Microwave-assisted solution-phase preparation and growth mechanism of FeMoO4 hierarchical hollow spheres†
Lei
Zhang
a,
Xiao-Feng
Cao
a,
Ying-Li
Ma
a,
Xue-Tai
Chen
*a and
Zi-Ling
Xue
b
aState Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, P. R. China. E-mail: xtchen@netra.nju.edu.cn; Fax: +86-25-83314502
bDepartment of Chemistry, The University of Tennessee, Knoxville, Tennessee 37996-1600, USA
First published on 28th August 2009
Abstract
In the present paper, we report successful synthesis of FeMoO4 hierarchical hollow spheres with 1.0 µm in diameter via a simple, rapid and reliable microwave-assisted solution-phase approach. The phases and morphologies of the products have been characterized by powder X-ray diffraction (XRD), energy dispersive spectrometry (EDS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM). Some factors influencing the morphology of the final product have been investigated. These experiments indicate that the self-assembly of FeMoO4 nanoparticles at the water/gas interface is responsible for the formation of a hierarchical hollow structure.
Introduction
Over the past decade, there has been much interest in the fabrication of nanostructures with desired morphologies and properties. Among the various morphologies of nanomaterials, hollow structures have attracted great attention because of their technological and fundamental scientific importance and potential applications.1–3 Up to now, many methods, including template-assisted synthesis,4–6 oriented attachment,7 the Kirkendall effect,8 and Ostwald ripening9–11 have been developed for the fabrication of hollow structures. Recently, particular attention has been paid to the fabrication of hierarchical hollow structures by the self-assembly of primary building units. These unique nanoarchitectures have exhibited excellent properties in catalysis and drug delivery and as artificial cell and light fillers, etc.12–15 However, most of the successes are limited to the metal, metal chalcogenide and binary metal oxides.16–22 It is still a challenge to fabricate hollow structures of ternary metal oxides.As a family of important functional materials, metal molybdates exbibit interesting optical, electronic and magnetic properties and thus have great potential applications in fields such as photoluminescent materials, microwave devices, optical fibers, scintillator materials, humidity sensors, and catalysis.23–25 Traditionally metal molybdates were prepared via high temperature solid-state reactions.23–25 Recently several solution-phase routes have been employed to controllably prepare this family of materials. For example, Ding et al.26 selectively synthesized molybdate hydrates MMoO4·nH2O (M = Co, Ni, Mn, n = 0, 3/4, 1) nano/microcrystals with different phases and morphologies by a hydrothermal process. Wang et al.27 successfully prepared hollow microspheres of cadmium molybdate via a template-free aqueous solution method with the assistance of NaCl at room temperature. However, it should be noted that these reported procedures are usually time-consuming. Therefore a facile and fast solution-based procedure is still highly desired for the preparation of metal molybdates. Here we report a rapid and economical route based on an efficient microwave-assisted process for preparing FeMoO4 hierarchical hollow microspheres. To the best of our knowledge, there has been no report on the preparation of FeMoO4 hierarchical hollow microspheres. By controlling the experimental parameters, such as reaction time, temperature, iron source and the volume ratio of water and glycerol, novel FeMoO4 hollow spheres have been successfully fabricated. Compared with the reported procedures for FeMoO4,28 the present approach has two main characteristics: (1) it is a simple, rapid and economical route for constructing hierarchical hollow structures; (2) no template, surfactant or other additive is used and no post-treatment is required.
Results and discussion
Fig. 1a depicts the XRD pattern of the product prepared at 190 °C for 10 min under microwave irradiation. All the reflection peaks could be indexed to monoclinic FeMoO4 with calculated lattice constants of a = 10.29 Å, b = 9.37 Å, c = 7.10 Å and β = 106.39°, which is in good agreement with the literature values (JCPDS file Card No. 89-2367). No other impurity peak is detected. Further evidence of the formation of FeMoO4 came from energy dispersion X-ray analysis. Fig. 1b shows the energy dispersion X-ray spectrum (EDS) of the as-prepared product. The peaks of Fe, Mo and O can easily be found. The C peak in the spectrum can be attributed to CO2 adsorbed by the sample. The Cu peak is attributed to the substrate. Quantitative analysis shows that the atomic ratio of Fe to Mo is about 1.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is close to the FeMoO4 stoichiometry.
1, which is close to the FeMoO4 stoichiometry.
 | ||
| Fig. 1 (a) The XRD pattern and (b) EDS spectrum of the product prepared at 190 °C for 10 min under microwave irradiation. | ||
As shown in Fig. 2a and 2b, the as-prepared products are composed of FeMoO4 hierarchical microspheres with relatively good dispersion and uniform diameters of ∼1 µm. Most of these microspheres are closed particles, but some incomplete and open spheres could also be found. A high magnification SEM image of an individual open microsphere reveals that the as-prepared hierarchical microspheres have hollow interiors and the shells are comprised of many irregular nanoparticles (Fig. 2c). The structure and morphology of the sample were also investigated by TEM. Fig. 2d shows a typical TEM image of the product, which further confirms its hollow nature. Based on the observation from Fig. 2, it is clear that the average shell thickness is about 300 nm and the diameters of the building blocks are in the range from 70 to 200 nm.
 | ||
| Fig. 2 (a)–(c) SEM and (d) TEM images of the as-prepared FeMoO4 at 190 °C for 10 min under microwave irradiation. | ||
As a general reducing agent with a relatively high boiling point, glycerol has been widely used in the so-called polyol synthesis of various metal and metal chalcogenide nanoparticles.29–32 In the present reaction, glycerol may play double roles as a reducing agent and solvent in the formation of hierarchical hollow structures. Obviously, the starting Fe(III) source was reduced to Fe(II) and FeMoO4 formed. As a part of the mixture solvent, the volume of glycerol in mixed solvent plays an important role in the preparation of FeMoO4 hierarchical hollow spheres. Irregular open hollow structures were obtained when the volume ratio of H2O/glycerol was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, keeping the total volume constant (Fig. 3a). When the volume ratio was decreased to 2
1, keeping the total volume constant (Fig. 3a). When the volume ratio was decreased to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, many polydispersed hollow spheres were produced (Fig. 3b). It appears that the shells have not been constructed completely and resulted in the formation of open structures. If the volume ratio was controlled at 8
1, many polydispersed hollow spheres were produced (Fig. 3b). It appears that the shells have not been constructed completely and resulted in the formation of open structures. If the volume ratio was controlled at 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, quasi-monodisperse hierarchical microspheres of FeMoO4 were successfully fabricated (Fig. 2a–c). The morphology was preserved while the ratio was controlled at 1
7, quasi-monodisperse hierarchical microspheres of FeMoO4 were successfully fabricated (Fig. 2a–c). The morphology was preserved while the ratio was controlled at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
 | ||
Fig. 3 SEM images of the as-prepared FeMoO4 with a different volume ratio of H2O and glycerol: (a) 4:1, (b) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (c) 1 1 and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, total volume = 15 mL. 2, total volume = 15 mL. | ||
It has been found that the reaction temperature can influence the morphology of the final product. When the reaction was carried out at 160 °C, nanoparticles and hollow spheres coexisted in the final product (Fig. 4a). When the temperature was decreased to 130 °C, larger open spherical structures were obtained (Fig. 4b). These experiments indicate that the reaction temperature plays an important role in the formation of FeMoO4 hierarchical hollow spheres and 190 °C is found to be optimum.
 | ||
| Fig. 4 SEM images of the as-prepared FeMoO4 at different reaction temperatures: (a) 160 °C and (b) 130 °C. | ||
Reaction time is another important factor affecting the chemical reaction. Fig. 5 shows the SEM images of the products obtained from the same system at 190 °C for 30 s, 3 min, and 20 min, respectively. When the reaction was controlled for 30 s or 3 min, spherical hollow structures and many nanoparticles coexisted in the final product (Fig. 5a and b). When the reaction time was prolonged to 10 min (Fig. 2a–c), many hierarchical hollow spheres were fabricated, accompanied by the disappearance of nanoparticles. After 20 min, the morphology of the final product was preserved (Fig. 5c). These experimental observations implied that the nanoparticles were the constructing units of the hollow spheres. With increasing the reaction time, these building units could self-assemble into intact hollow structures.
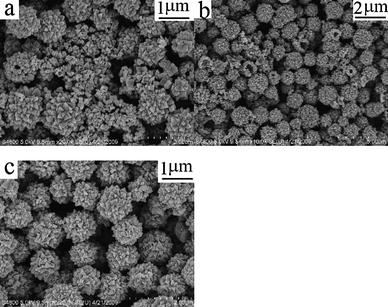 | ||
| Fig. 5 SEM images of the as-prepared FeMoO4 at 190 °C with different reaction times: (a) 30 s, (b) 3 min and (c) 20 min. | ||
Based on the present results, a possible growth mechanism involving bubble-templating coupled with a nanoparticles self-assembly process is proposed. Actually, the gas bubble templating method has already been demonstrated by Li33 and Xie's groups,34 respectively. This method has been proven to be feasible and facile for fabricating various functional hollow spheres.33–37 To demonstrate that the above mechanism could be used to explain the formation of FeMoO4 hollow spheres, a series of experiments were carried out. In the CEM system we used, the pressure of the reaction vessel before and after the reaction can be measured. When the reaction mixture was just heating to 60 °C, the pressure was 0 psi (ESI†, Fig. S1), indicating that the actual pressure was 1 atm. When the reaction terminated and then cooled down to 60 °C, the pressure was found to be 37 psi. This pressure difference (37 psi) implied that some gaseous by-products were simultaneously produced in our reaction system. Interestingly, when Fe(NO3)3·9H2O were replaced by Fe2(SO4)3·7H2O or FeCl3·6H2O, no hollow structure was obtained and the pressure difference was zero (ESI†, Fig. S2). When the experiment was repeated in the absence of (NH4)6Mo7O4·4H2O, the difference also reached 36 psi. The above experimental facts indicated that the gas was just produced in the presence of Fe(NO3)3·9H2O. It is not clear how the gaseous products were produced. Perhaps NO3− might react with glycerol to produce gas bubbles due to its strong oxidizing ability.38 The in-situ produced gas bubble would provide an aggregation center and serve as a growth template. The possible growth mechanism could be described below: (1) Fe(NO3)3 was reduced by glycerol to Fe2+ cations under microwave irradiation and resulted in the formation of FeMoO4 nanoparticles; (2) NO3− anions might react with glycerol to produce massive gas bubbles; (3) the new produced FeMoO4 nanoparticles would be self-assembled at the water/gas interface driven by the interfacial energy minimization, which led to the final FeMoO4 hierarchical hollow microspheres.
The production of a gaseous by-product is a necessity for the bubble template mechanism. However another important factor is that the gas bubble should survive during the self-assembly process. When the volume of glycerol exceeds 5 mL, the viscosity of the mixed solvent is high enough to keep the bubble “alive” for the self-assembly of nanoparticles at the water/gas interface. Therefore, a high medium viscosity is in favor of the self-assembly of nanoparticles and the formation of intact and spherical hollow structures. In contrast, the incomplete hollow structures were formed in a mixed solvent of low viscosity. As in the cases with the volume ratio of H2O and glycerol of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where the gas bubble could be “broken” before the finish of the self-assembly, only incomplete hollow structures were obtained (Fig. 3a and b).
1, where the gas bubble could be “broken” before the finish of the self-assembly, only incomplete hollow structures were obtained (Fig. 3a and b).
In comparison, the same reaction was also carried out under solvothermal conditions. As shown in Fig. S3 (ESI),† hollow structures of FeMoO4 were also obtained, which further confirms the rationality of our mechanism. However it should be mentioned that the as-prepared FeMoO4 spheres by the solvothermal route is not monodispersed and incomplete, which also indicates the uniqueness of the microwave radiation.
Conclusions
In summary, we have developed an economical and efficient microwave-assisted procedure for the synthesis of FeMoO4 hierarchical hollow microspheres. The gas bubbles produced in the process may provide an aggregation center, and these bubbles act as soft templates for the formation of hollow structures. Compared to the existing solution-phase synthetic methods using a solvothermal reaction, this combination of microwave radiation and bubble template provides a facile, high-yield, low-cost pathway to novel FeMoO4 nanoarchitectures.Experimental
All reagents were purchased from Shanghai Chemical Company and used without further purification. All samples were prepared in a microwave system (2.45 GHz, 200 W, Discover S-Class, CEM). The system was equipped with in situ magnetic stirring. The exposure time and temperature were programmed. The automatic temperature-control system allowed continuous monitoring and control (1 °C) of the internal temperature of the reaction systems. The preset profile (desired time and temperature) was followed automatically by continuously adjusting the applied microwave power. In a typical preparation procedure, Fe(NO3)3·9H2O (0.404 g, 1.0 mmol) and (NH4)6Mo7O4·4H2O (0.177 g, 0.143 mmol) was dissolved in 4 mL of distilled water, respectively. First, (NH4)6Mo7O4·4H2O solution was added dropwise to the Fe(NO3)3·9H2O solution under magnetic stirring; then glycerol (7 mL) was added. The solution was transferred to a 35 mL round-bottom flask. After treating the mixture at 190 °C for 10 min under microwave irradiation, it was cooled to room temperature naturally. The product was collected, washed with deionized water and absolute ethanol, and dried in vacuum at 60 °C for 6 h with a yield of 90%.The products were characterized by X-ray powder diffraction (XRD) with a Shimadzu XRD-6000 powder X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å), recorded with 2θ ranging from 10 to 60°. TEM were carried out on a JEM-2100 high resolution transmission microscope, employing an accelerating voltage of 200 kV. SEM images and EDS of the products were obtained on field emission scanning electron microananlysers (Hitachi S-4800), employing an accelerating voltage of 5 kV or 20 kV.
Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2006CB806104 and 2007CB925102), Natural Science Grant of China (No.20721002), and the US National Science Foundation.References
- X. L. Li, T. J. Lou, X. M. Sun and Y. D. Li, Inorg. Chem., 2004, 43, 5442 CrossRef CAS.
- Y. Sun and Y. N. Xia, Anal. Chem., 2002, 74, 5297 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- S. G. Jang, H. K. Yu, D. G. Choi and S. M. Yang, Chem. Mater., 2006, 18, 6103 CrossRef CAS.
- C. N. Lin and M. H. Huang, J. Phys. Chem. C, 2009, 113, 925 CrossRef CAS.
- G. Jia, M. Yang, Y. H. Song, H. P. You and H. J. Zhang, Cryst. Growth Des., 2009, 9, 301 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2004, 126, 8124 CrossRef CAS.
- J. H. Yang, L. M. Qi, C. H. Lu, J. M. Ma and H. M. Cheng, Angew. Chem., Int. Ed., 2005, 44, 598 CrossRef CAS.
- X. B. Cao, L. Gu, L. J. Zhuge, W. J. Gao, W. C. Wang and S. F. Wu, Adv. Funct. Mater., 2006, 16, 896 CrossRef CAS.
- H. G. Yang and H. C. Zeng, J. Phys. Chem. B, 2004, 108, 3492 CrossRef CAS.
- X. W. Lou, Y. Wang, C. L. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, A. R. Marquez and D. A. Weitz, Science, 2002, 298, 1006 CrossRef CAS.
- A. Bigi, E. Boanini, D. Walsh and S. Mann, Angew. Chem., Int. Ed., 2002, 41, 2163 CrossRef CAS.
- H. P. Cong and S. H. Yu, Adv. Funct. Mater., 2007, 17, 1814 CrossRef CAS.
- Z. T. Chen and L. Gao, Cryst. Growth Des., 2008, 8, 460 CrossRef CAS.
- X. X. Li, Y. J. Xiong, Z. Q. Li and Yi Xie, Inorg. Chem., 2006, 45, 3493 CrossRef CAS.
- J. H. Gao, B. Zhang, X. X. Zhang and B. Xu, Angew. Chem., Int. Ed., 2006, 45, 1220 CrossRef CAS.
- D. B. Zhang, L. M. Qi, J. M. Ma and H. M. Cheng, Adv. Mater., 2002, 14, 1499 CrossRef CAS.
- S. Y. Gao, H. J. Zhang, X. M. Wang, R. P. Deng, D. H. Sun and G. L. Zheng, J. Phys. Chem. B, 2006, 110, 15847 CrossRef CAS.
- J. H. Yang and T. Sasaki, Chem. Mater., 2008, 20, 2049 CrossRef CAS.
- B. Liu and H. C. Zeng, Chem. Mater., 2007, 19, 5824 CrossRef CAS.
- S. D. M. Jacques, O. Leynaud, D. Strusevich, A. M. Beale, G. Sankar, C. M. Martin and P. Barnes, Angew. Chem., Int. Ed., 2006, 45, 445 CrossRef CAS.
- W. X. Kuang, Y. N. Fan and Y. Chen, Langmuir, 2000, 16, 5205 CrossRef CAS.
- A. W. Sleight and B. L. Chamberland, Inorg. Chem., 1968, 7, 1672 CrossRef CAS.
- Y. Ding, Y. Wan, Y. L. Min, W. Zhang and S. H. Yu, Inorg. Chem., 2008, 47, 7813 CrossRef CAS.
- W. S. Wang, L. Zhen, C. Y. Xu, B. Y. Zhang and W. Z. Shao, J. Phys. Chem. B, 2006, 110, 23154 CrossRef CAS.
- ü. Kersen and L. Holappa, Appl. Phys. A: Mater. Sci. Process., 2006, 85, 431 CrossRef CAS.
- Q. Y. Yu, C. Y. Liu, Z. Y. Zhang and Y. Liu, J. Phys. Chem. C, 2008, 112, 2266 CrossRef CAS.
- Z. P. Liu, J. B. Liang, S. Li, S. Peng and Y. T. Qian, Chem.–Eur. J., 2004, 10, 634 CrossRef CAS.
- M. H. Ullah, K. Il and C. S. Ha, Mater. Lett., 2006, 60, 1496 CrossRef CAS.
- L. X. Yang, Y. J. Zhu, L. Li, L. Zhang, H. Tong, W. W. Wang, G. F. Cheng and J. F. Zhu, Eur. J. Inorg. Chem., 2006, 4787 CrossRef CAS.
- Q. Peng, Y. J. Dong and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3027 CrossRef CAS.
- C. Z. Wu, Y. Xie, L. Y. Lei, S. Q. Hu and C. Z. Yang, Adv. Mater., 2006, 18, 1727 CrossRef CAS.
- Y. S. Han, G. Hadiko, M. Fuji and M. Takahashi, Chem. Lett., 2005, 34, 152 CrossRef CAS.
- X. Fan, Z. Zhang, G. Li and N. A. Rowson, Chem. Eng. Sci., 2004, 59, 2639 CrossRef CAS.
- C. L. Jiang, W. Q. Zhang, G. F. Zou, W. C. Yu and Y. T. Qian, Nanotechnology, 2005, 16, 551 CrossRef CAS.
- X. Zhou, H. B. Yao, Q. Zhang, J. Y. Gong, S. J. Liu and S. H. Yu, Inorg. Chem., 2009, 48, 1082 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Variation curve of pressure as a function of time; variation curve of temperature as a function of time; SEM images of the as-prepared FeMoO4 at 190 °C with different iron sources; SEM image of the as-prepared FeMoO4 using solvothermal methods at 190 °C for 6 h. See DOI: 10.1039/b912555h |
| This journal is © The Royal Society of Chemistry 2010 |
