DOI:
10.1039/B905053C
(Paper)
CrystEngComm, 2010,
12, 166-171
Synthesis, characterization and optical properties of flower-like tellurium†
Received
11th March 2009
, Accepted 30th July 2009
First published on
27th August 2009
1. Introduction
The chemical and physical properties of micro-/nanomaterials and, therefore, their subsequent applications are strongly influenced by their dimensionality and shape.1–6 Morphological control of micro-/nanomaterials has attracted a great deal of attention in the past several decades.7–40 There is a considerable amount of research dedicated to one-dimensional (1D) tellurium (Te) nanostructures, such as nanobelts,7–9 nanorods,10–12 nanowires,13–19 nanotubes,20–24 and nanoribbons.25 Two-dimensional (2D) scroll-like Te nanocrystals and thin films have also been extensively studied.26–29 The assembly of those 1D or 2D building blocks to form three-dimensional (3D) complex functional architecture or ordered superstructures has become a key issue in developing more sophisticated micro-/nanodevices. Due to difficulty in controlling the growth of 3D nanocrystals, however, there are few reports on 3D Te superstructures.30–32
Qian and co-workers synthesized Te nanorod bundles and smectic-like arrays through a surfactant-assisted approach.11,30 Gautama and Rao prepared 3D feather-like and flower-like Te superstructures with a self-seeding solution process.31 Liu et al. fabricated bow-like Te microcrystals via PVP-assisted reducing route.32 Crystalline Te shows interesting chemical and physical properties and can be used as a holographic recording material, an infrared photoconductive detector, and for nonlinear-infrared optics.41–45 Until now the employment of appropriate polymer and/or surfactant appears to be necessary as the shape modifier in fabricating 3D Te superstructures. It is therefore desiring to develop more convenient routes and the success of such a study shall greatly facilitate the exploration of new applications of Te, including the use of Te in other functional materials such as PbTe, Nb3Te4, and CdTe.46–48
Herein, we report that 3D flower-like Te can be readily prepared through a simple, low temperature biphasic solvothermal reaction, in which TDEC is the Te source and DTBA is used as an organic reducing reagent. Significantly, no growth modifier such as surfactant, polymer or template is required with this new reaction route. Another unique feature of this new route is that DTBA is indeed a precursor of the reducing reagent, where the reaction of DTBA in alkaline solution provides a dynamic “control” on the concentration of the reducing agent sulfide ion and thus the reduction of TDEC.
2. Experimental
2.1 Synthesis of Te with 3D superstructures
Commercial grade TDEC, purchased from Zhejiang Ultrafine Powders & Chemicals Co. Ltd. (China), was recrystallized twice from high purity chloroform prior to its usage. Analytical grade DTBA, PVP (K30, polymerization degree 360), chloroform and sodium hydroxide were purchased from Aldrich. Deionized water from an 18.2 MΩ Milli-Q purity system was used in this study. In a typical procedure, 0.13 mmol of TDEC was dissolved in 27.7 ml CHCl3 to form a homogeneous solution in a 50 ml stainless steel autoclave with Teflon liner. Then 0.65 mmol of DTBA and 4.3 mL of NaOH aqueous solution were added in order without stirring. The concentration of NaOH was investigated as a control parameter here. The autoclave was sealed and maintained at 120 °C for 3 h, and then was rapidly cooled to room temperature with an ice–water mixture. The black solids formed at the interface of the two immiscible solvents were collected by filtration. The products were then washed several times with distilled water and absolute ethanol and were dried in vacuum at room temperature for several hours before further characterization.
2.2 Characterizations
The phase structure of the as-prepared products was investigated by X-ray diffraction (XRD) on a Bruker D8 Advance diffractometer using Cu Kα radiation (λ = 0.15406 nm). The data were collected in the 2θ range of 20–80° at a step size of 0.02°. The morphology was observed by scanning electron microscopy (SEM) on a FEI Nova Nanosem 200 microscope operated at an acceleration voltage of 10∼15 kV. The transmission electron microscopy (TEM) images, high-resolution transmission electron microscopy (HRTEM) image, the corresponding selected area electron diffraction (SAED) pattern and energy dispersive X-ray spectrometry (EDS) were taken on a JEOL 2010 high-resolution transmission electron microscope performed at 200 kV. Photoluminescence (PL) spectroscopy was carried out at room temperature on a Horiba Jobin Yvon Fluoromax-4 steady lifetime spectrofluoromerer from a Xe lamp. Micro-Raman spectroscopy was performed at room temperature using the 514.5 nm line of an argon ion laser. The scattered light was dispersed through a JY-T64000 triple monochromator system attached to an air-cooled CCD detector. The laser power was decreased for the measurement to avoid bringing any laser-induced effects to the sample. The scattered light from the sample was detected in the backscattering geometry with the wave vector of the incident laser beam parallel to the scattered light.
3. Results and discussion
3.1 Phase and morphology of the 3D Te
Fig. 1 shows a XRD pattern of the products obtained from the reduction of TDEC (1.3 × 10−4 mol) by DTBA (6.5 × 10−4 mol) in the immiscible solvent. All peaks can be indexed to the hexagonal phase of Te (t-Te) with lattice constants of a = 4.46 Å and c = 5.94 Å, which are consistent with the standard literature data (JCPDS card number: 36-1452). There are no impurity peaks detected. The immiscible solvent CHCl3/H2O has a volume ratio of 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the aqueous phase contains 0.93 M NaOH. While details of the reaction mechanism are still to be deciphered, the formation of a pure Te element is suggested through the following steps:
1 and the aqueous phase contains 0.93 M NaOH. While details of the reaction mechanism are still to be deciphered, the formation of a pure Te element is suggested through the following steps:| | | DTBA + NaOH → Na2S + other products | (1) |
| | | TDEC + Na2S → Te + other products | (2) |
 |
| | Fig. 1 XRD pattern of flower-like Te. It was obtained by the reduction of TDEC with DTBA in CHCl3/NaOH immiscible solvents at 120 °C for 3 h. | |
It has been confirmed that DTBA interacts with OH− to produce H2S.49 We confirmed in this study that Na2S can reduce TDEC to Te. The role of DTBA could therefore be offering a steady supply of the reducing reagent sulfide ions. Compared to the Te produced directly using Na2S as the reducing reagent, the result suggests that the dynamic supply of the reducing reagent sulfide ions is important for achieving a flower-like superstructure. We would like to point out that earlier studies suggest that compound TDEC may undergo both inter- and intramolecular exchange processes in solution,50 in which Te(Et2dtc)2 (Et2dtc = diethyldithiocarbamate), may be formed via the following reaction:51
| Te(Et2dtc)4 ↔ Te(Et2dtc)2 + Et4tds |
Thus, the production of Te could also arise from the reduction of Te(Et2dtc)2 by sulfide ions. Further studies are needed to discriminate the above two paths. In Fig. 2 EDS analysis was used to determine the compositions of the above 3D Te product, in which Cu and C peaks come from the carbon-coated copper grid, which is a normal observation for TEM samples. Importantly, strong Te peaks confirm that the product is Te.
Fig. 3 displays SEM, TEM and HRTEM images, along with the SAED pattern, of the Te products prepared at 120 °C. The SEM image in Fig. 3a shows that the product consists of uniform flower-like architectures. From the magnified SEM images in Figs 3b and 3c, it is clear that this 3D superstructure is formed by many needle-like nanorods growing radically from the core. It is further illustrated by the TEM image in Fig. 3e. These nanorods are about 150 to 350 nm wide, with length up to 5 µm. Notably, as shown in Fig. 3c, the cross sections of these needle-like nanorods have a hexagonal shape. In Fig. 3d, the SAED pattern taken along the [110] direction on an individual nanorod reveals that the nanorod is a single crystal with the growth direction along [001], which is further confirmed by the HRTEM image in Fig. 3f. The interplanar spacing is calculated to be about 0.594 nm, which corresponds to the separation between (001) lattice planes of the hexagonal Te and is in good agreement with the result reported in a previous study.24
3.2 Growth mechanism
To shed light on the formation mechanism of the flower-like superstructure, the Te product was analyzed at different reaction stages. Fig. 4 shows SEM images of the Te products which were prepared with different reaction times. Figs 4a and 4b are the SEM micrograph of the product collected at 15 min after the start of the reaction, where Te nanoparticles with a diameter of 30–120 nm could be observed. When the reaction was allowed to continue for a longer time period such as 20 min in Figs 4c and 4d, the Te product consisted of nanoparticles (diameter: 70–300 nm) and nanorods. These nanorods are very thin and short, only tens of nanometers in width and hundreds of nanometers in length. However, as shown in Figs 4e and 4f it only took another 10 min until 3D flower-like superstructures became the dominant morphology of the product. By prolonging the reaction time further to 90, 150 and 960 min, these needle-shaped nanorods grew continuously to reach ∼1 µm (Figs 4g and 4h), ∼3.5 µm (Figs 4i and 4j), and ∼7 µm (Figs 4k and 4l), respectively. After 16 h, the size and morphology of the products remained the same, suggesting that the reaction products, presumably including more stable sulfur compounds, have no effects on the as-prepared Te. The images in Fig. 4 clearly reveal that the flower-like t-Te products grow radically from Te nanoparticles.
 |
| | Fig. 4 SEM images of the Te samples produced at different reaction times: (a, b) 15, (c, d) 20, (e, f) 30, (g, h) 90, (i, j) 150, and (k, l) 960 min. The reaction temperature was kept at 120 °C. | |
In literature vapor–liquid–solid (VLS), vapor–solid (VS), and oxide–assistant (OA) mechanisms have been used to explain the growth of various nanostructures.52,53 For example, a VS mechanism was used to explain the growth of MgO nanoflowers,53 but it is not suitable for our products because the reaction takes place in a condensed phase. The images collected in Fig. 4 suggest that the flower-like Te grows through three stages. Stage (1) is the growth of Te particles during the initial reaction stage. In terms of surface-energy minimization, these initially produced Te particles are easy to aggregate into the Te clusters in the absence of polymeric material, which leads to stage (2)—the formation of Te clusters and nucleation of short Te nanorods. These newly formed nanorods attract Te atoms to diffuse toward their vicinity from the solution due to their intrinsic highly anisotropic properties,20,54 which consequently leads to stage (3)—the continuous growth of flower-like superstructures through the Ostwald ripening process. Fig. 5 demonstrates that the intensities of these diffraction peaks are greatly enhanced as the reaction time is prolonged.
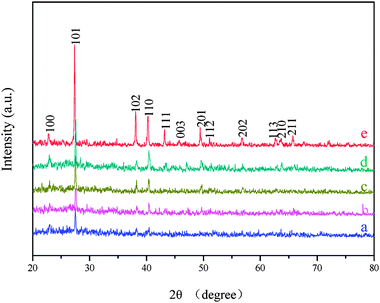 |
| | Fig. 5 XRD spectra of the Te samples prepared with different reaction times: (a) 20, (b) 30, (c) 90, (d) 150, and (e) 960 min. The reaction temperature is 120 °C. | |
3.3 Effect of the reaction conditions on morphology
Figs 6a and b illustrate that a product obtained at 110 °C is only of needle-like nanorods with diverse sizes. On the other hand, when the reaction temperature is increased to 150 °C, the length of these branched nanorods of the flower-like superstructure (Figs 6e and 6f) becomes shorter than that achieved at 130 °C. These experiments confirm that the reaction temperature is a key parameter and the suitable value for making flower-like superstructures is between 120 to 140 °C. The non-monotonic effect of the reaction temperature may arise from its effects on two separate reaction processes: (1) the formation of Na2S from DTBA; and (2) the reduction rate of TDEC by sulfide ions.
 |
| | Fig. 6 SEM images of the Te samples prepared at different reaction temperatures: (a, b) 110 °C, (c, d) 130 °C, and (e, f) 150 °C. The reaction time is 3 h in all cases. | |
As suggested earlier, Te is produced through the reduction of TDEC by sulfide ions, which are generated continuously from DTBA in an alkaline solution.49 Therefore, NaOH concentration is expected to be another critical factor in the growth of 3D superstructures. When NaOH concentration was low such as 0.23 M, no flower-like superstructure could be obtained and the product was dominated by a small quantity of Te microrods. As the concentration of NaOH was increased, a small quantity of Te nanorods mixed with a flower-like superstructure was obtained as the major product. The length to a diameter ratio of these Te nanorods increases with respect to NaOH concentration. In this study we found that when NaOH concentration is above 0.9 M flower-like 3D superstructures become the dominant product (see ESI, Fig. S1).†
3.4 Optical properties of the flower-like Te
In recent years, room-temperature photoluminescence spectroscopy of Te nanostructures has attracted much interest because of its shape-dependent light-emitting properties.18,24,30,31Fig. 7 displays the photoluminescence (PL) emission spectrum of the flower-like t-Te superstructure with an excitation wavelength of 341 nm. The spectrum consists of an obvious main emission peak at about 465 nm and some small peaks located at about 418, 436, 448, 521, and 538 nm, respectively. The strong, asymmetric and broad peak at 465 nm is found to be fitted well by two Gaussian line shapes on the constraint that each peak position fixed at 461 and 466 nm (ESI, Fig. S2).† The inset represents the excitation spectrum of the flower-like t-Te superstructures carried out with an emission wavelength of 465 nm. It shows two wide peaks: one is centered at 284 nm and the other is at about 341 nm. Apparently different from the PL spectrum of Te nanorods reported previously,31 the emission peak around 700 nm is not observed in our samples. To date, the emission peaks with a different wavelength in different nanostructures such as 405 and 426 nm in nanorods bundles,30 456 and 495 nm in nanotubes,24 334, 397, 460, and 507 nm in nanowires,18 411, 433, and 452 nm in ultrathin Te nanowires15 have been reported. For the flower-like superstructure prepared in this study, it was made up by many nanorods of non-uniform sizes. As to an individual nanorod, its diameter is also changed from top to bottom. Therefore the different emission peaks might be associated with the wide size distribution of the secondary branches (i.e. nanorods) and the crystallization behavior of one-D nanostructures.18,24,30,31 The exact luminescent mechanism still needs to be deciphered.
 |
| | Fig. 7 Photoluminescence emission spectrum of the flower-like Te with an excitation wavelength of 341 nm. The inset represents the excitation spectrum of the flower-like t-Te superstructures carried out with emission wavelength of 465 nm. | |
In Fig. 8, the Raman spectra of an individual needle-like nanorod were obtained at room temperature. The scanning was performed from the tip (a) to the bottom (e) of a t-Te nanorod as shown in Fig. 3b. Three characteristic vibration peaks at 92.4, 121.9, and 141.8 cm−1 are similar to the bands at 87.2, 114.8 and 134.4 cm−1 of t-Te nanotubes. The above bands shall correspond, respectively, to the E bond-stretching, A1 bond-stretching and E bond-stretching mode of the needle-like t-Te nanorod.33 When compared to t-Te nanotubes, the above three characteristic vibration peaks have ca. 7 nm red shift. According to the Campbell and Fauchet model,55 the smaller the crystalline grain, the bigger the frequency shifts and the more asymmetric and the broader the peak becomes. Because Raman measurements were performed from the bottom (e) to the tip (a) of a single t-Te nanorod, where the size became smaller and smaller, these Raman peaks shifted downward and became more asymmetric and broader.
4. Conclusions
It is well documented that reaction kinetics has a significant influence on the growth of the 3D superstructure.52,53 Te with a flower-like superstructure has been successfully fabricated here by a simple biphasic solvothermal reaction route. A unique feature of this new method is that the reducing reagent is supplied through a dynamic process, i.e. the reaction of DTBA and NaOH. In parallel experiments using Na2S as the starting reagent, no 3D flower-like Te could be achieved, underlining that the reactive supply of the reducing reactant is important. The continuous supply of the reducing reagent also favors the achievement of uniform superstructures. Significantly, this new approach does not require the presence of any templates. The 3D superstructure can be conveniently manifested by controlling the reaction temperature and the concentration of NaOH.
We would like to point out that the biphasic reaction route presented in this study can be combined with existing approaches to generate novel 3D Te superstructures. For example, we found that when a small amount of PVP was added to the above reaction system, the flower-like Te products would give way to Te nanorods (see Fig. S3 in the ESI).† Notably, the as-obtained Te nanorods have an average diameter of about 60 nm and a hexagonal cross section, the same as seen in Fig. 3c. The photoluminescence emission spectrum of the flower-like Te has a main intensive peak at 465 nm, different from those reported with other superstructures.
Acknowledgements
This work is supported through NSFC (20843007, 20471043), Zhejiang Provincial Natural Science Foundation of China (Y408177, Y404118), the ‘151’ Distinguished Person Foundation of Zhejiang Province of China, the ‘551’ Distinguished Person Foundation of Wenzhou and Zhejiang Technology Project Foundation of China (2007C21134).
References
- D. Wang and C. M. Lieber, Nat. Mater., 2003, 2, 355 CrossRef CAS.
- L. Manna, D. J. Milliron, A. Meisel, E. C. Scher and A. P. Alivisatos, Nat. Mater., 2003, 2, 382 CrossRef CAS.
- B. J. Wiley, Y. Chen, J. M. McLellan, Y. Xiong, Z. Y. Li, D. Ginger and Y. Xia, Nano Lett., 2007, 7, 1032 CrossRef CAS.
- J. M. McLellan, Z. Y. Li, A. R. Siekkinen and Y. N. Xia, Nano Lett., 2007, 7, 1013 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2004, 126, 7194 CrossRef CAS.
- X. Fang, Y. Bando, U. K. Gautam, C. Ye and D. Golberg, J. Mater. Chem., 2008, 18, 509 RSC.
- M. S. Mo, J. H. Zeng, X. M. Liu, W. C. Yu, S. Y. Zhang and Y. T. Qian, Adv. Mater., 2002, 14, 1658 CrossRef CAS.
- B. Zhang, W. Y. Hou, X. C. Ye, S. Q. Fu and Y. Xie, Adv. Funct. Mater., 2007, 17, 486 CrossRef CAS.
- Q. Wang, G. D. Li, Y. L. Liu, S. Xu, K. J. Wang and J. S. Chen, J. Phys. Chem. C, 2007, 111, 12926 CrossRef CAS.
- F. Gao, Q. Y. Lu and S. Komarneni, J. Mater. Res., 2006, 21, 343 CrossRef CAS.
- Z. P. Liu, Z. K. Hu, J. B. Liang, S. Li, Y. Yang, S. Peng and Y. T. Qian, Langmuir, 2004, 20, 214 CrossRef CAS.
- Y. J. Zhu, W. W. Wang, R. J. Qi and X. L. Hu, Angew. Chem., Int. Ed., 2004, 43, 1410 CrossRef CAS.
- H. Yu, P. C. Gibbons and W. E. Buhro, J. Mater. Chem., 2004, 14, 595 RSC.
- Q. Y. Lu, F. Gao and S. Komarneni, Adv. Mater., 2004, 16, 1629 CrossRef CAS.
- H. S. Qian, S. H. Yu, L. B. Luo, J. Y. Gong and L. F. Fei, Langmuir, 2006, 22, 3830 CrossRef CAS.
- G. C. Xi, Y. K. Liu, X. Q. Wang, X. Y. Liu, Y. Y. Peng and Y. T. Qian, Cryst. Growth Des., 2006, 6, 2567 CrossRef CAS.
- S. Sen, U. M. Bhatta, V. Kumar, K. P. Muthe, S. Bhattacharya, S. K. Gupta and J. V. Yakhmi, Cryst. Growth Des., 2008, 8, 238 CrossRef CAS.
- Z. H. Lin, Z. S. Yang and H. T. Chang, Cryst. Growth Des., 2008, 8, 351 CrossRef CAS.
- Z. H. Lin, Y. W. Lin, K. H. Lee and H. T. Chang, J. Mater. Chem., 2008, 18, 2569 RSC.
- B. Mayers and Y. N. Xia, Adv. Mater., 2002, 14, 279 CrossRef CAS.
- X. L. Li, G. H. Cao, C. M. Feng and Y. D. Li, J. Mater. Chem., 2004, 14, 244 RSC.
- G. C. Xi, Y. Y. Peng, W. C. Yu and Y. T. Qian, Cryst. Growth Des., 2005, 5, 325 CrossRef CAS.
- P. Mohanty, T. Kang, B. Kim and J. Park, J. Phys. Chem. B, 2006, 110, 791 CrossRef CAS.
- J. M. Song, Y. Z. Lin, Y. J. Zhan, Y. C. Tian, G. Liu and S. H. Yu, Cryst. Growth Des., 2008, 8, 1902 CrossRef CAS.
- Z. B. He and S. H. Yu, J. Phys. Chem. B, 2005, 109, 22740 CrossRef CAS.
- W. Zhu, W. Z. Wang, H. L. Xu, L. Zhou, L. S. Zhang and J. L. Shi, Cryst. Growth Des., 2006, 6, 2804 CrossRef CAS.
- Ph. Ott and J. R. Günter, Thin Solid Films, 2000, 366, 100 CrossRef CAS.
- Y. Wang, Z. Y. Tang, P. Podsiadlo, Y. Elkasabi, J. Lahann and N. A. Kotov, Adv. Mater., 2006, 18, 518 CrossRef CAS.
- G. H. Wang, P. P. Chen, Z. Y. Wang, M. Han, J. W. Fang and S. Y. Zhang, Thin Solid Films, 2000, 375, 33 CrossRef CAS.
- J. Li, J. H. Zhang and Y. T. Qian, Solid State Sci., 2008, 10, 1549 CrossRef CAS.
- U. K. Gautama and C. N. R. Rao, J. Mater. Chem., 2004, 14, 2530 RSC.
- Z. P. Liu, S. Li, Y. Yang, Z. K. Hu, S. Peng, J. B. Liang and Y. T. Qian, New J. Chem., 2003, 27, 1748 RSC.
- D. P. Li, Z. Zheng, Z. Y. Shui, M. Q. Long, J. Yu, K. W. Wong, L. Yang, L. Z. Zhang and W. M. Lau, J. Phys. Chem. C, 2008, 112, 2845 CrossRef CAS.
- L. Zhou, W. Z. Wang and H. L. Xu, Cryst. Growth Des., 2008, 8, 728 CrossRef CAS.
- D. Grützmacher, T. Fromherz, C. Dais, J. Stangl, E. Müller, Y. Ekinci, H. H. Solak, H. Sigg, R. T. Lechner, E. Wintersberger, S. Birner, V. Holý and G. Bauer, Nano Lett., 2007, 7, 3150 CrossRef.
- H. S. Qian, S. H. Yu, J. Y. Gong, L. B. Luo and L. L. Wen, Cryst. Growth Des., 2005, 5, 935 CrossRef CAS.
- Q. Q. Wang, G. Xu and G. R. Han, Cryst. Growth Des., 2006, 6, 1776 CrossRef CAS.
- D. B. Kuang, A. W. Xu, Y. P. Fang, H. Q. Liu, C. Frommen and D. Fenske, Adv. Mater., 2003, 15, 1747 CrossRef CAS.
- J. W. Cho, H. S. Kim, Y. J. Kim, S. Y. Jang, J. Park, J. G. Kim, Y. J. Kim and E. H. Cha, Chem. Mater., 2008, 20, 5600 CrossRef CAS.
- X. Fang, U. K. Gautam, Y. Bando and D. Golberg, J. Mater. Sci. Technol., 2008, 24, 520 CAS.
- P. Tangney and S. Fahy, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 054302 CrossRef.
- I. Shih and C. H. Champness, J. Cryst. Growth, 1978, 44, 492 CrossRef CAS.
- J. Beauvais, R. A. Lessard, P. Galarneau and E. J. Knystautas, Appl. Phys. Lett., 1990, 57, 1354 CrossRef CAS.
- A. W. Zhao, C. H. Ye, G. W. Meng, L. D. Zhang and P. M. Ajayan, J. Mater. Res., 2003, 18, 2318 CrossRef CAS.
- J. Lu, Y. Xie, F. Xu and L. Y. Zhu, J. Mater. Chem., 2002, 12, 2755 RSC.
- T. Mokari, M. J. Zhang and P. D. Yang, J. Am. Chem. Soc., 2007, 129, 9864 CrossRef CAS.
- H. K. Edwards, P. A. Salyer, M. J. Roe, G. S. Walker, P. D. Brown and D. H. Gregory, Cryst. Growth Des., 2005, 5, 1633 CrossRef CAS.
- E. S. Brigham, C. S. Weisbecker, W. E. Rudzinski and T. E. Mallouk, Chem. Mater., 1996, 8, 2121 CrossRef CAS.
- J. P. Danehy and K. N. Parameswaran, J. Org. Chem., 1968, 33, 568 CrossRef CAS.
- D. Dakternieks, R. D. Giacomo, R. W. Gable and B. F. Hoskins, J. Am. Chem. Soc., 1988, 110, 6753 CrossRef CAS.
- A. M. Bond, D. Dakternieks, R. D. Giacomo and A. F. Hollenkamp, Inorg. Chem., 1989, 28, 1510 CrossRef CAS.
- X. L. Yu and C. B. Cao, Cryst. Growth Des., 2008, 8, 3951 CrossRef CAS.
- X. S. Fang, C. H. Ye, L. D. Zhang, J. X. Zhang, J. W. Zhao and P. Yan, Small, 2005, 1, 422 CrossRef CAS.
- B. Mayers and Y. N. Xia, J. Mater. Chem., 2002, 12, 1875 RSC.
- I. H. Campbell and P. M. Fauchet, Solid State Commun., 1986, 58, 739 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the aqueous phase contains 0.93 M NaOH. While details of the reaction mechanism are still to be deciphered, the formation of a pure Te element is suggested through the following steps:
1 and the aqueous phase contains 0.93 M NaOH. While details of the reaction mechanism are still to be deciphered, the formation of a pure Te element is suggested through the following steps:







