A novel sensing platform based on periodate-oxidized chitosan†
Yan
Feng
,
Limin
Yang
and
Feng
Li
*
Shandong Provincial Key Laboratory of Biochemical Analysis, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao, 266042, PR China. E-mail: lifeng@qust.edu.cn; Fax: +86-532-84023927; Tel: +86-532-84023927
First published on 2nd November 2010
Abstract
A novel thionine (TH) sensing platform was proposed based on immobilization of TH on a periodate-oxidized chitosan (CS) modified glassy carbon electrode (GCE) surface. Oxidation of CS by periodate can lead to the cleavage of carbon–carbon bonds on the pyranosidic ring of CS, with the generation of dialdehyde groups. TH, an electroactive mediator, was selected as a model of amine compounds to react with the aldehyde group via Schiff-base reaction. Due to the resulting aldehyde-enriched surface and covalent immobilization of TH on periodate-oxidized CS, the CS-grafted-TH showed a pronounced electrochemical response with high stability. The application of the fabricated TH sensing platform as a hydrogen peroxide (H2O2) sensor was also investigated and it exhibited a rapid response to H2O2 within 4 s. The linear calibration ranged from 1.0 × 10−5 to 1.83 × 10−3 M with a detection limit of 4.0 × 10−6 M.
1. Introduction
Chitosan (CS) is a deacetylated derivative of chitin which is isolated from naturally occurring crustacean shells. CS has been proposed to have a unique set of properties, including little or no toxicity, biocompatibility, biodegradability, immunogenic activity and antibacterial effects, thus CS is recognized as an appealing material in the fields of biomedicine,1,2 pharmaceuticals,3,4 metal chelation,5,6 food additives,7 and especially in the fabrication of sensors8 or biosensors.9CS is a linear β-(1–4)-linked polysaccharide composed of repeating units of glucosamine and a small amount of N-acetylglucosamine residues.10 The charged state of the primary amine at the C-2 position of the glucosamine residues can be easily altered by pH. At lower pH (<6.5), the amino group is protonated in acidic solutions, and CS can act as a water-soluble cationic polyelectrolyte. At higher pH (>6.5), the polymer loses its charge and becomes insoluble.11 The soluble–insoluble transition occurs in physiological conditions, which makes CS quite suitable in bioanalysis.
While CS itself is a biocompatible and biodegradable material, it can also form membranes, films, and three-dimensional networks on underlying substrates via simple drop coating or electrodeposition.12 The membrane and film forming abilities can be attributed to the unique acid-soluble and alkaline-insoluble properties of CS. Also H-bonding, hydrophobic and electrostatic interactions contribute to the stability of the resulting membrane or film.13 In most CS containing sensors, CS was mainly acting as a biocompatible matrix to encapsulate proteins14 or nanoparticles15 with no intrinsic modification on the structure of CS, or cross-linking with aldehydes,16 epichlorohydrin,17 epoxides,18 and cyanates19 to form bridges between the polymeric chains, leading to a three-dimensional network which is insoluble over the entire pH range. Besides the above cross-linking of CS, trimethylated,20 thiolated,21 and azidated CS,22 as well as cyclodextrin-linked CS,23 and chemical grafting of CS24 endow CS with versatile functionalities.
Among the various approaches above, periodate oxidation of CS can be a convenient route to functionalize CS with aldehyde groups via opening of the pyranosidic rings of CS. As the periodate ion, IO4−, can attack vicinal diols, 1,2-dioxygenated groups and 1,2-amino alcohols to cleave the carbon–carbon bond by an oxidation reaction, leading to the formation of a dialdehyde,25 the freshly formed aldehyde groups can yield the corresponding aldimines with amine compounds via a Schiff-base reaction. The periodate oxidation process may generate a large amount of active binding sites, which holds great potential in multi-point covalent immobilization of amines or enzymes. As I−, IO4− and other side products may influence further reactions, it is essential to purify the periodate-oxidized CS by dialysis, which is a time-consuming procedure. CS can form a thin film on carbon glassy electrodes (GCE) due to its film forming ability, and the resulting CS modified electrode can be oxidized when immersed in periodate solution. The oxidation reaction can be stopped by removing the electrode from the periodate solution, followed by thoroughly rinsing with water. Moreover, the resulting aldehyde-enriched surface can be directly used without purification by dialysis. It has been reported that CS can be only partially oxidized by periodate, reaching a degree of oxidation around 0.5,25 thus the periodate-oxidized CS may still retain its biocompatibility.
Due to the specific affinity of an enzyme for its substrate, enzyme-based biosensors are of particular importance in bioanalysis. However, direct electron transfer is usually prohibited as the redox center is located deep inside the insulated protein shell. In addition, the enzyme needs a mild environment to retain its native structure, thus it may be an expensive way to fabricate enzyme-based biosensors. Alternatively, the participation of a mediator aiming to enhance the electron transfer is commonly adopted. Mediated electrochemical sensors also have the advantage of higher sensitivity, lower detection limits, lower operational potentials, and less interference from oxidizable species over unmediated sensors. Thionine (TH) is an electroactive and photoactive molecule, and is commonly used as an electron mediator due to its excellent electron transfer ability.26 It is a small planar molecule and contains one heterocyclic nitrogen atom and two amine groups symmetrically distributed on each side. TH is a pH-dependent electroactive dye with a pKa value around 8.0.27 A reversible two electron redox reaction occurs between TH and its reduced form in acidic or neutral aqueous solution. The electron transfer process between TH and the electrode surface is usually accompanied by the participation of protons, and the number of protons varies under different pH conditions. If the plot of formal potential vs. pH value gives a straight line with a slope near the theoretical value of 59 mV pH−1, the redox process is a two-electron and two-proton transfer process, while a slope near 29.5 mV pH−1 indicates a two-electron and one-proton process.28 Various strategies have been devised to confine TH onto the electrode surface to achieve electrochemical sensor fabrication, such as electrostatic interaction,29 entrapment,30 covalent binding,31 electropolymerization,32 and so on. Covalent binding can effectively preserve against TH loss, and thus it will be an alternative way to construct a novel sensing platform of TH based on periodate-oxidized CS via Schiff-base reaction.
In the current paper, TH was chosen as a model for amine compounds to be covalently immobilized on an aldehyde-enriched surface generated simply by immersion of CS film modified GCE in periodate solution. Due to the large amount of in situ generated aldehyde groups on the electrode surface and the imine bond between CS and TH, the amount and stability of immobilized TH were greatly increased. The capability of the as-prepared TH sensing platform for analysis of biological species was demonstrated by the electrochemical reduction of H2O2.The sensor exhibited a fast amperometric response to H2O2 with a wide linear range, low detection limit, high sensitivity, good reproducibility, long-term stability and anti-interference ability. For comparison, a TH modified electrode was prepared by direct immersion of a CS modified GCE in TH solution without pre-oxidation by periodate.
2. Experimental
2.1 Reagents
TH and potassium periodate (KIO4) were supplied by Sinopharm Chemical Reagent Co., Ltd. CS with 98% deacetylation and an average molecular weight of 6 × 104 g mol−1 was obtained from Yuhua Bio-medical Co., China. 0.1 M phosphate buffer solutions (PBS) were prepared by mixing standard stock solutions of Na2HPO4 and NaH2PO4 and adjusting with HCl or NaOH to various pH values. Double distilled water (DDW) was used throughout the measurements.2.2 Apparatus
Cyclic voltammetric (CV), electrochemical impedance spectroscopy (EIS) and amperometric measurements were performed with a CHI 660d electrochemical analyzer (Shanghai CH Instrument Company, China). A conventional three-electrode system was employed with GCE or modified GCE as the working electrode, Pt wire and Ag/AgCl (saturated with KCl) as the auxiliary and the reference electrodes, respectively. All experiments were carried out under the protection of high-purity nitrogen.2.3 Periodate oxidation of CS modified GCE and the immobilization of TH
0.15 g CS flakes were dissolved in 10 mL 0.05 M HAc solution and a solution with pH ca. 3.0 was obtained. After the undissolved material was filtered, the pH was adjusted to 5.5 using 1.0 M NaOH. An aqueous solution of CS was thus obtained.Prior to modification, GCEs were polished with 1.0, 0.3, and 0.05 μm alumina slurry, respectively. Then, the electrodes were rinsed thoroughly with DDW between each polishing step and were cleaned by ultrasonication. 10 μL CS solution was deposited on the electrode surface, and maintained for 12 h in a moist environment, followed by thorough rinsing with DDW. The obtained CS/GCE was then immersed in 10 mM KIO4 solution and kept in the dark under stirring for 6 h. Afterwards, the modified electrode was carefully rinsed with DDW and denoted as CHO-CS/GCE. The immobilization of TH was performed by directly transferring the CHO-CS/GCE into 2.0 mM TH solution (0.1 M PBS pH 7.0) followed by incubation for 4 h. The resulting electrode was washed with DDW to remove non-specifically adsorbed TH. The as-prepared electrode was denoted as TH–COH-CS/GCE and stored at 4 °C when not in use. For comparison, TH–CS/GCE was also prepared by direct immersion of CS/GCE in the same TH solution.
3. Results and discussion
3.1 Immobilization of TH on periodate oxidized CS modified GCE
Herein, TH was chosen as a model of amides and a novel sensing platform of CS-graft-TH was constructed based on the Schiff-base reaction between amino groups of TH and aldehyde groups of periodate-oxidized CS. Schematic representation of the fabrication process is given in Fig. 1. Periodate oxidation of CS opened the pyranosidic rings of CS with the cleavage of the carbon–carbon bond, which led to the formation of aldehyde groups on the electrode surface. Thus the resulting aldehyde-enriched surface afforded multiple binding sites for immobilization of TH. The Schiff-base reaction between the amine and the aldehyde group is a rapid reaction under appropriate conditions, and the formation of imine bonds between TH and periodate-oxidized CS endowed the sensing platform with high stability. Accordingly, the amount and stability of immobilized TH can be greatly increased via this facile strategy. | ||
| Fig. 1 Schematic representation for the immobilization of TH onto GCE. | ||
3.2 Characterization
CV was employed to probe the electrochemical characteristics of modified electrodes during stepwise modification. Fig. 2 shows CVs of different modified electrodes over the potential range from 0.2 to −0.6 V in 0.1 M PBS (pH 6.5) containing 0.1 M KCl at a scan rate of 50 mV s−1. There are no redox peaks observed at the bare GCE (Fig. 2a), CS/GCE (Fig. 2b) and CHO-CS/GCE (Fig. 2c), however there were very small redox peaks of TH appearing at the TH–CS/GCE by direct immersion of CS modified electrode in TH solution for 4 h (Fig. 2d). The peaks gradually degraded in the subsequent scans, which can be attributed to the loose attachment of TH. As CS has a relatively high chain stiffness, which may restrict its electrostatic interactions with oppositely charged molecules, only a small amount of TH can be immobilized. For comparison, a pair of well-defined redox peaks appeared at TH–COH-CS/GCE (Fig. 2e), with the anodic (Epa) and cathodic potential (Epc) of −0.110 V and −0.234 V, respectively. This can undoubtedly be attributed to the redox reaction of TH, indicating TH has been successfully immobilized on the electrode surface. The surface concentration of TH was estimated according to the equation Q = nFAΓ, where Q is the quantity of charge (C), calculated from the reduction peak area of the voltammogram, A is the electrode area (cm2), Γ is the surface coverage of the electrode reaction substance (mol cm−2), n is the electron transfer number, and F is the Faraday constant (96485 C mol−1). The surface concentration of TH on TH–COH-CS/GCE was estimated to be 6.39 × 10−11 mol cm−2. | ||
| Fig. 2 CVs of (a) bare GCE, (b) CS/GCE, (c) CHO-CS/GCE, (d) TH–CS/GCE and (e) TH–COH-CS/GCE in 0.1 M PBS (pH 6.5) at a scan rate of 50 mV s−1. | ||
EIS is a powerful tool for probing the interface features of the modified electrodes. Fig. 3 shows the typical Nyquist plots obtained on (a) the bare GCE, (b) CS/GCE, (c) CHO-CS/GCE and (d) TH–COH-CS/GCE in a solution of 0.1 M KCl containing 5 mM Fe(CN)63− and 5 mM Fe(CN)64− with the frequency ranging from 104 to 10−1 Hz. Compared with the bare GCE (Fig. 3a), CS/GCE showed a smaller electron transfer resistance (Ret) value (Fig. 3b). This can be attributed to the resulting positively charged surface after CS film formation on GCE, which accelerated the approach of the negatively charged redox probe to the electrode surface. When CS/GCE was periodate-oxidized to generate an aldehyde-enriched surface, the Ret value of CHO-CS/GCE was remarkably increased (Fig. 3c). It is probable that the lone pair electrons of the oxygen atoms increased the negative charge density, which made it difficult for the redox probe to access the electrode surface. As expected, TH–COH-CS/GCE showed a decreased value of Ret (Fig. 3d), due to the strong electrostatic adsorption between the negatively charged Fe(CN)63−/4− and the positively charged TH molecules.
 | ||
| Fig. 3 EIS for (a) the bare GCE, (b) CS/GCE, (c) CHO-CS/GCE and (d) TH–COH-CS/GCE in a solution of 0.1 M KCl containing 5.0 mM Fe(CN)63− and 5.0 mM Fe(CN)64−. | ||
Further study was performed to investigate the effect of scan rate on the response of TH in 0.1 M PBS (pH 6.5) (see Fig. S1, ESI†). It was obvious that CVs of TH–COH-CS/GCE gave nearly symmetric anodic and cathodic peaks. With increasing scan rate, the Epa and Epc were shifted slightly towards positive and negative potential, respectively. Both the anodic and the cathodic peak currents of the TH–COH-CS/GCE were proportional to the scan rate between 20 and 500 mV s−1, indicating a surface-controlled process.
Fig. 4 depicts the electrocatalytic response of TH–COH-CS/GCE in 0.1 M PBS (pH 6.5) without (a) and with (b) 1.0 mM H2O2 at a scan rate of 50 mV s−1. Upon the addition of H2O2 to the substrate solution, one can observe an increased reduction peak current and a decreased oxidation current (Fig. 4b), suggesting a typical electrocatalytic reduction process towards H2O2. The mechanism of H2O2 catalyzed by TH can be described as follows:
| Thionine(Ox) + 2e− + H+ → Thionine(Red) |
| Thionine(Red) + H2O2 → Thionine(Ox) + H2O |
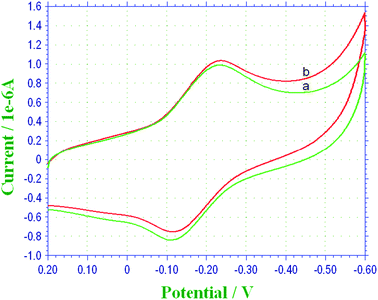 | ||
| Fig. 4 CVs of the TH–COH-CS/GCE (a) without H2O2 and (b) with 1.0 mM H2O2 in 0.1 M PBS (pH 6.5) at a scan rate of 50 mV s−1. | ||
3.3 Influence of pH and applied potential on the developed sensor responses
In acidic media, the amino group of CS is protonated, leading to the dissociation of CS from the electrode surface, which may influence the accuracy and stability of the sensor. Furthermore, TH is a pH-dependent dye,27 so the effect of solution pH on the electrochemical behavior of TH modification on GCE was also investigated. CVs of the TH–COH-CS/GCE in 0.1 M PBS from pH 4.0 to 9.0 at a scan rate of 50 mV s−1 are shown in Fig. 5. It was obvious that both Epc and Epa shifted in the negative direction with increasing pH, but the ΔEp changed slightly, indicating good reversibility of TH on the well-confined surface. In acidic solution, a relatively poor reversibility of TH was obtained, which may be caused by the partial dissolution of CS. The redox current increased from pH 4.0 and reached a maximum at pH 6.5. Then, the current response was found to decrease from pH 7.0 to 9.0. E°, which is estimated as the midpoint between Epa and Epc, shows a linear relationship with pH from 4.0 to 5.0 with a slope of −57 mV pH−1, which was very close to the anticipated Nernstian value of 59 mV pH−1, indicating a reversible two-electron and two-proton transfer process.28 However, a slope of −45 mV pH−1 was obtained when the pH increased from 6.0 to 9.0, which is in accordance with a two-electron and one-proton process.33 PBS with pH 6.5 was selected as the supporting electrolyte.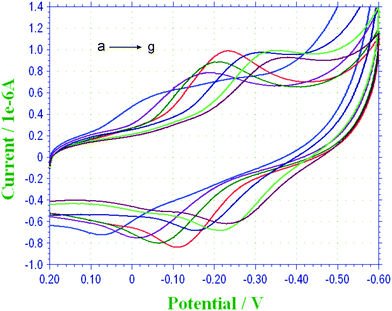 | ||
| Fig. 5 CVs of the TH–COH-CS/GCE in 0.1 M PBS containing 0.1 M KCl at pH (a) 4.0, (b) 5.0, (c) 6.0, (d) 6.5, (e) 7.0, (f) 8.0, and (g) 9.0 at a scan rate of 50 mV s−1. | ||
The effect of applied potential on the sensor performance was also investigated from −0.40 to −0.15 V by amperometric measurements in the presence of 0.5 mM H2O2 (see Fig. S2, ESI†). The current increased rapidly with the applied potential shifting from −0.40 to −0.22 V. At a potential more positive than −0.22 V, a significant decrease in the current was observed. The current approached a maximum value at −0.22 V, thus an operating potential of −0.22 V was chosen for further amperometric determination of H2O2.
3.4 Amperometric response of the developed sensor
Fig. 6 displays the amperometric response of the TH–COH-CS/GCE to successive addition of H2O2 in 0.1 M PBS (pH 6.5) with an applied potential of −0.22 V. Upon addition of H2O2 to the stirring solution, an apparent increase in the reduction current was observed. The modified electrode achieved 95% of the maximum steady-state current in less than 4 s, indicating a fast diffusion of the substrate towards the modified electrode. The inset in Fig. 6 shows the calibration curve of the sensor for H2O2 determination. Under the optimized experimental conditions, the linear range spans the concentration of H2O2 from 1.0 × 10−5 to 1.83 × 10−3 M. The linear regression equation is I (10−8A) = 4.982 C (10−3 M) + 0.0741 with a relative coefficient of 0.9996 (n = 16, inset in Fig. 6). The detection limit was estimated to be 4.0 × 10−6 M defined from a signal-to-noise ratio of 3. To demonstrate the analytical performance of the proposed sensor, a comparison of response time, linear range and lower detection limit with other TH-based sensors is listed in Table 1. It can be seen that the performance of the proposed sensor is comparable to most TH-based sensors. Most non-enzyme based H2O2 sensors commonly use carbon nanotubes, which usually include complicated pretreatment procedures. In this sense, the straightforward and simple strategy we proposed may exhibit a little superiority. In our previous work,31 a similar sensor was fabricated by direct immobilization of TH on potentiostatic activated GCE via a Schiff-base reaction, and the sensor exhibited a wider linear range and lower detection limit; however, potentiostatic activation of GCE in dilute nitric acid can largely damage the electrode, resulting in an increase in the cost. Taken in this sense, the proposed strategy can be a facile and inexpensive method.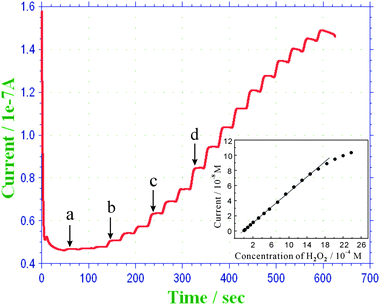 | ||
| Fig. 6 Typical amperometric response of the TH–COH-CS/GCE to successive addition of H2O2 (a) 1 × 10−5 M, (b) 6 × 10−5 M, (c) 1.2 × 10−4 M, (d) 1.8 × 10−4 M into stirred 0.1 M PBS (pH 6.5). The inset shows the linear part of the calibration curve between the current and the concentration of H2O2. | ||
3.5 The interference, reproducibility and stability of the developed sensor
The potential interference of some biological species, such as ascorbic acid, dopamine and uric acid, for the determination of H2O2 at TH–COH-CS/GCE was also investigated. A negligible increase in the reduction current was observed upon the injection of 1.0 mM of the above species to 0.5 mM H2O2, which can be attributed to the low detection potential, indicating the sensor possessed good anti-interference ability. The reproducibility and stability of the sensor were tested by the amperometric measurements to 0.5 mM H2O2. A relative standard deviation (R.S.D.) of 3.4% for 11 successive assays was obtained, which was a satisfactory reproducibility for the developed sensor. The fabrication reproducibility was investigated by preparing six biosensors independently using the same procedure to obtain a R.S.D. of 2.9%. After storage in 0.1 M PBS (pH 6.5) at 4 °C for 21 days, the sensor retained 96.6% of its initial response to the reduction of H2O2. It is reasonable that the biocompatible material CS provided a mild environment for retention of the electroactivity of TH, and the covalent bond between periodate-oxidized CS and TH endowed TH with high stability.4. Conclusions
A CS-graft-TH sensing platform was successfully constructed based on the Schiff-base reaction between amino groups of TH and aldehyde groups of periodate-oxidized CS. The conventional time-consuming process of dialysis was substituted by direct removal of the CS modified electrode from the periodate solution and thoroughly rinsing the surface with DDW, which largely simplified the experimental procedure. The developed CS-graft-TH sensing platform exhibited fast amperometric response to H2O2 with a wide linear range, low detection limit, high sensitivity, good reproducibility, long-term stability and anti-interference ability. The multiple binding sites of periodate-oxidized CS make it a promising material for the immobilization of other amine compounds, such as enzymes and DNA.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 20775039), the Natural Science Foundation of Shandong Province of China (No. ZR2009BM031), the Public Welfare Project of Marine Science Research (Nos. 200705011, 200805039), Science and Technology Project of Shandong Company, China National Tobacco Corporation (No. KN172), and the Scientific Research Fund of the First Institute of Oceanography, SOA (No. 2010T04).References
- J. Berger, M. Reist, J. M. Mayer, O. Felt, N. Peppas and R. Gurny, Eur. J. Pharm. Biopharm., 2004, 57, 19 CrossRef CAS.
- E. Khor and L. Y. Lim, Biomaterials, 2003, 24, 2339 CrossRef CAS.
- P. Guo, C. R. Martin, Y. P. Zhao, J. Ge and R. N. Zare, Nano Lett., 2010, 10, 2202 CrossRef CAS.
- M. N. V. Ravi Kumar, R. A. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. J. Domb, Chem. Rev., 2004, 104, 6017 CrossRef.
- X. Y. Xu, P. J. Dong, Y. Feng, F. Li and H. J. Yu, Anal. Methods, 2010, 2, 546 RSC.
- X. W. Liu, Q. Y. Hu, Z. Fang, X. J. Zhang and B. B. Zhang, Langmuir, 2009, 25, 3 CrossRef CAS.
- J. Y. Je and S. K. Kim, J. Agric. Food Chem., 2006, 54, 6629 CrossRef CAS.
- F. Xiao, F. Q. Zhao, Y. F. Zhang, G. P. Guo and B. Z. Zeng, J. Phys. Chem. C, 2009, 113, 84.
- F. Li, Z. Wang, W. Chen and S. S. Zhang, Biosens. Bioelectron., 2009, 24, 3030 CrossRef CAS.
- M. Bodnar, J. F. Hartmann and J. Borbely, Biomacromolecules, 2005, 6, 2521 CrossRef CAS.
- D. S. Rodrigues, A. A. Mendes, W. S. Adriano, L. R. B. Goncalves and R. L. C. Giordano, J. Mol. Catal. B: Enzym., 2008, 51, 100 CrossRef CAS.
- P. Fernandez-Saiz, J. M. Lagarón and M. J. Ocio, J. Agric. Food Chem., 2009, 57, 3298 CrossRef CAS.
- H. Yi, L. Q. Wu, W. E. Bentley, R. Ghodssi, G. W. Rubloff, J. N. Culver and G. F. Payne, Biomacromolecules, 2005, 6, 6.
- C. S. Shan, H. F. Yang, D. X. Han, Q. X. Zhang, A. Ivask and L. Niu, Biosens. Bioelectron., 2010, 25, 1070 CrossRef CAS.
- M. G. Zhang and W. Gorski, J. Am. Chem. Soc., 2005, 127, 2058 CrossRef CAS.
- R. S. Vieira and M. M. Beppu, Colloids Surf., A, 2006, 279, 196 CrossRef CAS.
- G. L. Huang, H. Y. Zhang, J. X. Shi and T. A. G. Langrish, Ind. Eng. Chem. Res., 2009, 48, 2646 CrossRef CAS.
- F. Li, Z. Wang and Y. Feng, Sci. China Chem., 2009, 52, 2269 Search PubMed.
- S. Lin-Gibson, H. J. Walls, S. B. Kennedy and E. R. Welsh, Carbohydr. Polym., 2003, 54, 193 CrossRef CAS.
- M. M. Thanou, A. F. Kotzé and T. Scharringhausen, J. Controlled Release, 2000, 64, 15 CrossRef CAS.
- A. Bernkop-Schnűrch, M. Hornof and D. Guggi, Eur. J. Pharm. Biopharm., 2004, 10, 9 CrossRef.
- M. Ishihara, K. Nakanishi and K. Ono, Biomaterials, 2002, 23, 833 CrossRef CAS.
- T. Tojima, H. Katsura and S. Han, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 1965 CrossRef CAS.
- H. L. Jiang, Y. K. Kim, R. Arote, J. W. Nah, M. H. Cho, Y. J. Choi, T. Akaike and C. S. Cho, J. Controlled Release, 2007, 117, 273 CrossRef CAS.
- I. M. N. Vold and B. E. Christensen, Carbohydr. Res., 2005, 340, 679 CrossRef CAS.
- K. Thenmozhi and S. S. Narayanan, Biosens. Bioelectron., 2007, 23, 606 CrossRef CAS.
- V. E. Nicotra, M. F. Mora, R. A. Iglesias and A. M. Baruzzi, Dyes Pigm., 2008, 76, 315 CrossRef CAS.
- A. Salimi, N. Amini, H. Danyali and R. Hallaj, Electroanalysis, 2006, 18, 1664 CrossRef CAS.
- H. Lin, H. M. Cheng, X. P. Miao, P. Papakonstantinou, D. Mihailovič and M. X. Li, Electroanalysis, 2009, 21, 2602 CrossRef CAS.
- J. Y. Ding, P. Y. Shih and C. K. Yin, Mater. Chem. Phys., 2004, 84, 263 CrossRef CAS.
- X. Y. Xu, Y. Feng, J. J. Li, F. Li and H. Y. Yu, Biosens. Bioelectron., 2010, 25, 2324 CrossRef CAS.
- Y. Xu, Y. Jiang, L. Yang, P. G. He and Y. Z. Fang, Chin. J. Chem., 2005, 23, 1665 CrossRef CAS.
- J. Clavilier, V. Svetlicic and V. Zutic, J. Electroanal. Chem., 1995, 386, 157 CrossRef.
- H. Lin, H. M. Cheng, X. P. Miao, P. Papakonstantinou, D. Mihailovič and M. X. Li, Electroanalysis, 2009, 21, 2602 CrossRef CAS.
- D. R. Shobha Jeykumari, S. Ramaprabhu and S. Sriman Narayanan, Carbon, 2007, 45, 1340 CrossRef.
- K. Y. Zhang, L. Zhang, J. G. Xu, C. Wang, T. Geng, H. Y. Wang and J. Zhu, Microchim. Acta, 2010, 171, 139 CrossRef CAS.
- A. Salimi, A. Noorbakhsh, H. Mamkhezri and R. Ghavami, Electroanalysis, 2007, 19, 1100 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Electrochemical data. See DOI: 10.1039/c0ay00499e |
| This journal is © The Royal Society of Chemistry 2010 |
