Low-temperature synthesis of CdSe nanocrystal quantum dots†‡
Jacqueline T.
Siy
,
Eric M.
Brauser
and
Michael H.
Bartl
*
Department of Chemistry, University of Utah, Salt Lake City, Utah 84112, USA. E-mail: bartl@chem.utah.edu; Fax: +1 801-581-8433; Tel: +1 801-585-1120
First published on 9th September 2010
Abstract
A method for fabricating colloidal CdSe nanocrystals at low reaction temperatures was developed. The transition from CdSe clusters to continuously-growing nanocrystals was found to be crucial in the formation of high-quality quantum dots with narrow size distribution and efficient, tunable optical properties.
The unique size and shape-related tunable functionalities of semiconductor nanocrystals (NCs) have dramatically altered our perception of fundamental material properties.1 These materials promise new applications in biological imaging,2 microlasing,3 displays4 and solar energy conversion5 with a combined projected market growth from about $30 million in 2008 to more than $720 million by 2013.6CdSe NCs have emerged as the most important (and by far most studied) system.7 This is largely due to their colorful optical properties in the visible and the pioneering development of robust syntheses, resulting in the breakthrough “hot injection” reaction method for nearly monodisperse colloidal NC quantum dots (NCQDs) by Murray et al.8 Subsequent research has focused on understanding the reaction mechanism and developing simpler and greener alternatives to the original reaction, resulting in high-quality NCs with narrow size distribution and tunable luminescence properties.9 However, despite recent progress in automated synthesis procedures,9h,i the high reaction temperatures (e.g., 195–350 °C for CdSe NCQDs) make large-scale production of NCs still difficult and expensive.6
Here we report a low-temperature organometallic method to enable synthesis of CdSe NCQDs at temperatures between 50 and 130 °C. A typical synthesis description is given in detail in the Electronic Supplementary Information (ESI)†. A key factor for our successful low-temperature synthesis is the simultaneous injection of cadmium and selenium precursors into a separate reaction flask containing a secondary ligand, as well as optimized ratio and concentration of reactants. While we are using reaction components similar to high-temperature methods, we find the range of synthesis conditions required to produce high-quality CdSe NCQDs is much narrower than in high-temperature routes. In particular, the transition from initially-formed clusters of fixed sizes to continuously-growing NCQDs is sensitive to the sequence of adding precursors to the growth solution, reactant concentration, and type of ligands. CdSe NCQDs formed under optimized conditions possess a wurtzite crystal structure, narrow size distributions (below 5% polydispersity), and display tunable optical properties spanning the visible range.
The temporal evolution of the NC nucleation and growth process is monitored by UV-vis absorption spectroscopy. Small aliquots were taken from the reaction mixture and spectra were recorded with an Ocean Optics USB2000 spectrometer. Fig. 1 shows representative results for CdSe NCQDs synthesized at 50, 100, and 130 °C. All three reaction temperatures lead essentially to the same optical features; however, with distinct formation kinetics ranging from minutes (130 °C) to days (50 °C). Initially, sharp absorption peaks appear at fixed wavelength positions, followed by the onset of typical CdSe NCQD excitonic absorption features (Fig. 1a). The sharp peaks at 415 and 445 nm can be attributed to CdSe clusters.9b,g The slowed kinetics at low temperatures (Fig. 1b) reveals successive appearance and disappearance of these two peaks. We interpret this as transformation of the initially-formed clusters into a second set of larger, yet still discretely-sized clusters, indicative of a step-wise growth mechanism at early stages of NCQD formation.9b The transition from cluster to NC growth is evidenced by the emergence of an absorption shoulder at around 470 nm and a fast decrease in the cluster peak intensities. This shoulder rapidly gains in intensity and develops into a typical first excitonic absorption feature of CdSe NCQDs, which slowly shifts to larger wavelength positions as the NCs continue to grow. The transition from clusters to NCs is also accompanied by a drastic change in the photoluminescence (PL) properties (recorded on a Fluorolog FL3-22 spectrometer and given as dotted spectra in Fig. 1). While during the initial period only weak cluster or defect-linked, broad emission was observed, with the appearance of the NC absorption shoulder, the corresponding PL emission peak sharpens and the broad background emission disappears as NC growth proceeds.
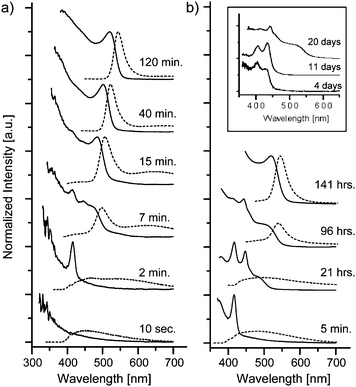 | ||
| Fig. 1 UV-vis absorption (full lines) and PL emission (dotted lines) spectra, showing the temporal evolution of the CdSe NC nucleation and growth process at 130 °C (a), 100 °C (b) and 50 °C (inset). The PL emission spectra were excited at 350 nm. Spectra were intensity-normalized for better comparison. | ||
The structural properties and morphology of these samples were investigated by powder X-ray diffraction (XRD) analysis and transmission electron microscopy (TEM). For XRD analysis dry NC-powder was deposited onto an X-ray sample holder and analyzed by a Bruker D8 Advanced X-ray diffractometer. A typical diffraction pattern is shown in Fig. 2a. Apart from strongly broadened reflections due to small CdSe crystallite size, a comparison with calculated line patterns reveals that the low-temperature CdSe NCQDs have a hexagonal wurtzite crystal structure. This is an interesting finding, since the wurtzite phase is typical of high-temperature methods8 or at lower temperatures (115–175 °C) in glycol-based reaction mixtures.10 In contrast, in growth mixtures similar to this work, only the cubic zinc blende phase was obtained at lower temperatures (150–200 °C).11TEM studies were performed on purified samples deposited on Carbon Type B coated copper grids and imaged using a FEI Tecnai F30 microscope operated at an acceleration voltage of 300 kV. Our results (Fig. 2b) show these NCs have approximately-spherical faceted shapes. Moreover, high-resolution TEM imaging of individual NCQDs shows lattice fringes visible along different crystal axes, confirming their single-crystalline nature.
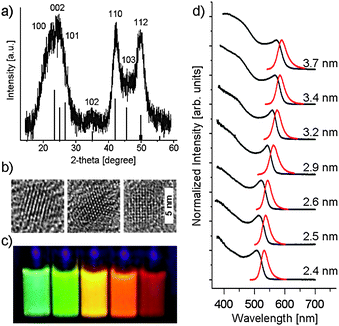 | ||
| Fig. 2 (a) Powder XRD pattern of low-temperature synthesized CdSe NCQDs. For comparison, the typical single-crystal wurtzite CdSe reflections are given as line-pattern below the recorded diffraction pattern. (b) Corresponding high-resolution TEM images taken along different crystallographic axes. (c) PL image of samples excited by a broadband UV lamp. (d) UV-vis absorption (black) and PL emission (red) spectra (excited at 350 nm) of CdSe NCQDs with different sizes (calculated according to ref. 13; diameter uncertainties are ±0.5 nm). | ||
Typical optical properties of a size-series of purified CdSe NCQDs synthesized at 130 °C in the presence of octadecylamine ligands are given in Fig. 2c and d. The samples display bright, tunable luminescence with emission band FWHM values between 28 and 35 nm (95–130 meV), corresponding to less than 5% size polydispersity.9e,f The samples display PL quantum efficiencies reaching 10 percent (measured using rhodamine 590 in ethanol as reference), which compares well with typical core-only CdSe NCQDs.9e,f However, it should be emphasized that synthesis of such high-quality CdSe NCQDs at low temperatures requires fine-tuning of the reaction parameters. The samples shown in Fig. 2 were synthesized under optimized reactant ratios and concentrations in the octadecene-based growth solution (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se
Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octadecylamine molar ratios of 1
octadecylamine molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.6
6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30–100 and cadmium concentrations of 1.6–11.1 mM). It is important to note that these reactant concentrations are lower than used in most high-temperature routes;9c,d synthesis attempts with higher reactant concentrations resulted in slower growth kinetics and NCs with poor size distributions (PL bands with FWHM values larger than 50 nm; see ESI†, Fig. S1) and weak PL emission.
30–100 and cadmium concentrations of 1.6–11.1 mM). It is important to note that these reactant concentrations are lower than used in most high-temperature routes;9c,d synthesis attempts with higher reactant concentrations resulted in slower growth kinetics and NCs with poor size distributions (PL bands with FWHM values larger than 50 nm; see ESI†, Fig. S1) and weak PL emission.
In general, we found low-temperature synthesis of high-quality CdSe NCQDs is less forgiving as compared to high-temperature routes. The type of ligand present in the growth solution is one important factor. We found that the NC growth kinetics and their final size-dispersion depend strongly on the choice and relative amounts of ligands added to the reaction mixture. Long-chain fatty acids such as oleic acid or stearic acid are required in the formation of the cadmium precursor and therefore are inherently present in the reaction mixture. However, the NCs formed at low temperatures in the presence of only fatty acid ligands are highly size-polydisperse with weak PL emission and full-width-half-maximum (FWHM) values larger than 100 nm (Fig. 3a). In contrast, when ligands such as fatty amines or alkylphosphine oxides are admixed to the reaction solution, NCQDs with strong PL and narrow size distribution are formed (Fig. 2c and d). With respect to ligand concentration, the highest-quality NCs were obtained at cadmium to ligand molar ratios of at least 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. At higher ratios (up to 1
10. At higher ratios (up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), NCs are still formed with excellent properties; however, their growth is increasingly slowed. In addition, for the formation of high-quality CdSe NCQDs, we find it is imperative to simultaneously add cadmium and selenium precursors to the ligand-containing growth solution. Premixing the secondary ligands with the cadmium precursor solution prior to adding the selenium precursor failed to produce size-monodisperse NCs (Fig. 3b).
100), NCs are still formed with excellent properties; however, their growth is increasingly slowed. In addition, for the formation of high-quality CdSe NCQDs, we find it is imperative to simultaneously add cadmium and selenium precursors to the ligand-containing growth solution. Premixing the secondary ligands with the cadmium precursor solution prior to adding the selenium precursor failed to produce size-monodisperse NCs (Fig. 3b).
 | ||
| Fig. 3 (a) UV-vis absorption (full) and PL emission (dotted) spectra of samples synthesized in the absence of fatty amine ligands. (b) UV-vis absorption spectra for samples synthesized with pre-mixed cadmium precursor/ligand-containing growth solution. (c) UV-vis absorption spectra of CdSe NCQD samples synthesized by double-injection of precursor solutions. Injections and growth times are listed in the spectra. Arrow indicates optical feature of 2nd generation NCQDs. | ||
Our observations show the defining step in the low-temperature synthesis of high-quality CdSe NCQDs is the transition from clusters to NCs. While CdSe clusters are formed within a broad range of reaction conditions (Fig. 3a and b), it is the transition from discretely-sized clusters to continuously-growing NCs that occurs only when both cadmium and selenium precursors are injected into a growth solution containing ligands with the ability to bind to both cadmium and selenium units (fatty amines and alkylphosphine oxides). Fatty acid-based ligands seem to stabilize the initially-formed clusters too strongly and the low reaction temperatures are not sufficient enough to induce transformation of these thermodynamically stable clusters into larger NCs. Moreover, a detailed investigation of the spectra reveals that in the presence of strongly-binding fatty acids as secondary ligands, only the smaller-sized clusters are formed and not even transformation to larger-sized clusters occurs (Fig. 3a).
Finally, we also studied the low-temperature (130 °C) behavior of growing CdSe NCQDs while injecting additional precursor solutions. Resulting UV-vis absorption spectra are shown in Fig. 3c. Ten minutes after the initial precursor injection (the formed NCs were about 2.3 nm in size) an additional 0.4 mL of each cadmium and selenium precursor solutions were added. When the absorbance of the sample was measured 5 minutes after the second injection, a second peak appeared (arrow in Fig. 3c), indicating a second set of NCs was formed. After 20 minutes the absorption spectrum again showed only a single feature, which further narrowed and red-shifted under prolonged growth time. These results are in excellent agreement with a theoretical model developed by Reiss in 1951.12 This work predicts a growth-regulating mechanism of particles in a competitive assembly by slowing the growth of larger particles while hastening that of smaller particles, leading to uniform colloidal particles with narrow size distributions. This behavior is exactly what was observed in our present study. The wavelength shift (growth rate) of 13 nm from 5 to 10 minutes after the first injection decreased to only 2 nm in a span of 15 minutes after the second injection. After a few minutes, the smaller NCs caught up to the larger ones and they continued to grow together, resulting in a narrow size distribution overall.
To conclude, we developed a method to enable synthesis of colloidal CdSe NCQDs at greatly reduced temperatures as compared to conventional routes. Low-temperature growth is attractive for large-scale fabrication of NCs and should be advantageous for industry-based production. In fact, our first scale-up attempts (by a factor of 100) have successfully been conducted and showed no decrease in NC yield and quality. Current investigations are focused on further gaining detailed mechanistic insights; in particular on molecular composition, size, and stability of the CdSe clusters formed in the initial synthesis stages.
We thank Jeffrey Farrer (BYU Microscopy Lab) for help with TEM studies and Christopher Kareis and Joel Miller for assistance with XRD measurements. This work was partially supported by the National Science Foundation under Award No. ECS-06-09244, a DuPont Young Professor Grant, and start-up funds from the University of Utah.
Notes and references
- (a) A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS; (b) L. E. Brus, J. Phys. Chem., 1986, 90, 2555 CrossRef CAS; (c) C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS.
- (a) A. P. Alivisatos, W. Gu and C. Larabell, Annu. Rev. Biomed. Eng., 2005, 7, 55 CrossRef CAS; (b) X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS.
- (a) J. N. Cha, M. H. Bartl, M. S. Wong, A. Popitsch, T. J. Deming and G. D. Stucky, Nano Lett., 2003, 3, 907 CrossRef CAS; (b) V. I. Klimov, S. A. Ivanov, J. Nanda, M. Achermann, I. Bezel, J. A. McGuire and A. Piryatinski, Nature, 2007, 447, 441 CrossRef CAS.
- N. Tessler, V. Medvedev, M. Kazes, S. Kan and U. Banin, Science, 2002, 295, 1506 CrossRef.
- (a) S. Kumar and G. D. Scholes, Microchim. Acta, 2008, 160, 315 CrossRef; (b) I. Gur, N. A. Fromer, M. L. Geier and A. P. Alivisatos, Science, 2005, 310, 462 CrossRef CAS; (c) D. J. Milliron, I. Gur and A. P. Alivisatos, MRS Bull., 2005, 30, 41 CAS.
- K. Sanderson, Nature, 2009, 459, 760 CrossRef.
- (a) C. B. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater. Sci., 2000, 30, 545 CrossRef CAS; (b) Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664 CrossRef CAS.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS.
- (a) C. R. Bullen and P. Mulvaney, Nano Lett., 2004, 4, 2303 CrossRef CAS; (b) S. Kudera, M. Zanella, C. Giannini, A. Rizzo, Y. Li, G. Gigli, R. Cingolani, G. Ciccarella, W. Spahl, W. J. Parak and L. Manna, Adv. Mater., 2007, 19, 548 CrossRef CAS; (c) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 183 CrossRef CAS; (d) L. Qu, Z. A. Peng and X. Peng, Nano Lett., 2001, 1, 333 CrossRef CAS; (e) S. Sapra, A. L. Rogach and J. Feldmann, J. Mater. Chem., 2006, 16, 3391 RSC; (f) S. L. Cumberland, K. M. Hanif, A. Javier, G. A. Khitrov, G. F. Strouse, S. M. Woessner and C. S. Yun, Chem. Mater., 2002, 14, 1576 CrossRef CAS; (g) M. Zanella, A. Z. Abbasi, A. K. Schaper and W. J. Parak, J. Phys. Chem. C, 2010, 114, 6205 CrossRef CAS; (h) E. M. Chan, C. Xu, A. W. Mao, G. Han, J. S. Owen, B. E. Cohen and D. J. Milliron, Nano Lett., 2010, 10, 1874 CrossRef CAS; (i) A. Toyota, H. Nakamura, H. Ozono, K. Yamashita, M. Uehara and H. Maeda, J. Phys. Chem. C, 2010, 114, 7527 CrossRef CAS.
- T. Wang, Z. Jin, T. Liu, W. Li and Y. Ni, J. Am. Ceram. Soc., 2010, 93, 1927 CAS.
- (a) N. Pradhan, D. Reifsnyder, R. Xie, J. Aldana and X. Peng, J. Am. Chem. Soc., 2007, 129, 9500 CrossRef CAS; (b) D. V. Talapin, J. H. Nelson, E. V. Shevchenko, S. Aloni, B. Sadtler and A. P. Alivisatos, Nano Lett., 2007, 7, 2951 CrossRef CAS; (c) N. Al-Salim, A. G. Young, R. D. Tilley, A. J. McQuillan and J. Xia, Chem. Mater., 2007, 19, 5185 CrossRef CAS; (d) Z. Deng, L. Cao, F. Tang and B. Zou, J. Phys. Chem. B, 2005, 109, 16671 CrossRef CAS; (e) D. Pan, Q. Wang, S. Jiang, X. Ji and L. An, J. Phys. Chem. C, 2007, 111, 5661 CrossRef CAS.
- H. Reiss, J. Chem. Phys., 1951, 19, 482 CrossRef CAS.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed nanocrystal synthesis description. Optical absorption and PL spectra of samples prepared under different reaction conditions. Low-resolution TEM images. See DOI: 10.1039/c0cc02304c |
| ‡ This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| This journal is © The Royal Society of Chemistry 2011 |
