Enhanced electrochemical catalytic activity of new nickel hydroxide nanostructures with (100) facet†
Xiaojun
Zhang
ab,
Aixia
Gu
a,
Guangfeng
Wang
a,
Bin
Fang
*a,
Qingyu
Yan
*b,
Jixin
Zhu
b,
Ting
Sun
b,
Jan
Ma
b and
Huey Hoon
Hng
*b
aCollege of Chemistry and Materials Science Anhui Normal University, Wuhu, 241000, P. R. China. E-mail: binfang_47@yahoo.com.cn; Fax: +86-553-3869303; Tel: +86-553-3869303
bSchool of Materials Science and Engineering, Nanyang Technological University, Singapore. E-mail: alexyan@ntu.edu.sg; ashhhng@ntu.edu.sg; Fax: +65-67904583; Tel: +65-67904583
First published on 27th August 2010
Abstract
Owing to their scientific and technological importance, inorganic single crystals with highly reactive surfaces have long been studied. Unfortunately, surfaces with high reactivity usually diminish rapidly during the crystal growth process as a result of surface energy minimization. The crystal planes of nickel hydroxide play an essential role in determining its catalytic oxidation properties. In this study, β-Ni(OH)2 nanocolumns with well-defined crystal planes have been synthesized by a facile solution-based hydrothermal method. TEM and XRD studies reveal that the assembled stacking of the Ni(OH)2 nanocrystals leads to the predominantly exposed planes as unusually reactive (100) facet rather than the stable (001) facet in the hexagonal nanoslice structures. Consequently, it is demonstrated that the β-Ni(OH)2 nanocolumns are more electrochemical catalytic active than their counterparts, nanoslices and nanoplates. The current study indicates that catalysts with well-defined reactive surface can be “designed” through controlled synthesis of nanostructures.
Introduction
The surface stability and reactivity of inorganic single crystals have long been thought to be dominated by their surface chemistry, which has a critical effect on the equilibrium morphology of single crystals with high reactivity. Useful examples, such as microcrystals of anatase (TiO2) with a large percentage of (001) facets, single crystals of the ZnO with (0001) and (000-1) facets and tetrahexahedral platinum nanocrystals with high-index facets have been reported.1–5Nickel hydroxide nano/microstructures are attractive materials, which have been used in a wide range of applications such as rechargeable batteries, heterogeneous catalysis, electrochemical sensors etc.6–13 In general, hexagonal crystals can be expressed with both polar and nonpolar facets. For β-Ni(OH)2, the (001) facet is polar and the (100) and (010) facets are nonpolar. In such structures, the polar surfaces (the ones with potentially high activity) diminish easily along with crystal growth as a result of surface energy minimization, which technically becomes a fundamental problem.5,14,15Growth of β-Ni(OH)2 nanostructures normally results in nanoslice shape, which is due to growth along the [001] direction of a hexagonal single crystal. Therefore, preparation of uniform, high purity hexagonal β-Ni(OH)2 single crystals with controllable crystallographic facets still remains a challenge.
Recently, nickel hydroxide with various morphologies have been synthesized including nanoplates,7,16 hollow spheres,17,18 ribbon-like and board-like structures,8,19 flower-like structures,20,21 and tubes.22 So far, complex structures with growth direction mainly along the [001] direction of nickel hydroxide have been reported. However, the functionality of the (100) and (010) facets of these complex structures is unclear, and this can greatly influence their application. In these reported methods, N2H4 are commonly used as a bridge to link the complex structure.23,24 However, N2H4 is known to be toxic and not environmental friendly. Furthermore, these complex structures are unstable and have the tendency to break down to nanoslices when used in aqueous solution, which can influence nickel hydroxide subsequent reliability performance and applications. Hence, a facile and safe method for large scale synthesis of stable β-Ni(OH)2 single crystals with controllable crystallographic facets is highly desired.
In this paper, we demonstrated the synthesis of Ni(OH)2 nanocolumns grown along the [001] direction. Each of the nanocolumns were formed by ordered stacking of oriented Ni(OH)2 single crystal nanoplates. These novel structures provide large area of the (100) facets. The as-prepared Ni(OH)2 nanocolumns showed better electrochemical catalytic properties as compared to those of Ni(OH)2 nanostructures with majority (001) facets. They were tested as an H2O2 sensor, and were found to show better sensitivity. This synthesis strategy by controlled constructing assembled nanostructures to attain desired crystal facets and functionality can be further extended to a variety of nanoarchitectures.
Experimental details
Chemicals. NiCl2·6H2O, ethanol, NaOH, NH3·H2O, and K3[Fe(CN)6] were purchased from Shanghai Chemical Corp. All chemicals were used as received without any further purification. Millipore water was used in all experiments.Synthesis of various Ni(OH)2 nanostructures
In a typical experiment, 0.0476 g of NiCl2·6H2O and 0.008 g of NaOH were separately dissolved in 10 mL of deionized water at room temperature. The two solutions were then mixed together, stirred vigorously for 1 h and transferred into 80 mL steel autoclave. The clave was sealed, maintained at 160 °C for 8 h, and then cooled in ambient to room temperature. The product was washed with distilled water and ethanol several times to remove the impurities before characterization.Electrochemical measurements
The modified electrode was prepared as follows: Glass Carbon (GC) electrodes (3 mm diameter) were carefully polished with a diamond pad/3 µm polishing suspension, rinsed with distilled water and ethanol, and then dried under ambient nitrogen gas. Ni(OH)2 nanostructures (nanocolumns, nanoplates, or nanoslices) (10 mg) were dissolved in a mixture of 0.1 mL of Nafion perfluorosulfonated ion-exchange resin and 0.9 mL of distilled water. Approximately 60 min of ultrasonication was necessary to obtain a uniformly dispersed Ni(OH)2 nanostructures. After dropping 10 µL of the Ni(OH)2 nanostructures (nanocolumns, nanoplates, and nanoslices) solution onto the electrode surface, the electrode was dried in air, and the modified electrode was then obtained and labeled as Ni(OH)2 nanocolumns/Nafion, Ni(OH)2 nanoplates/Nafion, Ni(OH)2 nanoslices/Nafion modified electrode, respectively.Electrochemical measurements were performed on a model CHI660B electrochemical analyzer (ChenHua Instruments Co. Ltd., Shanghai, China) controlled by a personal computer. By using the modified GC working electrode, the Cyclic Voltammetry (CV) and Cyclic Amperometric (CA) data were measured in a mixture of 1 mmolar H2O2 and 20 mmolar phosphate buffer solutions (PBS, pH 7.2). The CA measurements required the operation of the electrode at a constant applied potential of 0.40 V vs.SCE. Once the current reached a baseline in the absence of glucose, glucose was added every 50 s thereafter. Electrochemical impedance spectroscopy (EIS) measurements were performed in the presence of 2.5 × 10−3 mol/L [Fe(CN)6]4−/3− + 0.1 mol/L KCl + 10 × 10−3 mol/L PBS (pH 7.2) at a bias potential of 0.20 V by applying an AC voltage with 5 mV amplitude in a frequency range from 0.01 Hz to 100 kHz under open circuit potential conditions and plotted in the form of complex plane diagrams (Nyquist plots).
Characterization
X-ray powder diffraction (XRD) patterns of the products were recorded on a Shimadzu XRD-6000 X-ray diffractometer at a scanning rate of 0.05°/s with the 2θ range from 10 to 80°, with high-intensity Cu Kα radiation (λ) 0.154178 nm. Field-emission scanning electron microscopy (FESEM) were used (Hitachi S-4800 SEM, operated at 10 kV). The high-resolution transmission electron microscope (JEOL 2010) operating with an accelerating voltage of 200 kV was used. Infrared (IR) spectra of various Ni(OH)2 nanostructures were obtained on Vectortm 22 Fourier Transform spectrometer (FTIR) (Bruke, Germany).Results and discussion
Fig. 1 presents the powder XRD patterns of the as-prepared products. All the diffraction peaks can be indexed as β-phase hexagonal nickel hydroxide (JCPDS 14-0117). No peaks from α-Ni(OH)2 were observed in the XRD pattern, which confirms the high purity of the sample. An unusually high intensity of the (001) diffraction peak is observed, which indicates the preferential crystal growth direction of the samples.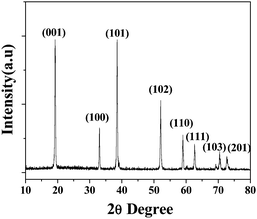 | ||
| Fig. 1 XRD pattern of the as-prepared Ni(OH)2 nanocolumns. | ||
The as-prepared Ni(OH)2 shows the interesting nanostructure with plate crystals stacking into columnar shape (Fig. 2). The diameters and the thicknesses of the Ni(OH)2 plate crystals are 200–400 nm and 25–50 nm, respectively. The TEM observation reveals the interconnection between the plate-shaped Ni(OH)2 crystals through the grain boundaries (Fig. 2c-d). This observation is completely different from previous reports, which show that nanocolumns were linked together through organic ligands.13,23–25 The selected area electron diffraction (SAED) pattern of a single Ni(OH)2 nanocolumn shows the spots pattern with 6-fold symmetry along the [001] zone-axis, which suggests that these plate crystals are stacking together with preferred orientation, e.g. along [001] direction. To understand the growth details of the β-Ni(OH)2 nanocolumns, we carried out the experiment with different reaction time and examined the resulting nanostructures.
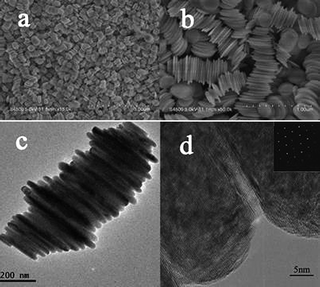 | ||
| Fig. 2 (a) Low- and (b) High-magnification SEM images of the Ni(OH) nanocolumns composed of nanoplates. (c) TEM image of a representative Ni(OH) nanocolumn composed of nanoplates. (d) HRTEM image shows the grain boundary region between two nanoplates, inset is the SAED pattern. | ||
The SEM images (Fig. 3) shows the evolution of the Ni(OH)2 nanocolumns from nanoparticles. First, when the two solutions (10 ml 0.02M NiCl2 and 10 ml 0.02M NaOH) mixed, Ni(OH)2 nanoparticles with sizes of 50–100 nm were formed (Fig. 3a). After the hydrothermal reaction at 160 °C for 2 hours, hexagonal nanoplates with thickness and diameter of 10–20 nm and 100–200 nm were observed (Fig. 3b). Here, only β-Ni(OH)2 phase was formed with low (001) peak intensity, which indicates no preferred alignment of nanocrystals along the [001] crystal axis (see supporting information† Fig. S1a). After four hours of hydrothermal reaction, the hexagonal platelets start to interconnect to each other through the overlapping of the plate surface (Fig. 3c), which may result from the reduction of the total surface energy.
 | ||
| Fig. 3 SEM images of samples prepared by hydrothermal reaction of a solution comprising NiCl2·6H2O (0.01 M), NaOH (0.01M) and double-distilled water (20 mL) in 160 °C for different reaction periods: (a) 0, (b) 2, (c) 4, (d) 8 h. | ||
The XRD pattern (see supporting information† Fig. S1b) confirms the β-Ni(OH)2 phase, although the intensity of (001) peak is higher than that of samples which were reacted for 2 hours. This indicates the onset of the preferred alignment of the Ni(OH)2 nanoplatelets through the interconnection. TEM observation (see supporting information† Fig. S2a-b) shows that these Ni(OH)2 nanoplates interconnected together through grain boundaries and not just simply stacked together through linkages with organic ligands. When the reaction time was extended to 8 h, the length of the columnar structure of Ni(OH)2 further increased (Fig. 3d).
The surface chemistry of the as-prepared samples is examined by FTIR (see supporting information† Fig. S3). The IR spectrum includes i) a broad peak centered around 3420 and 1620 cm−1 corresponding to interlamellar water and OH− bond vibration, ii) absorptions in the range of 1000–1500 cm−1 due to intercalated anions NO3−, and iii) absorptions at 3640 and 485 cm−1 due to the Ni–O–H bending and Ni–O stretching vibrations, respectively. No other peaks corresponding to other functional groups are observed in the spectra, which indicate interfaces of the nanoplatelets are clean and with no specific capping ligands.24
It is well-known that β-Ni(OH)2 has a layered crystal structure (Fig. 4). Two Ni ions in the adjacent (100) planes are linked through one OH− while two Ni ions in two adjacent (001) planes are separated by two layers of OH−. Thus, a study of the effect of pH values on the morphology of Ni(OH)2 nanostructure is necessary. At high pH (pH = 14), the products are mainly hexagonal nanoslices with thickness of 5–10 nm (see supporting information† Fig. S4a). It is suspected that the high concentration of OH− prevents the further growth of Ni(OH)2 along the (001) direction. Decreasing pH value to 12 leads to the formation of Ni(OH)2 nanoplates with thickness of 20–50 nm. These nanoplates stack together in columnar shape (see supporting information† Fig. S2a). The possible mechanism of the nanocolumn formation is mainly due to the dangling bonds on the surface resulting in plate stacking to reduce the total surface energy. Then, a second growth will occur that leads to the interconnection between the plate surfaces. When the pH value was further decreased to 10, we noticed that there are irregular shaped nanostructures (see supporting information† Fig. S4b) in the resulting products, which may be due to the insufficient amount of OH− available to allow the growth of proper crystal structure. On the basis of the above results, the scheme illustrated in Fig. 5 is proposed for the growth process of nickel hydroxide nanocolumns. Although the growth process is believed to be related to the reported orientated attachment of the nanoplates,24,25 the exact details may be different. Here no specific ligands, e.g. SDS24 or hydrazine,25 are required to link the nanoplates together. The alignment of the nanoplates along the [001] direction are preferred due to the reduction in the surface energy, which at high temperature leads to grain coarsening and interface diffusion to form grain boundaries.
 | ||
| Fig. 4 A ball and stick model for the β-Ni(OH)2 crystalline structure. | ||
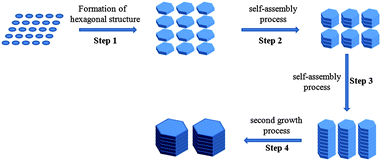 | ||
| Fig. 5 Schematic illustration of the morphological evolution of β-Ni(OH)2 nanostructures. | ||
In order to study the electrocatalytic activity of the samples, the electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) and cyclic amperometric (CA) spectra were obtained for samples with different area ratio of (100) facets.
EIS has been reported as an effective method for the understanding of chemical transformations and processes associated with a conductive electrode surface.26 The impedance spectra may include a semicircle portion and a linear portion. The semicircle portion at higher frequencies corresponds to the electron transfer limited process while the linear portion at lower frequencies corresponds to the ion diffusion process. The semicircle diameters correspond to the electron-transfer resistance (Ret). Fig. 6 shows the impedance spectroscopies of different electrodes with the same thickness. After Ni(OH)2 nanostructures, e.g. nanoslices (curve d), nanoplates (curve c), and nanocolumns (curve b) were attached onto the GC electrode, the semicircle diameter of EIS, Ret, increases as compared to the bare GC electrode (curve a). The impedance changes for different types of electrodes show that the nanostructure of Ni(OH)2 can affect the electron transfer process. For example, Ret is lower for Ni(OH)2 columns than that of nanoplates (see supporting information† Fig. S5c, d) and nanoslices (see supporting information† Fig. S5a, b), which may be related to the crystal structure of Ni(OH)2. As shown in Fig. 4, the two Ni ions along [100] direction are linked by one OH−group while two Ni ions along [001] direction are separated by two layer of OH−. Hence, the electron hopping along (100) direction is easier than that along (001) direction. It is suspected that increased portion of (100) facets, e.g. as in Ni(OH)2 nanocolumns, facilitate electron transfer. Furthermore, Ni(OH)2 nanocolumns have less open interface between the structures which reduce the electron scattering during the electron transfer, and may also result in smaller Ret. It should be mentioned that the as-prepared Ni(OH)2 nanocolumns/GCE electrode was found to be very stable, as illustrated by the redox process of [Fe(CN)6]3−/4− in the solution phase. The redox peak currents were essentially unchanged after continuously cycling the electrodes for more than 100 cycles.
![The electrochemical impedance spectroscopy of electrodes with the same thickness and surface area: (a) bare GCE; (b) Ni(OH)2 nanocolumns/GCE; (c) Ni(OH)2 nanoplates/GCE; (d) Ni(OH)2 nanoslices/GCE in 2.5 × 10−3 mol L−1[Fe(CN)6]4−/3− + 0.1 mol L−1 KCl + 0.01 M PBS (pH = 7.2).](/image/article/2011/CE/c003791p/c003791p-f6.gif) | ||
| Fig. 6 The electrochemical impedance spectroscopy of electrodes with the same thickness and surface area: (a) bare GCE; (b) Ni(OH)2 nanocolumns/GCE; (c) Ni(OH)2 nanoplates/GCE; (d) Ni(OH)2 nanoslices/GCE in 2.5 × 10−3 mol L−1[Fe(CN)6]4−/3− + 0.1 mol L−1 KCl + 0.01 M PBS (pH = 7.2). | ||
The CV curves (Fig. 7) show the response of the presence of H2O2 for the bare GCE, Ni(OH)2 nanocolumns/GCE, Ni(OH)2 nanoplates/GCE, Ni(OH)2 nanoslices/GCE. No amperometric response was observed for the bare GCE over the working potential range in 0.1 M PBS solution with a pH value of 7.2 that contained H2O2 (curve a). However, strong electrocatalytic activity were observed for Ni(OH)2 modified electrodes in response to the presence of H2O2. Comparing the electrochemical catalytic activity of Ni(OH)2 nanostructure electrodes tested in the solution with the same H2O2 molar concentration, the nanocolumns show the highest peak current, which is mainly attributed to the lower Ret as mentioned earlier.
 | ||
| Fig. 7 CV performance of (a) bare GCE, (b) Ni(OH)2 nanoslices/GCE; (c) Ni(OH)2 nanoplates/GCE; (d) Ni(OH)2 nanocolumns/GCE in the presence of the 1 µM of the H2O2 in 0.1 M PBS (pH 7.2) at a scan rate of 50 mV s−1. | ||
For amperometric sensing application, electrodes are generally evaluated by measuring current response at a fixed potential with the analysis added. Fig. 8 reveals the amperometric response (at 0.40 V) of the as-prepared Ni(OH)2 nanostructure electrodes exposed to PBS (pH = 7.2) solution with successive addition of 1 mM H2O2. As shown in the voltammetric data (see supporting information† Fig. S6), the Ni(OH)2 nanomaterial modified electrodes show a linear response to the changes in H2O2 concentration. The as-prepared Ni(OH)2 nanocolumns modified electrode gives a linear dependence in the H2O2 concentration range of 0.1 µM to 10 µM with a higher sensitivity of 3.995 µA µM−1 (see supporting information† Fig. S6a) as compared to that of nanoplates and nanoslices, e.g. 2.024 µA µM−1 and 1.050 µA µM−1, respectively.
 | ||
| Fig. 8 CA response of as-prepared Ni(OH)2 nanomaterials/GCE at 0.1 V upon subsequent addition of 1 mM H2O2 solution (a) Ni(OH)2 nanocolumns/GCE; (b) Ni(OH)2 nanoplates/GCE; (c) Ni(OH)2 nanoslices/GCE. | ||
Conclusion
In summary, we have achieved high-yield synthesis of Ni(OH)2 nanocolumns with a large portion of (100) facets by a facile wet chemical method. Characterizations of EIS, CV and CA on these Ni(OH)2 nanocolumns electrodes show easier electron transfer processes than nickel hydroxide with two other nanostructures, e.g. nanoplates and nanoslices. These Ni(OH)2 nanocolumns show better sensitivity to H2O2 than that of nanoslices or nanoplates nickel hydroxide, which may be due to the larger (100) facet area.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 20675001, 20901003), Anhui Key Laboratory of Controllable Chemistry Reaction & Material Chemical Engineering, the Young Teacher Program of Anhui Normal University (2009xqnzc19), and the Natural Science Foundation of Educational Department of Anhui Province (No. KJ2008B167, No. KJ2009B013Z) and AcRF Tier 1 RG 31/08 from MOE, Singapore.References and notes
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261 CrossRef CAS.
- O. Bikondoa, C. L. Pang, R. Ithnin, C. A. Muryn, H. Onishi and G. Thornton, Nat. Mater., 2006, 5, 189 CrossRef CAS.
- X. Q. Gong, A. Selloni, M. Batzill and U. Diebold, Nat. Mater., 2006, 5, 665 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- P. Oliva, J. Leonardi, J. F. Laurent, C. Delmas, J. J. Braconnier, M. Figlarz, F. Fievet and A. de Guibert, J. Power Sources, 1982, 8, 229 CrossRef CAS.
- Z. H. Liang, Y. J. Zhu and X. L. Hu, J. Phys. Chem. B, 2004, 108, 3488 CrossRef CAS.
- D. N. Yang, R. M. Wang, M. S. He, J. Zhang and Z. F. Liu, J. Phys. Chem. B, 2005, 109, 7654 CrossRef CAS.
- Y. Wang, Q. S. Zhu and H. G. Zhang, Chem. Commun., 2005, 5231 RSC.
- L. X. Yang, Y. J. Zhu, H. Tong, Z. H. Liang and W. W. Wang, Cryst. Growth Des., 2007, 7, 2716 CrossRef CAS.
- L. P. Xu, Y. S. Ding, C. H. Chen, L. L. Zhao, C. Rimkus, R. Joesten and S. L. Suib, Chem. Mater., 2008, 20, 308 CrossRef CAS.
- S. M. Zhang and H. C. Zeng, Chem. Mater., 2009, 21, 871 CrossRef CAS.
- S. Ida, D. Shiga, M. Koinuma and Y. Matsumoto, J. Am. Chem. Soc., 2008, 130, 14038 CrossRef CAS.
- X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, Nature, 2009, 458, 746 CrossRef CAS.
- P. J. Thomas and P. O'Brien, J. Am. Chem. Soc., 2006, 128, 5614 CrossRef CAS.
- Y. Li, B. Tan and Y. Wu, Chem. Mater., 2008, 20, 567 CrossRef CAS.
- D. Wang, C. Song, Z. Hu and X. Fu, J. Phys. Chem. B, 2005, 109, 1125 CrossRef CAS.
- G. Duan, W. Cai, Y. Luo and F. Sun, Adv. Funct. Mater., 2007, 17, 644 CrossRef CAS.
- D. Yang, R. Wang, J. Zhang and Z. Liu, J. Phys. Chem. B, 2004, 108, 7531 CrossRef CAS.
- M. Cao, X. He, J. Chen and C. Hu, Cryst. Growth Des., 2007, 7, 170 CrossRef CAS.
- Y. Luo, G. Li, G. Duan and L. Zhang, Nanotechnology, 2006, 17, 4278 CrossRef.
- F. S. Cai, G. Y. Zhang, J. Chen, X. L. Gou, H. K. Liu and S. X. Dou, Angew. Chem., Int. Ed., 2004, 43, 4212 CrossRef CAS.
- J. X. Zhu, Z. Gui, Y. Y. Ding, Z. Z. Wang, Y. Hu and M. Q. Zou, J. Phys. Chem. C, 2007, 111, 5622 CrossRef CAS.
- W. Zhou, M. Yao, L. Guo, Y. M. Li, J. H. Li and S. H. Yang, J. Am. Chem. Soc., 2009, 131, 2959 CrossRef CAS.
- C. Coudun and J. F. Hochepied, J. Phys. Chem. B, 2005, 109, 6069 CrossRef CAS.
- R. J. Pei, Z. L. Cheng, E. K. Wang and X. R. Yang, Biosens. Bioelectron., 2001, 16, 355 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, SEM, FTIR, linear response. See DOI: 10.1039/c003791p |
| This journal is © The Royal Society of Chemistry 2011 |
