DOI:
10.1039/B911401G
(Paper)
CrystEngComm, 2010,
12, 199-206
Received
10th June 2009
, Accepted 13th August 2009
First published on
3rd September 2009
Abstract
Yttrium fluoride such as Na(Y1.5Na0.5)F6 and α-NaYF4; YF3 has been successfully synthesized via a salt-assisted hydrothermal method in the presence of sodium nitrate. The morphology and chemical composition of the as-obtained products can be controlled by monitoring the reaction parameters inclusive of the time-zero concentration of salt solution, reaction temperature and time. The as-prepared products cover Na(Y1.5Na0.5)F6, α-NaYF4 and YF3, while the corresponding morphology changes from spherical aggregation to octahedron, which were characterized by XRD, SEM, and TEM. Moreover, LnF3 (Ln = La, Nd, Eu, Gd) can also be synthesized using the same method. Further studies reveal that the morphology and composition of the as-synthesized lanthanide fluoride can be determined mainly by the ionic radius of rare earth and the concentration of NaNO3. It is emphasized that the sodium nitrate plays an important role in such control of the differentiated products. Apart from these findings, the photoluminescent properties of Eu3+:YF3 octahedral crystals are further investigated.
Introduction
It is of importance to control the inorganic crystals of uniform dimension and shape so as to obtain their differentiated properties.1 To date, many chemical methods have been developed for the synthesis of varied inorganic crystals in well-defined shapes, including rods, whiskers and microboxes.2–4 The commonly used methods take advantage of monitoring the nucleation and growth processes, for example, manipulating the controllable growth parameters using different molecular precursors and capping agents at reaction temperature for certain growth time. In general, organic additives functioning as the “shape modifiers” are favoring the control of the morphology and size of the crystals because they either promote or inhibit crystal growth through modifying the crystal facets dynamically.5 Besides, a cost-effective and environmentally-friendly synthetic method becomes more popular for obtaining such nano-crystals. Nowadays, one of the challenges facing the chemists and scientists is to develop a facile approach for the creation of controllable morphology.
Although organic surfactants or polymers appear to be effective in tailoring the crystal growth and the morphology of products through controlling the reaction rate and selectively altering the growth kinetics of different crystal facets, some organic additives are required for different purposes, which pose potential dangers of changing the properties of products. Therefore, it is indispensable to choose suitable and novel additives in the synthetic process. In the past several years, some attractive attempts have been made in the synthesis of nanomaterials through a salt-mediated process.6 The addition of salt avoids the occurrence of impurities and effectively controls the morphology of the final product. On the other hand, when a metal ionic compound (such as precursor) is dissolved in the aqueous solution, the metal ions are usually covalently bonded to other ions or water molecules because of their particular value of charge and some characteristic “combining power” according to Alfred Werner's proposal. The specific number of molecules or ions combined with a metal ion is now referred to as the coordination numbers of the element (typically four or six). The water molecules covalently bonded to the central metal ion are called hydrate ligands. It is worth mentioning that NO3− ions can replace water molecules and combine with the rare earth metal ion to form a new ligand in high NO3−(∼8 M) ionic solution, which would further affect the chemical reactions.7 In the salt-mediated process, the effects of inorganic salts on the inorganic materials include: first, the inorganic ions (in the salt-assisted synthesis) are expected to have a more pronounced influence on the nucleation process of nanocrystallites than that of organic surfactants or polymers because of their smaller size, which can increase the ability to form complexes with reactive species and decrease the reaction rate of reaction ions in the solution; second, the addition of inorganic ions might also affect the growth stages through selectively altering the growth kinetics of different crystal facets of the final products, similar to the role of surfactants in controlling the formation of nanomaterials.8 Herein, sodium nitrate is chosen to synthesize rare earth fluorides and is proven to be useful for controlling the composition and morphology.
YF3, a well-known functional rare earth fluoride, has been extensively studied and employed in various fields including lamps and display devices,9 phosphors,10 and scintillators.11 Stimulated by both the promising applications and the interesting properties, much attention has been directed to the controlled synthesis of YF3 nanostructures. Over the past few years, considerable progress has been made in the synthesis of YF3 nanostructures of different shapes and in the investigation of their size/shape-dependent properties.12 YF3 crystals with different morphologies according to different procedures and synthetic techniques are obtained, such as truncated octahedral-, quadrilateral-, hexagonal-, spherical-, octahedral-, and bundle-like nanocrystals.10,13 Of these methods, the hydrothermal method is by far the most reported for its merits. However, in previous reports about the nanosized YF3 prepared by the hydrothermal method, some special organic surfactants or templates were used.12, 14 So, it is necessary to develop a facile, economical and effective method for preparing YF3 with a controllable composition and morphology. On the other hand, though various morphologies of YF3 have been obtained, the investigation of the relationship between different morphologies is still crucial for realizing the morphology-controlled synthesis.
In this paper, a salt-assisted hydrothermal method has been developed to control-synthesize yttrium fluorides, such as Na(Y1.5Na0.5)F6 shuttles, α-NaYF4 nanospheres, YF3 particles with a spindle, octahedral and truncated octahedral shape. It is found that the composition and morphology of the obtained product can be controlled using inorganic species like sodium nitrate. Furthermore, other light rare earth fluorides (LaF3, PrF3, EuF3, GdF3) have also been prepared by this method.
Experimental
Synthesis of YF3 nanooctahedron
All reagents (analytical grade) were purchased from Shanghai Chemical Reagent Company and used without further purification. In a typical procedure, yttrium nitrate hexahydrate (Y(NO3)3·6H2O) (0.107 g, 0.28 mmol) was first dissolved into 28 ml NaNO3 (7 M) solution, and then 0.035 g (0.84 mmol) NaF was added and stirred for 10 min. The clear and colorless solution was transferred into a 35 ml Teflon-lined autoclave, and then the autoclave was filled up to 80% of its total volume. Afterwards the autoclave was heated up to 140 °C and held for 20 h, then allowed to cool down to room temperature naturally. The obtained samples were collected after being centrifugally separated at 3500 rpm for 20 min, washed with deionized water and dried at 60 °C in air. The sample Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 (molar ratio Eu3+/Y3+ = 1
YF3 (molar ratio Eu3+/Y3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was prepared using similar procedures. The detailed reaction parameters and the corresponding results are summarized in Table 1.
9) was prepared using similar procedures. The detailed reaction parameters and the corresponding results are summarized in Table 1.
Table 1 All reaction parameters and the corresponding resultsa
| Sample |
[NaNO3]/M |
Time/h |
Temperature/°C |
Compounds |
Morphology of product |
|
All samples were obtained with NaF and Ln(NO3)3·6H2O (Ln = Y, La, Nd, Eu, Gd) as precursors.
|
| S1 |
0 |
20 |
25 |
YF3 |
nanobones |
| S2 |
0.1 |
20 |
25 |
YF3 |
nanospindles |
| S3 |
1 |
20 |
25 |
Na(Y1.5Na0.5)F6 |
nanoshuttles |
| S4 |
7 |
20 |
25 |
α-NaYF4 |
nanospheres |
| S5 |
7 |
20 |
60 |
α-NaYF4 |
nanospheres |
| S6 |
7 |
20 |
100 |
YF3 |
truncated and regular octahedra |
| S7 |
7 |
20 |
140 |
YF3 |
monodisperse octahedra |
| S8 |
7 |
1 |
140 |
α-NaYF4 |
nanospheres |
| S9 |
0 |
20 |
140 |
YF3 |
nanobones |
| S10 |
0.1 |
20 |
140 |
YF3 |
nanoplates |
| S11 |
3 |
20 |
140 |
YF3 |
nanoparticles |
| S12 |
7 |
20 |
140 |
LaF3 |
nanoplates |
| S13 |
7 |
20 |
140 |
NdF3 |
nanoplates |
| S14 |
7 |
20 |
140 |
EuF3 |
nanoplates |
| S15 |
7 |
20 |
140 |
GdF3 |
olive-like nanoparticles |
Structural characterization
The phase of as-prepared products was carried out using powder X-ray diffraction (XRD, Shimadzu XRD-6000) with Cu Kα radiation (λ = 1.5406 Å) 2θ from 10 to 80° at a scanning rate of 6° min−1. The X-ray tube voltage and current were set at 40 kV and 30 mA, respectively. The morphologies and structure of the samples were characterized using high-resolution transmission electron microscopy (HRTEM; JEOL, 2100F, with an accelerating voltage of 200 kV) and field-emission scanning electron microscopy (FESEM; JEOL, JSM-6700F with an accelerating voltage of 5 kV), respectively. The obtained solid samples were suspended in absolute ethanol by an ultrasonic treatment for 5 min. Then a drop of the well-dispersed suspension was trickled on a piece of carbon-coated copper grid, and the copper grid was air-dried in ambient conditions. Photoluminescence (PL) was investigated on Perkin Elmer LS50B photoluminescence spectrophotometer by dispersing samples in alcohol.
Results and discussion
LnF3 (Ln = Y, La, Nd, Eu, Gd) was synthesized in a salt-assisted solution, as summarized in Table 1. A typical XRD pattern of the as-obtained products (sample 7) is shown in Fig. 1a. It can be seen that the pattern fits well with the orthorhombic type YF3 [space group: pnma (62)] with lattice constants a = 6.353, b = 6.850 and c = 4.393 Å (JCPDS 74–0911). No peaks of any other phases or impurities can be detected, indicating its high purity. The chemical composition of the as-prepared product was further confirmed by EDX. It can be seen from Fig. 1b that only yttrium and fluorine are detected in the spectrum, and the atomic ratio of F to Y is 73.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.66, which is very close to the stoichiometric YF3.
26.66, which is very close to the stoichiometric YF3.
The as-obtained product was further characterized by field emission scanning electron microscopy (FESEM) and high-resolution transmission electron microscopy (HRTEM). A typical SEM image of sample 6 is shown in Fig. 2a. The monodisperse YF3 particles can be prepared on a large scale, and the as-synthesized particles possess a regular and well-defined octahedral shape. It is detected that YF3 octahedra have triangular surfaces and sharp edges, and the edge length ranges from 3 to 3.5 µm (Fig. 2b). The bright field TEM image of an individual YF3 particle also reveals that the as-obtained YF3 is in rhombic shape (Fig. 2c). The diffraction spots are indexed to the (020) and (211) planes of orthorhombic YF3, respectively, indicating that the YF3 octahedron is a well-developed single crystal (inset of Fig. 2c). As illustrated in Fig. 2d, the 0.33 nm interplanar lattice shown in HRTEM image (recorded on the tip of the rhombus) corresponds to the (111) planes of YF3, indicating that the well-defined octahedral YF3 crystal is enclosed by eight {111} planes.
 |
| | Fig. 2 Images of YF3 particles prepared at 140 °C for 20 h: (a) low-magnification SEM image; (b) enlarge SEM image; (c) TEM image of an individual YF3 octahedron, the inset gives the corresponding SAED; (d) HRTEM image of the YF3 octahedron. | |
In the salt-assisted synthetic process, the concentration of NaNO3, the reaction temperature and time have a great effect on the chemical composition and morphology of the final product. A series of condition-dependent experiments have been carried out to understand the characteristic effects of inorganic salt on the nucleation and crystal growth processes, and further to uncover the underlying mechanism for the controllable composition and shape.
The effect of NaNO3 concentration
The influence of NaNO3 concentration on the chemical composition and morphology of the final products was investigated. When the reaction was carried out at room temperature, a large quantity of uniform and dumbbell-like YF3 (sample 1) structures, with 160 nm in diameter and 3.5 µm in length, could be obtained in the absence of NaNO3 salt. The obtained nanobones consist of many nanowires with a small size (Fig. 3a). However, when 0.1 M NaNO3 was added into the reaction system, spindle-like YF3 (sample 2) particles were obtained with length ranging from 150 nm to 3.5 µm. They are still composed of bundles of small nanowires (Fig. 3b). Further increasing the concentration of NaNO3 to 1 M, nearly monodiseperse Na(Y1.5Na0.5)F6 (sample 3) nanoshuttles were obtained in large quantity (see the ESI, Fig. S1).† These shuttles of 1.2–2 µm in length actually comprised many little nanoparticles (Fig. 3c). When the concentration of NaNO3 was increased to 7 M, monodisperse α-NaYF4 (sample 4) nanospheres (Fig. 3d) formed (see the ESI, Fig. S2).† These nanospheres of 100–500 nm in diameter also aggregated from many primary nanoparticles.
 |
| | Fig. 3 SEM images of the products prepared at room temperature for 20 h with different concentrations of NaNO3: (a) YF3, 0 M; (b) YF3, 0.1 M; (c) Na(Y1.5Na0.5)F6, 1 M; (d) α-NaYF4, 7 M. | |
By increasing the reaction temperature up to 140 °C, YF3 could be obtained even in the higher concentration of NaNO3. However, the concentration of NaNO3 still affected the morphology of the obtained products. When the reaction was carried out without NaNO3, bone-like YF3 (sample 9) nanostructures were obtained (Fig. 4a). In comparison with the sample prepared at room temperature, these uniform bone-like YF3 particles are assembled by the original nanoparticles of about 400–600 nm in diameter and 700–1500 nm in length, respectively. By increasing NaNO3 concentration to 0.1 M, the YF3 nanoplates of 40–500 nm in size (sample 10) formed and aggregated loosely (Fig. 4b). When the concentration of NaNO3 increased up to 3 M, irregular YF3 particles (sample 11) conglomerated into larger particles in an octahedral shape (Fig. 4c). When a higher concentration of NaNO3 (7 M) was used, monodisperse YF3 octahedra were prepared on a large scale (Fig. 2a). To further confirm the uniqueness in changing the morphology and composition of YF3, other salts such as KNO3 and NaNO2 at an equal molar concentration were used. It was found that no YF3 or other pure compounds could be obtained, suggesting that these salts could not play the same role as did NaNO3 in the synthesis process. The reasonable explanation for the uniqueness of NaNO3 include: first, NaNO3 can replace water molecules and attach the Y3+ ion to form a ligand; second, it can affect the reaction rate according to Le Chatelier's principle; third, the properties of NaNO3 (such as the ionic radius, molecular weight, solubility, etc) have some influence on the synthesis.
 |
| | Fig. 4 SEM images of YF3 obtained at 140 °C for 20 h with different concentrations of NaNO3: (a) 0 M; (b) 0.1 M; (c) 3 M. | |
In traditional crystal growth, both nucleation and growth get involved in the preparation of nanostructured materials. To meet the requirements of homogeneous nucleation, the reaction should be carried out at high temperature to surmount the energy barrier, and the source materials should reach high supersaturation to promote nucleation. After the formation of nuclei, the constituents will be incorporated to the existing nuclei with less energy than that for nucleation, and such post-growth would be largely controlled by the kinetic parameters adopted in the reaction system and responsible for the morphology variation.15,16 Based on the above results, an acceptable explanation can be presented in the salt-assisted process. In a low salt concentration, the improvement of the reaction temperature may diminish the separation between the two stages. At room temperature, the rare earth ions are presumably more favorable to nucleation and oriented growth, resulting in formation of nanowires. On the contrary, when the reaction temperature increases, particle nucleation is fast followed by the aggregation of small particles caused by nanoparticles with reduced surface energy. This result proves that temperature is crucial to the shape of products in a low salt concentration. The higher concentration of salt would increase the ionic intensity and decrease the diffusion of ions or growing units, which would further reduce the net reaction rate according to Le Chatelier's principle and suppress the nucleation formation of YF3 nanocrystal at room temperature. Thus YF3 can be obtained with less NaNO3 at lower reaction temperature or with more NaNO3 at high reaction temperature (140 °C) because it is easy to reach a degree of supersaturation for the nucleation of YF3. However, the thermodynamic stable hexagonal Na(Y1.5Na0.5)F6 shuttle and cubic α-NaYF4 nanospheres are apt for the formation at high NaNO3 concentration and lower reaction temperature because they reach easily their supersaturation degree in advance in the micro-environment.17–19 The formation of shuttles and nanospheres can be well understood by the reported two-stage growth model in which nanosized Na(Y1.5Na0.5)F6 and α-NaYF4 are nucleated first in supersaturated solution and then the initially formed original particles aggregate into large secondary particles.19 The above experiments clearly demonstrate the presence of NaNO3 is beneficial for the control of both the shape and chemical composition of the products.
The effect of the reaction temperature
Under a high concentration of NaNO3, the reaction temperature is also crucial to the composition and morphology of the final products. Monodisperse α-NaYF4 (sample 5) nanospheres were obtained if the reaction temperature was lower than 60 °C (Fig. 5a). Their size is also similar to that of sample 4. However, YF3 (sample 6) particles in truncated and regular octahedral shape, with an average edge size of about 700 nm in length, were obtained when the reaction was carried out at 100 °C under similar conditions (Fig. 5b). It can be observed that the truncated octahedra also have a smooth surface and sharp edges (inset in Fig. 5b). Regular and well-defined YF3 octahedra could be obtained if the reaction temperature was further increased to 140 °C (Fig. 2a). The size of the obtained YF3 is increased to 3–3.5 µm. On the basis of the above experiment, it is reasonable to conclude that the elevated reaction temperature accelerates the motion of ions and facilitates the nucleation of YF3 between the two constituent ions in solution. These experiments further demonstrate the high motion/diffusion of ions is in favor of the formation of YF3 and the NaNO3 can effectively regulate its morphology.
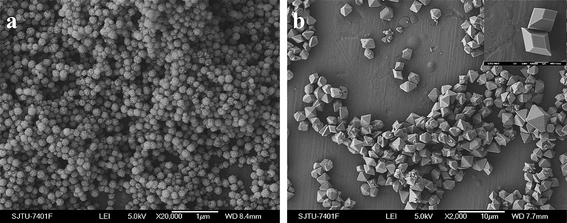 |
| | Fig. 5 SEM images of the products obtained with 7 M NaNO3 for 20 h at different reaction temperatures: (a) α-NaYF4, 60 °C; (b) YF3, 100 °C. | |
The effect of reaction time
To further investigate the role of NaNO3 in the composition and shape of the as-prepared products, samples taken from different time intervals at a high NaNO3 concentration (7 M) were characterized by XRD and TEM. The XRD patterns reveal that only α-NaYF4 (sample 8) is obtained after 1 h. With increasing reaction time, YF3 appears gradually and then α-NaYF4 disappears. α-NaYF4 completely transforms into YF3 as the reaction time is up to 20 h (Fig. 6a). This result implies that a longer reaction time would be favorable for the formation of the YF3 product at high NaNO3 concentration. Similarly, the corresponding SEM images are consistent with the observation. Monodisperse α-NaYF4 nanospheres are obtained when the reaction is 1 h (Fig. 6b). Then, truncated or regular octahedral YF3 particles appear gradually with the reaction time increasing to 15 h and the size of YF3 particles grows from 1 to 2 µm (Fig. 6c,d). As the reaction proceeds to 20 h, octahedral YF3 particles are obtained with all nanospheres vanishing and the size of octahedral YF3 particles in the range of 3–3.5 µm (Fig. 6e). It is evident to conclude that the YF3 octahedra formed through the dissolution-crystallization process at the cost of α-NaYF4 nanospheres and the transformation of chemical composition was from the metastable α-NaYF4 phase to the stable YF3 phase with extending the reaction time.
 |
| | Fig. 6 (a) XRD patterns of the products synthesized at 140 °C with 7 M NaNO3 for different reaction times; (b–e) SEM images of the as-prepared product for (b) 1 h; (c) 5 h; (d) 15 h; (e) 20 h, respectively. Other reaction parameters were unchanged. | |
The formation mechanism of octahedron
For the growth of inorganic crystals, the organic additives including surfactants or polymers play an important role in the controlled synthesis of the final products because of their coordination and selective absorption effects on different crystal facets. Furthermore, most of the reactions occur in an aqueous solution, thus the ion concentrations and the effects from salts also exert a great influence on the crystal growth. In our experiment, when Y(NO3)3 and NaF are dissolved in the aqueous of NaNO3, the Y3+ and F− ions would be surrounded by the opposite charge NO3− and Na+ ions owing to electrostatic interactions. This result would decrease the collision probability and the net reaction rate between the Y3+ and F− ions, and further reduce the nucleation of YF3 crystallites. However, increasing the reaction temperature and time can overcome these shortcomings, and promote the nucleation of YF3. On the other hand, the added NaNO3 also has similar influence on the shape-controlled crystal growth to the organic additives selectively modifying the different facets of inorganic crystal. Furthermore, from the crystal structure of YF3 (Fig. 7), the zirconium type of structure of YF3 consists of YF8 dodecahedron, and the YF8 units are stacked alternately along its axes. The interplanar lattice of (100), (010) and (001) is 6.353, 6.858 and 4.393 Å, respectively, which is much larger than that of (111) faces (3.1959 Å), leaving much space along the axes for other ions attacking. This structure results in a relatively low growth rate in the [111] direction because the active points of (111) faces are greatly restricted by NaNO3, and further leads to the formation of YF3 octahedron. As illustrated in Scheme 1, the formation of the YF3 octahedron can be rationally expressed as a kinetically controlled dissolution-crystallization mechanism through the reaction temperature and time.20 In conclusion, the main effect of NaNO3 on the growth of YF3 nanocrystals comprises two aspects, one is to limit the rate of the chemical reaction through interaction between the yttrium and fluorine ions; the other is to exert dynamic effect on the growth of different crystal facets.
![Crystal structure of YF3 octahedral nanocrystals viewed (a) along the [111] direction and (b) along the [010] direction.](/image/article/2010/CE/b911401g/b911401g-f7.gif) |
| | Fig. 7 Crystal structure of YF3 octahedral nanocrystals viewed (a) along the [111] direction and (b) along the [010] direction. | |
 |
| | Scheme 1 Schematic illustration of the formation of YF3 octahedron. | |
Optical properties of Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) YF3 crystals
YF3 crystals
Fig. 8 shows the room temperature emission spectra of Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 nanocrystals excited at 224 nm short-wavelength. All emission peaks at λ = 579 (5D0-7F0), 592 (5D0-7F1), 612 (5D0-7F2), 657 nm (5D0-7F3) have been assigned in Fig. 8, and there is no notable shift in the positions of emission peaks compared to other Eu3+-doped system,21 because the 4f energy levels of Eu3+ are hardly affected by the crystal field.12 The electric dipole transition 5D0-7F2 is the primary group in the emission spectrum of the sample. The 5D0-7F1 and 5D1-7F1 transitions are magnetic dipole allowed and their intensities are almost independent of the local environment around Eu3+ ions.22 The 5D0-7F2 transition is allowed due to an admixture of opposite parity 4fn−15d states by odd parity crystal-field component.21,23 Therefore, its intensity is sensitive to the local structure around the Eu3+ cation. The 5D0-7F3 transition exhibits a mixture of magnetic dipole and electric dipole character.21 Reduction of particle size and the high concentration of Eu3+ cause the degree of lattice distortion and corresponding lower symmetry of the crystal field around the Eu3+ ions, results in the blue shift of excitation, the primary emission of 5D0-7F2 transition and no splitting of 5D0-7Fj (J = 1, 2, 3) transition.12,24
YF3 nanocrystals excited at 224 nm short-wavelength. All emission peaks at λ = 579 (5D0-7F0), 592 (5D0-7F1), 612 (5D0-7F2), 657 nm (5D0-7F3) have been assigned in Fig. 8, and there is no notable shift in the positions of emission peaks compared to other Eu3+-doped system,21 because the 4f energy levels of Eu3+ are hardly affected by the crystal field.12 The electric dipole transition 5D0-7F2 is the primary group in the emission spectrum of the sample. The 5D0-7F1 and 5D1-7F1 transitions are magnetic dipole allowed and their intensities are almost independent of the local environment around Eu3+ ions.22 The 5D0-7F2 transition is allowed due to an admixture of opposite parity 4fn−15d states by odd parity crystal-field component.21,23 Therefore, its intensity is sensitive to the local structure around the Eu3+ cation. The 5D0-7F3 transition exhibits a mixture of magnetic dipole and electric dipole character.21 Reduction of particle size and the high concentration of Eu3+ cause the degree of lattice distortion and corresponding lower symmetry of the crystal field around the Eu3+ ions, results in the blue shift of excitation, the primary emission of 5D0-7F2 transition and no splitting of 5D0-7Fj (J = 1, 2, 3) transition.12,24
 |
| | Fig. 8 Room temperature (a) excitation (λem = 614 nm) and (b) emission (λex = 224 nm) spectra of a Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 sample. YF3 sample. | |
The preparation of light lanthanide fluoride LnF3 (Ln = La, Nd, Eu, Gd)
It is well known that the series of rare earth element is frequently divided into two groups based on the atomic weight and chemical properties. The “light” rare earths consist of elements with atomic number 57 to 63, while the “heavy” rare earths consist of elements 64–71 as well as scandium and yttrium because of similar chemical behavior. Although the crystal phase of LnF3 (Ln = La–Lu) belongs to the hexagonal or orthorhombic system, it is interesting to find that only light rare earth fluoride, such as LnF3 (Ln = La, Nd, Eu, Gd) (sample 12–15), can be hydrothermally synthesized at 7 M NaNO3 under similar conditions (see ESI, Fig. S3†). The TEM of LaF3, NdF3, EuF3 and GdF3 are shown in Fig. 9, nanoplates of LaF3, NdF3 and EuF3 can be obtained (Fig. 9a-c). While the as-prepared GdF3 (Fig. 9d) has an olive-like structure with about 250–600 nm in length and 300 nm in diameter, assembled by many nanoplates, which is much different from the YF3 octahedron prepared in similar conditions. Owing to the lanthanide contraction, there is a gradual decrease in the radius of rare earth with the increase of its atomic number. So LnF3 (Ln = La, Nd, Eu, Gd) can also be prepared at high NaNO3 concentration because the larger radius of light rare earth element increases the probability of ionic collision between Ln3+ and F− and the nucleation of LnF3. These results imply that the shape and composition of LnF3 are principally dependent on the radius of rare earth and the concentration of NaNO3.
 |
| | Fig. 9 TEM images of LnF3 obtained at 140 °C with 7 M NaNO3 for 20 h: (a) LaF3; (b) NdF3; (c) EuF3; (d) GdF3. | |
Conclusion
In summary, different kinds of yttrium fluoride (Na(Y1.5Na0.5)F6; α-NaYF4; YF3) have been successfully fabricated by using a one-step and facile hydrothermal procedure. The results indicate that a different chemical composition and morphology of yttrium fluoride can be controlled effectively by adjusting the reaction condition. A possible growth mechanism of the YF3 octahedron is proposed. This method can be extended to synthesizing light rare earth fluoride, and their chemical composition and morphology can also be controlled effectively. Further studies reveal that the morphology and composition of the as-synthesized LnF3 are determined mainly by the ionic radius of rare earth and the concentration of NaNO3. This work may present a way for the shape-controlled synthesis of other inorganic materials. The simple composition and morphology controllable synthesis of yttrium fluoride holds promise for application in photoluminescence devices.
Acknowledgements
The work described here was supported by the National Science Foundation of China (No: 20671061), the Programme for New Century Excellent Talents of Education Ministry of China, and National Basic Research Programme of China (2009CB930400 and 2007CB209705).
References
-
(a) H. Li, R. Liu, R. X. Zhao, Y. F. Zheng, W. X. Chen and Z. D. Xu, Cryst. Growth Des., 2006, 6, 2795 CrossRef CAS;
(b) C. X. Li, X. M. Liu, P. P. Yang, C. M. Zhang, H. Z. Lian and J. Lin, J. Phys. Chem. C, 2008, 112, 2904 CrossRef CAS;
(c) Z. L. Wang, Z. W. Quan, P. Y. Jia, C. K. Lin, Y. Luo, Y. Chen, J. Fang, W. Zhou, C. J. O'Connor and J. Lin, Chem. Mater., 2006, 18, 2030 CrossRef CAS.
- J. Q. Hu, Q. Chen, Z. X. Xie, G. B. Han, R. H. Wang, B. Ren, Y. Zhang, Z. L. Yang and Z. Q. Tian, Adv. Funct. Mater., 2004, 14, 183 CrossRef CAS.
- J. Q. Hu, Q. Li, N. B. Wong, C. S. Lee and S. T. Lee, Chem. Mater., 2002, 14, 1216 CrossRef CAS.
- F. H. Zhao, W. J. Lin, M. M. Wu, N. S. Xu, X. F. Yang, Z. R. Tian and Q. Su, Inorg. Chem., 2006, 45, 3256 CrossRef CAS.
-
(a) X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Adv. Mater., 2006, 18, 2031 CrossRef CAS;
(b) C. X. Li, J. Yang, P. P. Yang, X. M. Zhang, H. Z. Lian and J. Lin, Cryst. Growth Des., 2008, 8, 923 CrossRef CAS.
-
(a) S. K. Im, Y. T. Lee, B. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2154 CrossRef;
(b) L. Yan, R. B. Yu, J. Chen and X. R. Xin, Cryst. Growth Des., 2008, 8, 1474 CrossRef CAS;
(c) Q. Wu, F. Zhang, P. Xiao, H. S. Tao, X. Z. Wang, Z. Hu and Y. N. Lü, J. Phys. Chem. C, 2008, 112, 17076 CrossRef CAS;
(d) T. Xia, Q. Li, X. D. Liu, J. Meng and X. Q. Cao, J. Phys. Chem. B, 2006, 110, 2006 CrossRef CAS;
(e) C. X. Li, Z. W. Quan, P. P. Yang, J. Yang, H. Z. Lian and J. Lin, J. Mater. Chem., 2008, 18, 1353 RSC.
-
Q. Su, Rare Earth of Chemistry, Science and Technology Press of Chinese Henan Province, 1993, p 108 Search PubMed.
-
(a) T. Herricks, J. Chen and Y. Xia, Nano Lett., 2004, 4, 2367 CrossRef CAS;
(b) K. E. Korte, S. E. Skrabalak and Y. Xia, J. Mater. Chem., 2008, 18, 437 RSC;
(c) M. H. Kim, B. Lim, E. P. Lee and Y. Xia, J. Mater. Chem., 2008, 18, 4069 RSC;
(d) S. H. Yu and H. Cölfen, J. Mater. Chem., 2004, 14, 2124 RSC;
(e) S. H. Yu, H. Cölfen and M. Antonietti, J. Phys. Chem. B, 2003, 107, 7396 CrossRef CAS;
(f) S. H. Yu, Top. Curr. Chem., 2007, 271, 79 CAS.
- L. Beauzamy, B. Moine and P. Gredin, J. Lumin., 2007, 127, 568 CrossRef CAS.
- R. X. Yan and Y. D. Li, Adv. Funct. Mater., 2005, 15, 763 CrossRef CAS.
- M. Nikl, A. Yoshikawa, A. Vedda and T. Fukuda, J. Cryst. Growth, 2006, 292, 416 CrossRef CAS.
-
(a) M. F. Zhang, H. Fan, B. J. Xi, X. Y. Wang, C. Dong and Y. T. Qian, J. Phys. Chem. C, 2007, 111, 6652 CrossRef CAS;
(b) F. Tao, Z. J. Wang, L. Z. Yao, W. L. Cai and X. G. Li, J. Phys. Chem. C, 2007, 111, 3241 CrossRef CAS.
-
(a) J. L. Lemyre and A. M. Ritcey, Chem. Mater., 2005, 17, 3040 CrossRef CAS;
(b) Y. Cheng, Y. S. Wang, Y. H. Zheng and Y. Qin, J. Phys. Chem. B, 2005, 109, 11548 CrossRef CAS;
(c) X. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3497 CrossRef CAS;
(d) X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Nature, 2005, 437, 121 CrossRef CAS;
(e) Y. W. Zhang, X. Sun, R. Si, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2005, 127, 3260 CrossRef CAS;
(f) M. Wang, Q. L. Huang, J. M. Hong and X. T. Chen, Mater. Lett., 2007, 61, 1960 CrossRef CAS;
(g) M. Wang, Q. L. Huang, H. X. Zhong, X. T. Chen, Z. L. Xue and X. Z You, Cryst. Growth Des., 2007, 7, 2106 CrossRef CAS.
-
(a) C. X. Li, J. Yang, P. P. Yang, H. Z. Lian and J. Lin, Chem. Mater., 2008, 20, 4317 CrossRef CAS;
(b) F. Tao, Z. J. Wang, L. Z. Yao, W. L. Cai and X. G. Li, Cryst. Growth Des., 2007, 7, 854 CrossRef CAS.
- X. Ji, X. Song, J. Li, Y. Bai, W. Yang and X. Peng, J. Am. Chem. Soc., 2007, 129, 13939 CrossRef CAS.
- Z. P. Peng, Y. S. Jiang, Y. H. Song, C. Wang and H. J. Zhang, Chem. Mater., 2008, 20, 3153 CrossRef CAS.
- L. Y. Wang and Y. D. Li, Nano Lett., 2006, 6, 1645 CrossRef CAS.
- C. X. Li, J. Yang, Z. W. Quan, P. P. Yang and J. Lin, Chem. Mater., 2007, 19, 4933 CrossRef CAS.
-
(a) J. P. Ge, Y. X. Hu, M. Baisini, W. P. Beyermann and Y. D. Yin, Angew. Chem., Int. Ed., 2007, 46, 4342 CrossRef CAS;
(b) S. Libert, V. Gorshkov, D. Goia, E. Matijević and V. Privman, Langmuir, 2003, 19, 10679 CrossRef CAS;
(c) H. Deng, X. L. Li, Q. Peng, X. Wang, J. P. Chen and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 2782 CrossRef CAS;
(d) X. Liang, X. Wang, Y. Zhuang, B. Xu, S. M. Kuang and Y. D. Li, J. Am. Chem. Soc., 2008, 130, 2736 CrossRef CAS.
- C. X. Li, Z. W. Quan, J. Yang, P. P. Yang and J. Lin, Inorg. Chem., 2007, 46, 6329 CrossRef CAS.
- G. S. Ofelt, J. Chem. Phys., 1962, 37, 511 CrossRef.
- S. Ray, P. Pramanik, A. Singha and A. Roy, J. Appl. Phys., 2005, 97, 094312 CrossRef.
- B. R. Judd, Phys.
Rev., 1962, 127, 750 CrossRef CAS.
- N. Zhang, W. B. Bu, Y. P. Xu, D. Y. Jiang and J. L. Shi, J. Phys. Chem. C, 2007, 111, 5014 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 (molar ratio Eu3+/Y3+ = 1
YF3 (molar ratio Eu3+/Y3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was prepared using similar procedures. The detailed reaction parameters and the corresponding results are summarized in Table 1.
9) was prepared using similar procedures. The detailed reaction parameters and the corresponding results are summarized in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.66, which is very close to the stoichiometric YF3.
26.66, which is very close to the stoichiometric YF3.






![Crystal structure of YF3 octahedral nanocrystals viewed (a) along the [111] direction and (b) along the [010] direction.](/image/article/2010/CE/b911401g/b911401g-f7.gif)

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) YF3 crystals
YF3 crystals![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 nanocrystals excited at 224 nm short-wavelength. All emission peaks at λ = 579 (5D0-7F0), 592 (5D0-7F1), 612 (5D0-7F2), 657 nm (5D0-7F3) have been assigned in Fig. 8, and there is no notable shift in the positions of emission peaks compared to other Eu3+-doped system,21 because the 4f energy levels of Eu3+ are hardly affected by the crystal field.12 The electric dipole transition 5D0-7F2 is the primary group in the emission spectrum of the sample. The 5D0-7F1 and 5D1-7F1 transitions are magnetic dipole allowed and their intensities are almost independent of the local environment around Eu3+ ions.22 The 5D0-7F2 transition is allowed due to an admixture of opposite parity 4fn−15d states by odd parity crystal-field component.21,23 Therefore, its intensity is sensitive to the local structure around the Eu3+ cation. The 5D0-7F3 transition exhibits a mixture of magnetic dipole and electric dipole character.21 Reduction of particle size and the high concentration of Eu3+ cause the degree of lattice distortion and corresponding lower symmetry of the crystal field around the Eu3+ ions, results in the blue shift of excitation, the primary emission of 5D0-7F2 transition and no splitting of 5D0-7Fj (J = 1, 2, 3) transition.12,24
YF3 nanocrystals excited at 224 nm short-wavelength. All emission peaks at λ = 579 (5D0-7F0), 592 (5D0-7F1), 612 (5D0-7F2), 657 nm (5D0-7F3) have been assigned in Fig. 8, and there is no notable shift in the positions of emission peaks compared to other Eu3+-doped system,21 because the 4f energy levels of Eu3+ are hardly affected by the crystal field.12 The electric dipole transition 5D0-7F2 is the primary group in the emission spectrum of the sample. The 5D0-7F1 and 5D1-7F1 transitions are magnetic dipole allowed and their intensities are almost independent of the local environment around Eu3+ ions.22 The 5D0-7F2 transition is allowed due to an admixture of opposite parity 4fn−15d states by odd parity crystal-field component.21,23 Therefore, its intensity is sensitive to the local structure around the Eu3+ cation. The 5D0-7F3 transition exhibits a mixture of magnetic dipole and electric dipole character.21 Reduction of particle size and the high concentration of Eu3+ cause the degree of lattice distortion and corresponding lower symmetry of the crystal field around the Eu3+ ions, results in the blue shift of excitation, the primary emission of 5D0-7F2 transition and no splitting of 5D0-7Fj (J = 1, 2, 3) transition.12,24

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 sample.
YF3 sample.
