c shows a crystalline character with a lattice spacing of 0.2 nm, which can be indexed to the (111) plane of fcc Ni. A SAED pattern in inset of Fig. 2d taken from the edge of microflower reveals highly single crystalline nature of the Ni microflowers.
 | ||
| Fig. 1 XRD pattern of the as-obtained Ni products. | ||
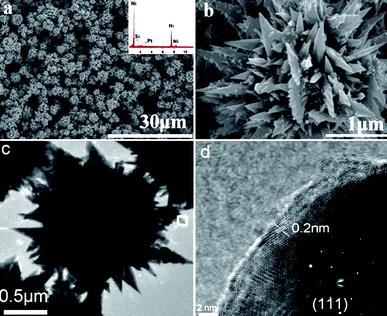 | ||
| Fig. 2 (a) Low magnification SEM image of Ni microflowers and the EDS pattern (inset). (b) High magnification SEM image of Ni microflowers. (c) Representative TEM image of the Ni microflowers. (d) HRTEM image of the edge area of the microflower shown in (c). Inset: SAED pattern of the microflowers. | ||
Several experiments were carried out to determine the parameters that might affect the formation of Ni hierarchical nanostructures. We have studied the influence of several parameters (such as additives, concentration of additives, reaction time and temperature) on the evolution of this unusual structure. It was found that the presence of the alkali salts played a crucial role in the formation of metal Ni flower-like architectures. To prove this, control experiment was repeated without Na2SO4. Irregular microspheres were the dominant products in the batch solution. When the amount of Na2SO4 is 0.142 g (1 mmol), although the dominant product remains irregular microspheres, quasi-flower-like architectures could be detected. When the addition of Na2SO4 is up to 0.5 g (3.5 mmol), 3D flower-like architectures are the only morphology. And no more changes with further additional quantity of Na2SO4. From Fig. 3, it was found that once Na2SO4 was added into the reaction system, a significant change took place in the morphologies of the products. Therefore, the quantity of salts had great impacts on the formation of well-defined hierarchical nanostructures.
 | ||
| Fig. 3 SEM images of Ni samples with different amounts of Na2SO4 keeping the other conditions constant: (a) 0 mmol. (b) 1 mmol. (c) 3.5 mmol. | ||
To fully understand the role of salts on the morphologies of the products, a series of experiments with different salts were performed, such as KCl, NaCl, NaNO3, KNO3, K2SO4, Na2SO4, (NH4)2SO4, NaF, KF, and NH4F. The FESEM images of the samples obtained were shown in Fig. 4. The typical shapes and corresponding morphologies of the different products were listed in Table 1 (also see ESI).† From the results, we can see that it was not Na+ but SO42− that led to the regular Ni microflowers. This result can be proved by introducing K2SO4 instead of Na2SO4 into the reactant solution. In this case, similar regular Ni flower-like architectures are obtained as expected (Fig. 4b). The effects of metal salts of different anions on the morphologies of the products can also confirm the result. This phenomenon may be attributed to the stronger interaction between SO42− and Ni2+ cations than that of the other counter anions. In addition, it has been well-documented that growth habits always directly determine the final crystal shape, which in turn is greatly influenced by the growth conditions.7 In our approach, owing to the addition of strong electrolytes salts, ion concentration in solution will increase accordingly, then in turn the interaction between ions will be increased, thereby the ion activity can be decreased.8 These factors will influence the nucleation and growth of the nanocrystals, which is favorable for the formation of high-quality and regular shape of nanocrystals.9 Therefore, it is reasonable to conclude that, the mineralizers have obvious influence on the morphology and size of the product. However, the exact mechanism for the role of salts is still not fully understood, and further studies are needed to explain the observed phenomena.
 | ||
| Fig. 4 SEM images of the samples obtained at different addition of salts: (a) Na2SO4; (b) K2SO4;(c) (NH4)2SO4; (d) NaCl; (e) KCl; (f) NH4Cl; (g) NaNO3; (h) KNO3; (i) NH4NO3; (j) NaF; (k) KF; (l) NH4F. | ||
| Sample | 300 K | 5 K | ||||
|---|---|---|---|---|---|---|
| M s/emu g−1 | M r/emu g−1 | H c/Oe | M s/emu g−1 | M r/emu g−1 | H c/Oe | |
| Ni | 54 | 7.7 | 166 | 58 | 11.7 | 240 |
| Co | 157 | 7.4 | 125 | 158 | 14.7 | 295 |
In the case of ammonium salt system, interesting phenomena can be observed that different ammonium salt lead to different kinds of morphological results due to the peculiar properties of ammonium. In a word, the morphology change derived from the cooperation of both cations and anions.10 It is well known that ammonium can act as complexing agents in solution to affect the deposition rate of crystals.11 In addition, the salt also acts as a mineralizer which could provide a suitable chemical environment for the growth of the desired crystals.12
The quantity of NaOH was also found to have a remarkable influence on the morphology of the final product. Without NaOH, the final products were all spheres with rugged surfaces. Increasing the amount of NaOH (1 and 2 mL) in the reaction mixture led to the formation of the well-defined Ni microflowers (Fig. 5a and b). As the concentration of NaOH was increased further (3 and 5 mL), the product was dominated by Ni spherical microcrystals (Fig. 5c and d), and few flowers could be detected. In the meantime, concentration controlled experiments were also performed, and similar result were obtained (ESI, Fig. S1).† Thus, it can be concluded that the formation of 3D microflowers can be obtained only by keeping the NaOH concentrations in a proper range. Because the concentration of OH− had a great influence on the redox rate of Ni(NO3)2 with N2H4·H2O. Obviously, at a higher concentration of OH−, the generation rate of Ni particles can accelerate largely, thereby leading to the fast growth of Ni crystals and finally forming irregular morphologies.13
 | ||
| Fig. 5 SEM images of Ni samples with different amounts of NaOH keeping the other conditions unchanged. (a) 1 mL; (b) 2 mL; (c) 3 mL; (d) 5 mL. | ||
The selection of reducing agents is crucial in the preparation of metal nanostructures. Here, N2H4·H2O was chosen as the preferred reductant because of its unique features. Hydrazine can coordinate to Ni2+ and then form a nickel-hydrazine complex [Ni(N2H4)3]2+, which helps to form a uniform and transparent aqueous solution before reaction.14 Compared with other reducing agent, hydrazine is favourite for its high reductive activity and the oxidation product of hydrazine, N2, and can be easily separated form solution without introducing any impurity.15 As a comparison, NaBH4 and NaH2PO2 were selected to replace N2H4 to conduct control experiments. Only indiscernible plates or particles were obtained (ESI, Fig. S2).†
In order to understand the formation mechanism of the metal Ni microflowers, time-dependent experiments were carried out by quenching the Teflon-lined autoclave using cold water at different reaction stages. Fig. 6 shows the XRD patterns of the products prepared at different reaction times. The XRD patterns of the sample obtained at the initial stage (0 and 20 min) show that sharp peaks correspond to [Ni(N2H4)3]SO4 (JCPDS no. 20-0793). In the early stage of the reaction (0–20 min), a nickel-hydrazine complex are formed (Fig. 6a and b). When the reaction time extends to 40 min or longer, all diffraction peaks of the as-obtained samples can be indexed to the cubic phase of Ni (Fig. 6d–f), that is, pure Ni is formed.
 | ||
| Fig. 6 XRD patterns of the as-prepared samples at 180 °C for different reaction times: (a) 0 min; (b) 20 min; (c) 30 min; (d) 40 min; (e) 2 h and (f) 5 h. | ||
The SEM images of the products obtained at different time intervals were shown in Fig. 7. When the sample was collected immediately after deposition at room temperature without any thermal treatment, purple precipitates were obtained which consisted of irregular particles with an average size of about 50 nm (Fig. 7a). As the reaction time was increased to 20 min, no obvious change of the nanoparticles was observed (Fig. 7b). After 30 min of hydrothermal treatment, the colour of precipitates changed from purple to black which means that Ni complex have been decomposed to metal Ni. We could observe that primary flower-like crystals started to emerge, but irregular nanoparticles still existed (Fig. 7c). Notably, the enlarged image of an individual flower is presented in the inset of Fig. 7c and several small particles on the surface of the petals can be found, which indicate that the flowers are assembled by small particles. When the reaction proceeded to 40 min (Fig. 7d), only flower-like morphology was observed with sizes of 2 μm in width although these flowers are ill-defined and under-developed. With a longer reaction time, the flower-like morphology gradually became perfect (Fig. 7e, f). According to the above experimental results, one possible formation mechanism of the metal Ni microflowers is proposed as shown schematically in Scheme 1. In the initial stage, N2H4 was first coordinated with Ni2+ to produce a complex [Ni(N2H4)3]SO4 at lower temperature, which spontaneously precipitated to become the nuclei and quickly grew into the primary particles approximately 50 nm in size. In the second stage, Ni complex has been decomposed to form initial Ni nuclei at high temperature which confirmed by the color change and XRD characterization of the precipitates. It has been reported that under hydrothermal conditions (high temperature and pressure), the complex chelating would be weakened and Ni would be released gradually.14Ni particles gradually form at the expense of destroying the coordination between N2H4 and Ni2+. The Ni primary microflowers are formed through the self-assembly aggregation of the nanoparticles at higher hydrothermal temperatures, driven by the minimization of interfacial energy.16 We believe that key factors affecting this self-assembly process are the addition of mineralizers Na2SO4.17 The exact role of Na2SO4 in the solution process has long been an issue of debate, and extensive work is under way towards its further clarification.
 | ||
| Fig. 7 SEM images for the shape-evolution process of Ni samples at different reaction times: (a) 0 min; (b) 20 min; (c) 30 min and high magnification SEM image of single microflowers (inset); (d) 40 min; (e) 2 h and (f) 5 h. | ||
 | ||
| Scheme 1 Schematic illustration of the proposed formation mechanism of 3D Ni microflowers. | ||
Characterization of cobalt microspheres
Similarly, uniform Co microspheres were obtained by this salt-assisted hydrothermal route (Fig. 8). The overall morphology of the samples, as shown in Fig. 8a, indicates that large quantities of microspheres with good monodispersity are achieved by using this simple method. As can be seen from the high magnification SEM image (Fig. 8b), all the micropsheres have the diameters of about 1–2 μm. More interesting, each microsphere has a rough surface consisting of thin nanopetals with an average size of about 50–100 nm. The chemical composition of these microspheres is also further characterized by using EDS (Fig. 8a, inset). Only peaks of the element Co are detected in the EDS pattern. The TEM image further confirms the co-spherical structures. Fig. 8c indicates a typical low-magnification TEM image of a single Co microspheres with thin nanopetals were epitaxially grew on those surfaces. In the HRTEM image of Fig. 8d recorded from Fig. 8c, the fringe spacing of 0.22 nm observed is close to the (100) lattice plane of Co. Furthermore, the SAED pattern (inset of Fig. 8d) taken from the edge of microsphere can be indexed as a single-crystalline structure. | ||
| Fig. 8 (a) Low magnification SEM image of Co microspheres. And the EDS pattern (inset); (b) High magnification SEM image of Co microspheres; (c) Representative TEM image of the Co microspheres; (d) HRTEM image of the edge area of the microspheres shown in (c). Inset: SAED pattern of the microspheres. | ||
To shed light on the function of Na2SO4 in the formation of Co microspheres, control experiment was carried out in which Na2SO4 was not used. The obtained sample was composed of irregular particles (ESI, Fig. S3).† To fully understand the role of salts on the morphologies of the products, a series of experiments with different salts were also performed, such as KCl, NaCl, NaNO3, KNO3, K2SO4, Na2SO4, (NH4)2SO4, NaF, KF, and NH4F. The corresponding typical morphology of the products in the addition of different salts is shown in Fig. 9. Note that various morphologies including rough cuboids with diameters of around 1 μm, rods with lengths in the range of 300 nm to 20 μm, uniform plates with about 500 nm length and 50 nm thicknesses, well-defined snowflakes with size of about 2μm were synthesized in large quantities through the addition of different mineralizers. Furthermore, we surmised that the case of metal Co gave very similar results as the case of metal Ni, suggesting that the effect of different salts on the morphologies of the products is analogous for the metal materials.
 | ||
| Fig. 9 SEM images of the samples obtained at different addition of salts: (a) Na2SO4; (b) K2SO4;(c) (NH4)2SO4; (d) NaCl; (e) KCl; (f) NH4Cl; (g) NaNO3; (h) KNO3; (i) NH4NO3; (j) NaF; (k) KF; (l) NH4F. | ||
Magnetic properties of nickel and cobalt nanomaterials
Magnetic properties of flower-like Ni and Co co-spherical architectures have been investigated, respectively, and we found that both Ni and Co have similar magnetic properties whether from M–H data or from M–T data. Magnetic parameters of the samples with special morphologies were listed in Table 1. The results demonstrate that all hysteresis loops of the samples measured at 5 and 300 K show typical ferromagnetic behaviours with non-zero remnant magnetization and coercivity (Fig. 10a, c).18 To further study the magnetic property, zero-field-cooled (ZFC) and field-cooled (FC) measurements were carried out from 5 to 350 K under an applied field of 100 Oe as shown in Fig. 10b and d. The characteristics of the ZFC and FC curves are similar, but the ZFC decreases more rapidly than FC, and the ZFC transformation region is larger than the FC, which results in a significant split during the whole temperature region. Moreover, from the ZFC/FC curves, it can be found that the Curie temperatures (TC) of the Ni and Co nanomaterials are higher than 350 K (bulk Nickel: Tc = 627 K, bulk Cobalt: Tc = 1388 K).19 It is inclined with the M–H results, that both of the samples show ferromagnetism behaviour. Under external field (Hext), the samples reaches their saturation magnetization (Ms) quickly, which is 54 emu g−1 and 58 emu g−1 for Ni nano flowers at 5 K and 300 K, respectively, and that is 157 emu g−1 and 158 emu g−1 for Co nanoparticles (Table. 1). The little difference of Ms at 5 K and 300 K is inclined to the results of M–T measurement also. Because the Tc of the samples is much higher than 300 K, the Ni and Co samples at 300 K and 5 K have spontaneous magnetism due to the molecular field (Hmol), and μ0μB Hmol/KBT ≫1 (here μ0 and μR referred to magnetic constant and Bohr magneton, respectively), which means that the thermal perturbation shows little effect on the Ms compared with Hext, and so the measured Ms of the samples are similar to each other at 5 and 300 K.20 | ||
| Fig. 10 (a, c) Magnetic hysteresis curves for Ni microflowers and Co microspheres measured at 5 and 300 K, and the down-right inset are the magnified hysteresis loops at low applied field (−400 Oe < H < 400 Oe). (b, d) Temperature dependence of ZFC and FC magnetization of the samples under an applied field of 100 Oe. | ||
Conclusions
In summary, well-defined Ni microflowers and Co microspheres have been successfully fabricated by a simple one-pot hydrothermal method with high yield. Na2SO4 was introduced into the reaction system to act as a mineralizer and played a key role in the formation process of these nanomaterials. We also demonstrate that salt addition in the solution induces drastic changes in the particular shape. In addition, these synthesized Ni and Co nanocrystals exhibit room-temperature enhanced magnetic properties. Such a facile one-pot approach may also be applied for the synthesis of other metal materials.Acknowledgements
The authors are grateful for financial aids from the National Natural Science Foundation of China (grant no. 20631040) and the MOST of China (‘973’ Program, grant no. 2006CB601103).References
- (a) T. M. Whitney, J. S. Jiang, P. C. Searson and C. Chien, Science, 1993, 261, 1316 CAS; (b) S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989 CrossRef CAS; (c) S. Odenbach, J. Phys.: Condens. Matter, 2004, 16, R1135 CrossRef CAS; (d) S. Mornet, S. Vasseur, F. Grasset and E. Duguet, J. Mater. Chem., 2004, 14, 2161 RSC.
- (a) H. Deng, X. Li, Q. Peng, X. Wang, J. Chen and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 2782 CrossRef CAS; (b) L. J. Zhao, H. J. Zhang, L. Zhou, Y. Xing, S. Y. Song and Y. Q. Lei, Chem. Commun., 2008, 3570 RSC; (c) M. T. Chang, L. J. Chou, C. H. Hsieh, Y. L. Chueh, Z. L. Wang, Y. Murakami and D. Shindo, Adv. Mater., 2007, 19, 2290 CrossRef CAS.
- (a) N. Spaldin, Cambridge University Press, Cambridge, UK 2003; (b) R. C. O'Handley, Wiley, New York 2000; (c) A. H. Lu, E. L. Salabas and F. SchÜth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS; (d) S. Ghosh, M. Ghosh and C. N. R. Rao, J. Cluster Sci., 2007, 18, 97 CrossRef CAS.
- (a) Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS; (b) E. Vesselli, L. D. Rogatis, X. L. Ding, A. Baraldi, L. Savio, L. Vattuone, M. Rocca, P. Fornasiero, M. Peressi, A. Baldereschi, R. Rosei and G. Comelli, J. Am. Chem. Soc., 2008, 130(34), 11417 CrossRef CAS; (c) K. Nielsch, F. J. Castano, S. Matthias, W. Lee and C. A. Ross, Adv. Eng. Mater., 2005, 7, 217 CrossRef CAS; (d) J. C. Bao, C. Y. Tie, Z. Xu, Q. F. Zhou, D. Shen and Q. Ma, Adv. Mater., 2001, 13, 1631 CrossRef CAS.
- (a) Q. Xie, Z. Dai, W. W. Huang, J. B. Liang, C. L. Jiang and Y. T. Qian, Nanotechnology, 2005, 16, 2958 CrossRef CAS; (b) R. Xu, T. Xie, Y. G. Zhao and Y. D. Li, Cryst. Growth Des., 2007, 7, 1904 CrossRef CAS; (c) X. H. Liu, R. Yi, Y. T. Wang, G. Z. Qiu, N. Zhang and X. G. Li, J. Phys. Chem. C, 2007, 111, 163 CrossRef CAS; (d) L. P. Zhu, H. M. Xiao, W. D. Zhang, Y. Yang and S. Y. Fu, Cryst. Growth Des., 2008, 8, 1113 CrossRef CAS; (e) N. Chakroune, G. Viau, C. Ricolleau, F. Fiévet-Vincent and F. Fiévet, J. Mater. Chem., 2003, 13, 312 RSC; (f) D. Ung, Y. Soumare, N. Chakroune, G. Viau, M. J. Vaulay, V. Richard and F. Fiévet, Chem. Mater., 2007, 19, 2084 CrossRef CAS; (g) Q. Li, H. J. Liu, M. Han, J. M. Zhu, Y. Y. Liang, Z. Xu and Y. Song, Adv. Mater., 2005, 17, 1995 CrossRef CAS; (h) Z. G. An, S. L. Pan and J. J. Zhang, J. Phys. Chem. C, 2009, 113(7), 2715 CAS; (i) X. L. Hu and J. C. Yu, Chem. Mater., 2008, 20, 6743 CrossRef CAS; (j) L. X. Sun and Q. W. Chen, J. Phys. Chem. C, 2009, 113(7), 2710 CrossRef CAS.
- (a) H. Wu, R. Zhang, X. X. Liu, D. D. Lin and W. Pan, Chem. Mater., 2007, 19, 3506 CrossRef CAS; (b) R. Brayner, M. J. Vaulay, F. Fivet and T. Coradin, Chem. Mater., 2007, 19(5), 1190 CrossRef CAS; (c) Y. P. Sun, H. W. Rollins and R. Guduru, Chem. Mater., 1999, 11(1), 7 CrossRef CAS; (d) R. Xu, T. Xie, Y. G. Zhao and Y. D. Li, Cryst. Growth Des., 2007, 7, 1904 CrossRef CAS.
- (a) Y. Z. Shao, J. Sun and L. Gao, J. Phys. Chem. C, 2009, 113(16), 6566 CrossRef CAS; (b) Z. P. Liu, R. Z. Ma, M. Osada, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2005, 127, 13869 CrossRef CAS; (c) X. Wang, F. L. Yuan, P. Hu, L. J. Yu and L. Y. Bai, J. Phys. Chem. C, 2009, 113(30), 13456 CrossRef CAS.
- (a) R. F. Qin, H. W. Song, G. H. Pan, X. Bai, B. Dong, S. H. Xie, L. N. Liu, Q. L. Dai, X. S. Qu, X. G. Ren and H. F. Zhao, Cryst. Growth Des., 2009, 9(4), 1750 CrossRef CAS; (b) R. Liu, X. R. Xu, Y. R. Cai, A. H. Cai, H. H. Pan, R. K. Tang and K. Cho, Cryst. Growth Des., 2009, 9(7), 3095 CrossRef CAS; (c) A. N. Baranov, G. N. Panin, T. W. Kang and Y. J. Oh, Nanotechnology, 2005, 16, 1918 CrossRef CAS.
- (a) L. Yan, R. B. Yu, J. Chen and X. R. Xing, Cryst. Growth Des., 2008, 8(5), 1474 CrossRef CAS; (b) B. Tang, L. H. Zhuo, J. C. Ge, J. Y. Niu and Z. Q. Shi, Inorg. Chem., 2005, 44, 2568 CrossRef CAS.
- (a) Z. P. Liu, S. Li, Y. Yang, S. Peng, Z. K. Hu and Y. T. Qian, Adv. Mater., 2003, 15, 1946 CrossRef CAS; (b) Q. Y. Lu, F. Gao and D. Y. Zhao, Nanotechnology, 2002, 13, 741 CrossRef CAS; (c) H. L. Niu, Q. W. Chen, M. Ning, Y. S. Jia and X. J. Wang, J. Phys. Chem. B, 2004, 108(13), 3996 CrossRef CAS.
- (a) Z. H. Liang, Y. J. Zhu and X. L. Hu, J. Phys. Chem. B, 2004, 108, 3488 CrossRef CAS; (b) D. B. Kuang, B. X. Lei, Y. P. Pan, X. Y. Yu and C. Y. Su, J. Phys. Chem. C, 2009, 113(14), 5508 CrossRef CAS; (c) H. B. Li, L. L. Chai, X. Q. Wang, X. Y. Wu, G. C. Xi, Y. K. Liu and Y. T. Qian, Cryst. Growth Des., 2007, 7(9), 1918 CrossRef CAS.
- (a) C. J. Jia, L. D. Sun, Z. G. Yan, L. P. You, F. Luo, X. D. Han, Y. C. Pang, Z. Zhang and C. H. Yan, Angew. Chem., 2005, 117, 4402 CrossRef; (b) S. Yamabi and H. Imai, J. Mater. Chem., 2002, 12, 3773 RSC.
- (a) W. D. Shi, L. Zhou, S. Y. Song, J. H. Yang and H. J. Zhang, Adv. Mater., 2008, 20, 1892 CrossRef CAS; (b) Z. B. Zhuang, Q. Peng, J. Zhuang, X. Wang and Y. D. Li, Chem.–Eur. J., 2006, 12, 211 CrossRef CAS; (c) Z. G. An, S. L. Pan and J. J. Zhang, J. Phys. Chem. C, 2009, 113, 1346 CrossRef CAS; (d) Y. Wang, Q. S. Zhu and H. G. Zhang, J. Mater. Chem., 2006, 16, 1212 RSC.
- (a) X. H. Liu, R. Yi, Y. T. Wang, G. Z. Qiu, N. Zhang and X. G. Li, J. Phys. Chem. C, 2007, 111(1), 163 CrossRef CAS; (b) L. P. Zhu, H. M. Xiao, W. D. Zhang, Y. Yang and S. Y. Fu, Cryst. Growth Des., 2008, 8(4), 1113 CrossRef CAS.
- (a) F. Cao, W. D. Shi, L. J. Zhao, S. Y. Song, J. H. Yang, Y. Q. Lei and H. J. Zhang, J. Phys. Chem. C, 2008, 112, 17095 CrossRef CAS; (b) C. M. Liu, L. Guo, R. M. Wang, Y. Deng, H. B. Xu and S. H. Yang, Chem. Commun., 2004, 2726 RSC.
- (a) Z. Zhang, H. Sun, X. Shao, D. Li, H. Yu and M. Han, Adv. Mater., 2005, 17, 42 CrossRef CAS; (b) Y. Q. Lei, G. H. Wang, S. Y. Song, W. Q. Fan and H. J. Zhang, CrystEngComm, 2009, 11, 1857 RSC.
- (a) A. Filankembo and M. P. Pileni, J. Phys. Chem. B, 2000, 104(25), 5865 CrossRef CAS; (b) N. Sui, Y. Z. Duan, X. L. Jiao and D. R. Chen, J. Phys. Chem. C, 2009, 113, 8560 CrossRef CAS.
- (a) F. L. Jia, X. Y. Zhang and Y. Yang, Adv. Mater., 2008, 20, 1050 CrossRef CAS; (b) Y. Z. Mi, D. S. Yuan, Y. L. Liu, J. X. Zhang and Y. Xiao, Mater. Chem. Phys., 2005, 89, 359 CrossRef CAS.
- (a) M. J. Aus, B. Szpunar, A. M. Elsherik, U. Erb, G. Palumbo and K. T. Aust, Scr. Metall. Mater., 1992, 27, 1639 CrossRef CAS; (b) Y. Hou, H. Kondoh and T. Ohta, Chem. Mater., 2005, 17, 3994 CrossRef CAS.
- (a) M. Respaud, J. M. Broto, H. Rakoto, A. R. Fert, L. Thomas, B. Barbara, M. Verelst, E. Snoeck, P. Lecante, A. Mosset, J. Osuna, T. Ould Ely, C. Amiens and B. Chaudret, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 2925 CrossRef CAS; (b) L. L. Diandra and D. R. Reuben, Chem. Mater., 1996, 8(8), 1770 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of the Ni products prepared with different amounts of NaOH, different addition of reducing agents; SEM image of the sample Co obtained without addition of Na2SO4. See DOI: 10.1039/c0ce00074d |
| This journal is © The Royal Society of Chemistry 2011 |
