Determination strategies of phytohormones: recent advances
Yu
Bai
,
Fuyou
Du
,
Yu
Bai
and
Huwei
Liu
*
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, Institute of Analytical Chemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, P. R. China. E-mail: hwliu@pku.edu.cn; Tel: +086-010-62754976
First published on 14th October 2010
Abstract
Phytohormones are key regulators in various physiological processes of plant growth and development. To better understand their regulatory actions, fast, sensitive and accurate methods for phytohormone analysis are critically required, and great efforts have been invested in this field. In this review, recent advances and developments in phytohormone determination strategies are presented, in terms of sample preparation and analytical approaches. Many types of analytical techniques are involved, including chromatography, mass spectrometry, immunoassay and others. Some challenges and prospects are also discussed.
 Yu Bai | Yu Bai completed her B.S. degree at Nankai University in 2007 and now she is studying for her doctorate in analytical chemistry at Peking University. Her research is oriented towards the development of new chromatographic and mass spectrometric methods for the fast, accurate and sensitive determination of plant hormones. |
 Fuyou Du | Fuyou Du obtained his PhD in chemistry from Sun Yat-Sen University and joined the research group of Prof. Huwei Liu as a postdoctoral fellow at Peking University in 2008. His major research interests focus on the analysis of plant peptide hormones by the combined methods of immunology, chromatography and mass spectrometry. |
 Yu Bai | Yu Bai is currently an Associate Professor at Peking University. She obtained her PhD in chemistry from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences in 2004. Her major research interests focus on proteomics, disease metabolomics and analysis of bio-active molecules using liquid chromatography-mass spectrometry approaches. |
 Huwei Liu | Huwei Liu received his PhD degree from the Beijing Institute of Technology in 1990, and now he is a professor at the Institute of Analytical Chemistry, Peking University, His research interests mainly focus on pharmaceutical and clinical analysis, and the analysis of bio-active compounds in complex matrices, by hyphenated techniques based on chromatography, capillary electrophoresis and mass spectrometry. |
1. Introduction
Phytohormones are a group of naturally occurring, organic substances which influence physiological processes at low concentrations.1 They take part in the regulation of almost all phases of plant growth and development, such as seed germination,2 cell division, organ formation,3 stem elongation,4 senescence5 and so on. Besides, phytohormones also mediate the responses of the plant to various environmental stresses. On the basis of diverse structures and physiological functions, it is commonly accepted that plant hormones can be mainly grouped into several major classes which include auxins, ethylene, cytokinins (CKs), gibberellins (GAs), abscisic acid (ABA), salicylic acid (SA), jasmonates, brassinosteroids (BRs) and peptide hormones. More new phytohormones are also likely to be found with better understanding of plant regulation in the future. While each of them has characteristic biological effects, several kinds of phytohormones are also interconnected in a cross-talk way, which means multiple hormones can mediate the plant growth and development synergistically or antagonistically.1,6For a better understanding of the functions of the phytohormones and the interactions between them, exhaustive determination of the hormone contents is thus of great importance. However, to settle this issue, there are some considerable challenges. One of the challenges is that different hormones have diverse chemical and structural properties, which makes the simultaneous and equitable evaluation of their quantities difficult. Besides, because their active concentrations are very low, usually at the ppb level, high sensitivity of the method is highlighted, especially when only limited amounts of sample are available for analysis. Therefore, development of a rapid, sensitive, accurate and efficient method for the analysis of phytohormones from complex biological samples is necessary and significant for botany research.7
2. State of the art in phytohormone analysis
Since the discovery of phytohormones, great efforts have been invested in the determination of hormone levels in various kinds of plants. Several review articles have briefly presented the general status of phytohormone analysis,8,9 or detailed one aspect such as cytokinin determination10–12 or mass spectrometry (MS) profiling of hormones.13 In this article, we review the recent advances in plant hormone analysis by a variety of techniques.Bioassays are a classical method for hormone analysis14 and were first used in the determination of auxins in 1928. Because of tedious sample preparation, low specificity and poor repeatability, the application of bioassays has attracted little attention in recent years. Immunoassays15 have also been employed for phytohormone determination, considering that the specificity of assays is favorable. However, the presence of cross reactions and the long period of antibody preparation limit their further application. In addition, the specific antibody is not suitable for the simultaneous detection of multiple plant hormones in different classes. Chromatographic methods, especially when coupled with MS, have shown good separation and qualitative abilities and have become powerful tools for the detection of hormone contents from complex plant samples. These studies started from the analysis of one compound16 or one specified class,17 and gradually extended to two or more classes of phytohormones, some of which even comprised the quantification of related metabolites.18 Therefore, in this review, recent developments of chromatographic methods are discussed in detail, along with some advancements in other techniques such as immunoassays, spectrometry, electrochemical methods and so on.
2.1 Sample preparation
Phytohormones are difficult to analyze not only because they exist in trace amounts but also because some more abundant substances present in plant tissues can interfere with the analysis. The process of sample treatment can affect the sensitivity of a whole method significantly.19 Therefore, it is pivotal to choose proper approaches for the extraction and purification of plant hormones before their analysis.Considering many kinds of phytohormones are distributed in different plants at a trace level, multiple procedures for sample preparation are often required to develop an effective method for phytohormone analysis. A typical process for the determination of phytohormones in plant tissues is shown in Scheme 1, which comprises general steps of sample preparation and analysis, such as homogenization, extraction, purification and so on. In the homogenization step, the collected plant samples need to be stored at low temperature immediately and ground into powder in liquid nitrogen,20 to avoid chemical degradation or metabolic change of the hormones of interest.13 As for the extraction, since the plant hormones are structurally and chemically diverse compounds, in order to extract the target hormones as efficiently as possible, various extraction solvents have been employed such as water,16 methanol,21 ethyl acetate22 and so on. Bieleski solvent,23 which consists of methanol, water and formic acid, is widely used in plant extraction,17,24–26 as it can prevent the degradation of plant hormones and block the extraction of too many lipids.24 When it comes to the purification step, three main measures are usually adopted: solid phase extraction (SPE), liquid–liquid extraction (LLE) and immunoextraction.
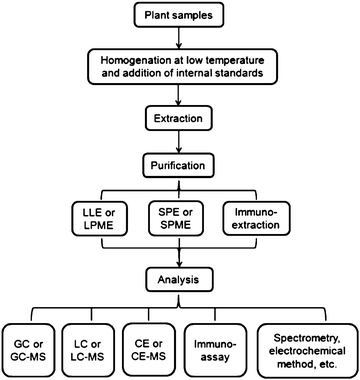 | ||
| Scheme 1 A typical process for phytohormone analysis in plant samples. | ||
SPE with various kinds of sorbent or solid phase microextraction (SPME) with different fibers, which utilize the retention differences of compounds on a stationary phase to separate the compounds of interest, are common procedures for further purification of the crude plant extract.16,21,22,27–31 C18 cartridges are capable of retaining lipids and plant pigments in the crude extract. One type of mixed-mode extraction cartridge, which combines the reversed-phase retention features with ion-exchange ability, is well exploited for a rapid and effective purification process,25,27,32–34 and it is in good conformity with the analysis of multiple phytohormones which present diverse structural characteristics. Besides, it is reported that the mixed-mode cartridges are able to significantly decrease the ion suppression effects of the plant matrix in MS detection.25
Taking advantage of the analyte solubility differences in different solvents, LLE is an effective way to purify the plant extract35–37 as well. At the early stage, utilization of LLE might result in the use of a large volume of solvent and cumbersome concentration steps, as a large quantity of plant tissues was used in the extraction. However, with advancement of detection techniques and decreasing demand for sample amount, LLE becomes easier to handle since the extraction solvent volume is reduced, sometimes to as little as 1 mL,20,36 which facilitates the fast and high throughput analysis of phytohormones. Furthermore, liquid phase microextraction (LPME) methods for sample pretreatment have been applied in the simultaneous determination of several hormones.38,39 One of them39 was realized through the hollow fiber-based liquid–liquid–liquid microextraction (HF-LLLME), which utilized the pH difference between the donor and acceptor phases to extract and concentrate the analytes. Results showed good enrichment factors and ideal sensitivity even with only low-sensitivity UV detection.
Immuno-based extraction has also been applied for further sample purification.40–42 Antibodies with high specificity for the target analytes are raised and prepared in an immunoaffinity column (IAC), which allows the specific capture and efficient concentration of the compounds of interest. On the other hand, the specificity of the antibodies confines the analytes to one class of plant hormones that exhibit similar structures, and the lack of durability of IAC makes it unsuitable for high throughput analysis.
Meanwhile, some new materials and methods have been also tried in the sample preparation. A durable polydimethylsiloxane (PDMS) film was fabricated and used for the extraction of methyl jasmonate in leaf extracts.43 The high extraction efficiency and good reproducibility of the film provided an impressive detection limit of 0.2 ng mL−1 for the method with gas chromatography-flame ionization detection (GC-FID). Thus it is no exaggeration to say that an appropriate sample pretreatment step is able to shape the whole analysis method. It is worth mentioning that not all these steps of sample preparation are needed in every case of phytohormone analysis and some of them can be simplified.44,45 The strategies actually adopted for hormone determination greatly depend on the characteristics and content distribution of the targets, the number of phytohormone classes of interest and the analytical methods to be applied next.
2.2 Analytical approaches
Two-dimensional (2D) LC has been employed in the quantification of plant hormones. Dobrev et al.33 developed a 2D-LC method in ‘heart cutting’ mode, which efficiently removed the co-eluting contaminants with indole-3-acetic acid (IAA) and ABA. And thus even with non-selective detectors, the on-line quantification was still reliable.
An alternative means to the traditional UV method is fluorescence detection.38,49,50 Chen et al.49 developed 6-oxy(acetylpiperazine) fluorescein (APF) as a new fluorescent labeling reagent for carboxylic acids and realized the quantification of auxins in vegetable samples. Besides, determination of auxin with chemiluminescence (CL) detection was also realized51 through the CL reaction of auxins with immobilized Ru(bpy)32+-KMnO4.
Combined with MS, LC-MS is the most widely employed method for plant hormone analysis.19,24,35,41,52–57 One of the most prominent advantages of LC-MS is that the accurate quantitation and qualitation for analytes can be both achieved without many purification or derivatization steps. Fletcher et al.58 developed a method for profiling the phytohormones in dormant Macadamia integrifolia with liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (LC-QToF-MS/MS) and studied their correlations with abnormal vertical growth (AVG). With higher mass accuracy and better resolution, QToF-MS is suitable for wide-range hormone profiling. Ma et al.59 described an approach to simultaneously analyze four classes of phytohormones including auxins, ABA, GA and CKs with liquid chromatography-ion trap tandem mass spectrometry (LC-IT-MS/MS), and applied it to screen putative endogenous phytohormones present in coconut water. Endogenous jasmonic acid (JA), SA and their related compounds were successively quantified in tobacco plants by UPLC-MS/MS.18 In particular, measuring the accumulation patterns of glucosides and other related compounds is valuable for the understanding of their interactions and the biosynthetic pathway of JA and SA. Sensitive quantification of several classes of phytohormones and their derivatives was achieved by a nanoflow-LC-ESI-IT-MS/MS method.25 With the detection limits in the sub-fmol range, this method was successfully employed to analyze endogenous hormones in 1 mg dry tobacco seeds, which indicated that the improvement of detection sensitivity could greatly increase the analysis efficiency. Derivatization measures are also used in LC-MS analyses. Kojima et al.32 developed a high-throughput method to simultaneously quantify 43 molecular species from four classes of phytohormones including CKs, auxins, ABA and GAs. After derivatization of acidic compounds with bromocholine, the response of targets was improved and all compounds of interest could be analyzed in a single run. Pan et al.20,36 established a rapid and sensitive method for the determination of a number of phytohormones which covered six major classes of hormones and related metabolites in plant extracts. After double extraction, the sample was subjected to analysis with LC-MS/MS without further derivatization, which realized the differentiation between hormone esters and their acid forms.
In LC-MS analysis of phytohormones, electrospray ionization (ESI)20,25,32,45 and atmospheric pressure chemical ionization (APCI)60 are two main choices of ion source. ESI is preferable and more popular in practice since the electrospray ionization process happens in the liquid phase and is more suitable for the analysis of polar compounds. Compared to the full scan MS mode, tandem mass spectrometry (MS/MS) with multiple reaction monitoring (MRM) mode provides better selectivity, specificity and sensitivity13 for phytohormone qualitation and quantification and thus has obtained more application.18,36,57 Usually, isotope labeled compounds are appropriate as internal standards in target quantification, as they have almost the same physical and chemical properties as their corresponding analytes, which provides correction for analyte loss during sample preparation and also compensates for the suppression of ion yield caused by co-eluting components in the sample matrix.13,19 Employment of appropriate internal standards contributes to an accurate evaluation of method recovery and ensures more reliable results. However, selection of proper internal standards is sometimes arduous as the availability of some compounds is limited.18 Thus the process of synthesizing internal standards brings an extra workload for a whole analysis.
An open-tubular capillary electrochromatography method based on permanent polydopamine coating was first developed and successfully used in the determination of four auxins.64 Micellar electrokinetic capillary chromatography (MEKC) methods were applied in the quantification of several CKs in coconut water65,66 and tobacco flowers.67 Besides, monitoring the purity of systemin in preparation process68 was realized by a simple capillary zone electrophoresis (CZE) mode. To improve the sensitivity of CE-UV, some on-line concentration approaches such as large volume sample stacking67,69 have been integrated into hormone analysis and satisfactory limits of detection (LOD) have been achieved. Combining the efficiency of CE and the selectivity of LC, pressurized capillary electrochromatography (pCEC) methods have also been exploited in phytohormone determination69,70 and used to quantify multiple classes of plant hormones including auxin, ABA, GA and CK.
To enhance the method sensitivity, other types of detection such as laser-induced fluorescence (LIF) and CL detection have also been employed.44,71,72 Moreover, the combination of CE and MS shows good sensitivity and structural identification, which is appropriate for hormone analysis and it thus becomes another technique complementary to LC-MS. Cytokinins73 and cytokinin nucleotides74 in coconut water were analyzed by CZE-MS/MS together with on-line sample stacking. Eleven species of GAs were completely separated and identified in 25 min in a polymer-coated capillary by CE-MS.75 MEKC is a widely used mode in CE separation. However, because of the nonvolatility of the surfactant, the direct coupling of MEKC to MS is limited. Thus the partial filling (PF) technique was developed, in which the nonvolatile surfactants are prevented from entering the ion source.76 A PF-MEKC-MS approach was utilized to quantify eight GAs in coconut water.77 And Ge et al. further employed another PF-MEKC-MS/MS method to determine 13 structurally similar CKs and achieved a satisfactory separation and identification in 25 min.78
Generally, the sensitivity and reproducibility of CE-MS are lower than that of LC-MS, because in CE-MS the nanolitre injection volumes as well as the dilution effect of the sheath liquid cause difficulties for the detection. In addition, the properties of the capillary inner wall are not easily controllable, which results in the instability of electroosmotic flow and irreproducible separation.
Amperometric immunosensors for auxin,82 ABA83 and CK84 analysis in hybrid rice grain samples have been developed. The determination was based on a competitive immunoreaction between the target and horseradish peroxidase (HRP) labeled target to bind with the antibody immobilized on the electrode surface, in which the response signal was expressed as percentage current reduction.82 In addition, impedance immunosensors for ABA have also been investigated.85,86 The ABA target bound to the antibody immobilized on a porous nanogold film electrode,85 bringing about a response of the impedance signal related to the degree of binding.
As a result of the unique ligand–antibody binding, the immuno-based method presents good specificity and high sensitivity. However, besides the time-consuming process of antibody preparation, the cross-reactivity of antibodies often results in reduced specificity and accuracy, especially when they are applied to crude plant extracts. Additionally, the specificity of antibodies conflicts with the demand for simultaneous detection of multiple classes of hormones. On the other hand, since the immuno-based method is less dependent on large equipment and more amenable to miniaturization, it is more portable and suitable for in situ screening of targets in plant tissues.
Although GC is efficient for the structural identification and accurate quantification in multiple phytohormone analysis, the requirement for sample volatility limits its application for all classes of phytohormones. Thus extra derivatization steps are commonly needed in the process of sample preparation, to increase sample volatility and sensitivity. When methyl esters coexist in the plant with free carboxylic acids,36 the derivatization of acids into methyl esters is not appropriate as it cannot distinguish the original methyl esters from free acids. Besides, some thermally labile components are likely to break down at the high temperature of the GC injector and column, which limits the range of plant hormones fit for GC analysis.
Recently, some new spectral methods have also been exploited for phytohormone analysis. Ethylene content in tomatoes was quantified by visible/short-wave near-infrared (Vis/SW NIR) spectroscopy, combined with some chemometrics methods including partial least squares regression (PLSR) and stepwise multiple linear regression (SMLR).92 An excitation-emission matrix fluorescence coupled to second-order calibration method was developed and applied to detect ABA and GA in the ginkgo leaves and buds.93 Quantum dots (QDs) have also been adopted in the determination of ABA,94 which is based on the fluorescent quenching mechanism of QDs. Guo et al.95 described a colorimetric method for the differentiation of IAA and indolebutyric acid (IBA). The color change resulted from the Ehrlich reaction between the auxins and p-(dimethylamino)benzaldehyde (PDAB). Additionally, the method was successfully used to monitor the IAA change in the growth of mung bean sprout.
As for the electrochemical detection of plant hormones, de Toledo et al.96 proposed a method for quantification of IAA, with a graphite-polyurethane composite electrode and square wave voltammetry, and Wu et al.97 developed an amperometric sensor for IAA based on multi-walled carbon nanotubes (MWNTs)-film coated glassy carbon electrode. Investigation of electrochemical behavior and determination of IAA was also carried out on a nanoAu/MWNTs/chitosan-modified electrode.98 The determination of 6-benzyl adenine (6-BA) has been studied at a mercury electrode99 and it was applied to analyze 6-BA in bean sprout samples.
These spectral methods and electrochemical strategies are proved to be simple, fast and effective. The number of targets in most of them are limited to only one or two, as these schemes are designed to be of some specificity. However, in some cases, the specificity is susceptible to impurities present in real samples. In addition, the lack of universality makes these protocols not easily adaptable for the analysis of other plant hormones.
3. Conclusions and prospects
The advanced instrumental techniques, separation strategies and detection approaches in analytical science have led to the continuing improvements in multiple phytohormone analysis. Among these strategies, chromatographic methods including LC/LC-MS, GC/GC-MS and CE/CE-MS have been mostly employed with the advantages of good sensitivity, high throughput, excellent accuracy and favorable precision. Coupling diverse separation techniques with various types of detection, chromatographic methods are capable of simultaneously analyzing multiple plant hormones in different plant samples. At the same time, other detection techniques, such as immunoassays, spectrometry, electrochemical approaches and so on, are necessary alternatives for plant hormone analysis, as they can explore other features of the hormones of interest. All of these analysis approaches have greatly promoted the biological research of phytohormones at the molecular level.Meanwhile, challenges still remain in several aspects. Firstly, as different phytohormones act in a highly interconnected manner in a complex dynamic network, and changes of one component in the hormone system may bring about a chain of influences on other hormones, it is necessary to develop a comprehensive determination strategy for monitoring all classes of phytohormones, along with their metabolites and precursors. For this purpose, higher speed, efficiency and universality in the sample preparation are needed. However, at present there are various kinds of extraction solvent and purification steps for phytohormones of diverse characteristics in different complex matrices, and no method is reported to extract all the hormones equally well, which is not compatible with the comprehensive determination of all classes of phytohormones. Therefore, more universal and effective sample pretreatment procedures with high throughput are in urgent need. Secondly, though the sensitivity of the methods has been greatly improved, the minimum quantity of fresh sample consumption is about dozens of milligrams,20 which is still a large quantity since in most cases the amount of available sample is quite limited. Thus, to further evaluate hormone levels in specific cells, ultra-high sensitivity as well as microsurgical techniques for harvesting several cell populations is required. In addition, detaching the leaves or tissues from the plants may induce some changes in hormone levels. Also, the samples are usually homogenized in the extraction solvent with the quantified results showing only the average hormone levels in the whole tissue. So, to determine the local hormone contents more accurately and precisely, some ultrasensitive, in vivo real-time detection methods are desired. For this purpose, some miniaturized electrochemical probes or MS imaging techniques may contribute to its realization.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 90717002, 20905005, and 20805001) and by the Science Foundation of China Postdoctoral Grant (No. 20090450231).Notes and references
- P. J. Davies, The plant hormones: their nature, occurrence, and functions. In: P. J. Davies (Ed.), Plant hormones: biosynthesis, signal transduction, action!, Kluwer Academic Publishers, Dordrecht, Boston, London, pp. 1–15, 2004 Search PubMed.
- C. Sánchez-Rodríguez, I. Rubio-Somoza, R. Sibout and S. Persson, Trends Plant Sci., 2010, 15, 291–301 CrossRef CAS.
- K. Magyar-Tábori, J. Dobránszki, J. A. T. da Silva, S. M. Bulley and I. Hudák, Plant Cell, Tissue Organ Cult., 2010, 101, 251–267 CrossRef CAS.
- L. N. Mander, Nat. Prod. Rep., 2003, 20, 49–69 RSC.
- B. De Rybel, D. Audenaert, T. Beeckman and S. Kepinski, ACS Chem. Biol., 2009, 4, 987–998 CrossRef CAS.
- R. Aloni, E. Aloni, M. Langhans and C. I. Ullrich, Ann. Bot., 2006, 97, 883–893 CrossRef CAS.
- Z. H. Xu and J. Y. Li, Chinese Bull. Bot., 2006, 23, 433–442 Search PubMed.
- Y. W. Li and L. T. Xiao, Life Sci. Instrum., 2007, 5, 10–14 Search PubMed.
- Y. Bai, F. Y. Du, Y. Bai and H. W. Liu, Chinese Bull. Life Sci., 2010, 22, 36–44 Search PubMed.
- L. Y. Ge, S. Tan, J. W. H. Yong and S. N. Tan, Electrophoresis, 2006, 27, 4779–4791 CrossRef CAS.
- Y. Liang, M. P. Zhao and H. W. Liu, Chin. J. Anal. Chem., 2009, 37, 1232–1239 CAS.
- P. Tarkowski, L. Y. Ge, J. W. H. Yong and S. N. Tan, TrAC, Trends Anal. Chem., 2009, 28, 323–335 CrossRef CAS.
- X. Q. Pan and X. M. Wang, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2009, 877, 2806–2813 CrossRef CAS.
- B. Volksch, F. Bublitz and W. Fritsche, J. Basic Microbiol., 1989, 29, 463–468 CrossRef.
- G. Sandberg, A. Crozier and A. Ernstsen, Indole-3-acetic acid and related compounds. In: L. Rivier and A. Crozier (Ed.), Principles and practice of plant hormone analysis, Academic Press, London, pp, 233, 1987 Search PubMed.
- R. Meyer, G. F. Rautenbach and I. A. Dubery, Phytochem. Anal., 2003, 14, 155–159 CrossRef CAS.
- O. Novák, P. Tarkocwski, D. Tarkowská, K. Doležal, R. Lenobel and M. Strnad, Anal. Chim. Acta, 2003, 480, 207–218 CrossRef CAS.
- H. Matsuura, A. Aoi, C. Satou, M. Nakaya, C. Masuta and K. Nabeta, Plant Growth Regul., 2009, 57, 293–301 CrossRef CAS.
- M. López-Carbonell, M. Gabasa and O. Jáuregui, Plant Physiol. Biochem., 2009, 47, 256–261 CrossRef CAS.
- X. Q. Pan, R. Welti and X. M. Wang, Nat. Protoc., 2010, 5, 986–992 Search PubMed.
- C. Zadra, A. Borgogni and C. Marucchini, J. Agric. Food Chem., 2006, 54, 9317–9321 CrossRef CAS.
- F. J. Zhang, Y. J. Jin, X. Y. Xu, R. C. Lu and H. J. Chen, Phytochem. Anal., 2008, 19, 560–567 CrossRef CAS.
- R. L. Bieleski, Anal. Biochem., 1964, 9, 431–442 CrossRef CAS.
- S. Giannarelli, B. Muscatello, P. Bogani, M. M. Spiriti, M. Buiatti and R. Fuoco, Anal. Biochem., 2010, 398, 60–68 CrossRef CAS.
- Y. Izumi, A. Okazawa, T. Bamba, A. Kobayashi and E. Fukusaki, Anal. Chim. Acta, 2009, 648, 215–225 CrossRef CAS.
- K. Hoyerová, A. Gaudinová, J. Malbeck, P. I. Dobrev, T. Kocábek, B. Šolcová, A. Trávníčková and M. Kamínek, Phytochemistry, 2006, 67, 1151–1159 CrossRef CAS.
- P. I. Dobrev and M. Kamínek, J. Chromatogr., A, 2002, 950, 21–29 CrossRef.
- L. S. Barkawi, Y. Y. Tam, J. A. Tillman, B. Pederson, J. Calio, H. Al-Amier, M. Emerick, J. Normanly and J. D. Cohen, Anal. Biochem., 2008, 372, 177–188 CrossRef CAS.
- H. T. Liu, Y. F. Li, T. G. Luan, C. Y. Lan and W. S. Shu, Chromatographia, 2007, 66, 515–520 CrossRef CAS.
- M. L. R. del Castillo and G. P. Blanch, J. Sep. Sci., 2007, 30, 2117–2122 CrossRef.
- J. Rolčík, J. Řečinská, P. Barták, M. Strnad and E. Prinsen, J. Sep. Sci., 2005, 28, 1370–1374 CrossRef CAS.
- M. Kojima, T. Kamada-Nobusada, H. Komatsu, K. Takei, T. Kuroha, M. Mizutani, M. Ashikari, M. Ueguchi-Tanaka, M. Matsuoka, K. Suzuki and H. Sakakibara, Plant Cell Physiol., 2009, 50, 1201–1214 CrossRef CAS.
- P. I. Dobrev, L. Havlíček, M. Vágner, J. Malbeck and M. Kamínek, J. Chromatogr., A, 2005, 1075, 159–166 CrossRef CAS.
- P. Tansupo, P. Suwannasom, D. L. Luthria, S. Chanthai and C. Ruangviriyachai, Phytochem. Analysis, 2010, 21, 157–162 Search PubMed.
- A. Durgbanshi, V. Arbona, O. Pozo, O. Miersch, J. V. Sancho and A. Gómez-Cadenas, J. Agric. Food Chem., 2005, 53, 8437–8442 CrossRef CAS.
- X. Q. Pan, R. Welti and X. M. Wang, Phytochemistry, 2008, 69, 1773–1781 CrossRef CAS.
- A. R. S. Ross, S. J. Ambrose, A. J. Cutler, J. A. Feurtado, A. R. Kermode, K. Nelson, R. Zhou and S. R. Abrams, Anal. Biochem., 2004, 329, 324–333 CAS.
- Q. M. Lu, L. H. Chen, M. H. Lu, G. N. Chen and L. Zhang, J. Agric. Food Chem., 2010, 58, 2763–2770 CrossRef CAS.
- Y. L. Wu and B. Hu, J. Chromatogr., A, 2009, 1216, 7657–7663 CrossRef CAS.
- E. Hauserová, J. Swaczynová, K. Doležal, R. Lenobel, I. Popa, M. Hajdúch, D. Vydra, K. Fuksová and M. Strnad, J. Chromatogr., A, 2005, 1100, 116–125 CrossRef CAS.
- V. Hradecká, O. Novák, L. Havlíček and M. Strnad, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 847, 162–173 CrossRef CAS.
- A. Pěnčík, J. Rolčík, O. Novák, V. Magnus, P. Barták, R. Buchtík, B. Salopek-Sondi and M. Strnad, Talanta, 2009, 80, 651–655 CrossRef.
- F. Wei, J. H. Cheng, S. Y. Liu, J. Y. Huang, Z. Wang, X. Y. Dong, P. P. Li, F. P. Kong, Y. Wu, Y. H. Li, Y. Q. Feng and H. Chen, Phytochem. Anal., 2010, 21, 290–297 CrossRef CAS.
- Z. L. Zhang, X. Liu, D. F. Li and Y. T. Lu, Anal. Bioanal. Chem., 2005, 382, 1616–1619 CrossRef CAS.
- G. Segarra, O. Jáuregui, E. Casanova and I. Trillas, Phytochemistry, 2006, 67, 395–401 CrossRef CAS.
- V. Diopan, V. Adam, L. Havel and R. Kizek, Molecules, 2009, 14, 1825–1839 Search PubMed.
- G. P. Blanch, G. Flores, M. D. Caja and M. L. R. del Castillo, J. Sep. Sci., 2009, 32, 180–184 CrossRef CAS.
- H. L. Jiang, L. Y. Ying, Q. Wang, H. Y. Shen, M. Ying and T. T. Zeng, Chinese J. Anal. Chem., 2007, 35, 1327–1330 Search PubMed.
- H. Chen, Z. X. Zhang, G. M. Zhang, X. F. Guo, H. S. Zhang and H. Wang, J. Agric. Food Chem., 2010, 58, 4560–4564 CrossRef CAS.
- J. H. Fu, J. F. Chu, J. D. Wang and C. Y. Yan, Chinese J. Anal. Chem., 2009, 37, 1324–1327 Search PubMed.
- Z. J. Xi, Z. J. Zhang, Y. H. Sun, Z. L. Shi and W. Tian, Talanta, 2009, 79, 216–221 CrossRef CAS.
- L. Ge, J. W. H. Yong, N. K. Goh, L. S. Chia, S. N. Tan and E. S. Ong, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2005, 829, 26–34 CrossRef CAS.
- F. Vilaró, A. Canela-Xandri and R. Canela, Anal. Bioanal. Chem., 2006, 386, 306–312 CrossRef CAS.
- S. Forcat, M. H. Bennett, J. W. Mansfield and M. R. Grant, Plant Methods, 2008, 4, 16 CrossRef.
- S. J. Hou, J. Zhu, M. Y. Ding and G. H. Lv, Talanta, 2008, 76, 798–802 CrossRef CAS.
- Q. M. Lu, L. Zhang, T. W. Chen, M. H. Lu, T. Ping and G. N. Chen, Rapid Commun. Mass Spectrom., 2008, 22, 2565–2572 CrossRef CAS.
- M. Kallenbach, I. T. Baldwin and G. Bonaventure, Plant Methods, 2009, 5, 17 CrossRef.
- A. T. Fletcher and J. C. Mader, J. Plant Growth Regul., 2007, 26, 351–361 CrossRef CAS.
- Z. Ma, L. Ge, A. S. Y. Lee, J. W. H. Yong, S. N. Tan and E. S. Ong, Anal. Chim. Acta, 2008, 610, 274–281 CrossRef CAS.
- X. G. Chen, Z. L. Jiang, Y. Zhu and J. Y. Tan, Chromatographia, 2007, 65, 141–147 CrossRef CAS.
- M. Unger, Planta Med., 2009, 75, 735–745 CrossRef CAS.
- T. F. Jiang, Z. H. Lv, Y. H. Wang and M. E. Yue, Anal. Sci., 2006, 22, 811–814 CrossRef CAS.
- N. A. Assuncao, S. C. C. Arruda, A. P. Martinelli and E. Carrilho, J. Braz. Chem. Soc., 2009, 20, 183–187 CAS.
- X. B. Yin and D. Y. Liu, J. Chromatogr., A, 2008, 1212, 130–136 CrossRef CAS.
- L. Y. Ge, J. W. H. Yong, S. N. Tan, X. H. Yang and E. S. Ong, J. Chromatogr. A, 2004, 1048, 119–126 CrossRef CAS.
- L. Y. Ge, J. W. H. Yong, S. N. Tan, X. H. Yang and E. S. Ong, Electrophoresis, 2005, 26, 1768–1777 CrossRef CAS.
- B. F. Liu, X. H. Zhong and Y. T. Lu, J. Chromatogr., A, 2002, 945, 257–265 CrossRef CAS.
- P. Mucha, P. Rekowski, G. Kupryszewski and J. Barciszewski, J. Chromatogr., A, 1996, 734, 410–415 CrossRef CAS.
- S. J. Wang, L. Jia, D. Xing, D. P. Chen and J. S. Zhao, J. Sep. Sci., 2008, 31, 859–864 CrossRef CAS.
- Q. M. Lu, L. Zhang, L. H. Chen, M. H. Lu, P. Tong and G. N. Chen, J. Sep. Sci., 2010, 33, 651–657 CrossRef CAS.
- X. Liu, L. Ma, Y. W. Lin and Y. T. Lu, J. Chromatogr., A, 2003, 1021, 209–213 CrossRef CAS.
- X. B. Yin, J. M. Guo and W. Wei, J. Chromatogr., A, 2010, 1217, 1399–1406 CrossRef CAS.
- L. Y. Ge, J. W. H. Yong, S. N. Tan and E. S. Ong, Electrophoresis, 2006, 27, 2171–2181 CrossRef CAS.
- L. Y. Ge, J. W. H. Yong, S. N. Tan, X. H. Yang and E. S. Ong, J. Chromatogr., A, 2006, 1133, 322–331 CrossRef.
- L. Y. Ge, C. Y. C. Peh, J. W. H. Yong, S. N. Tan, L. Hua and E. S. Ong, J. Chromatogr., A, 2007, 1159, 242–249 CrossRef CAS.
- W. M. Nelson and C. S. Lee, Anal. Chem., 1996, 68, 3265–3269 CrossRef CAS.
- L. Y. Ge, J. W. H. Yong, S. N. Tan, L. Hua and E. S. Ong, Electrophoresis, 2008, 29, 2126–2134 CrossRef CAS.
- L. Y. Ge, S. N. Tan, J. W. H. Yong, L. Hua and E. S. Ong, Electrophoresis, 2008, 29, 2024–2032 CrossRef CAS.
- R. Maldiney, B. Leroux, I. Sabbagh, B. Sotta, L. Sossountzov and E. Miginiac, J. Immunol. Methods, 1986, 90, 151–158 CrossRef CAS.
- J. Zhao, G. Li, G. X. Yi, B. M. Wang, A. X. Deng, T. G. Nan, Z. H. Li and Q. X. Li, Anal. Chim. Acta, 2006, 571, 79–85 CrossRef CAS.
- J. Swaczynová, O. Novák, E. Hauserová, K. Fuksová, M. Šíša, L. Kohout and M. Strnad, J. Plant Growth Regul., 2007, 26, 1–14 CrossRef CAS.
- J. Li, L. T. Xiao, G. M. Zeng, G. H. Huang, G. L. Shen and R. Q. Yu, Anal. Chim. Acta, 2003, 494, 177–185 CrossRef CAS.
- R. Z. Wang, Y. W. Li, Q. Li, G. L. Shen and L. T. Xiao, Anal. Lett., 2009, 42, 2893–2904 CrossRef CAS.
- J. Li, L. T. Xiao, G. M. Zeng, G. H. Huang, G. L. Shen and R. Q. Yu, Anal. Biochem., 2003, 321, 89–95 CrossRef CAS.
- Y. W. Li, K. Xia, R. Z. Wang, J. H. Jiang and L. T. Xiao, Anal. Bioanal. Chem., 2008, 391, 2869–2874 CrossRef CAS.
- Q. Li, R. Z. Wang, Z. G. Huang, H. S. Li and L. T. Xiao, Chin. Chem. Lett., 2010, 21, 472–475 CrossRef CAS.
- C. Birkemeyer, A. Kolasa and J. Kopka, J. Chromatogr., A, 2003, 993, 89–102 CrossRef CAS.
- G. Flores, G. P. Blanch and M. L. R. del Castillo, J. Sep. Sci., 2008, 31, 1207–1214 CrossRef CAS.
- A. Müller, P. Düchting and E. W. Weiler, Planta, 2002, 216, 44–56 CrossRef CAS.
- E. A. Schmelz, J. Engelberth, H. T. Alborn, P. O'Donnell, M. Sammons, H. Toshima and J. H. Tumlinson, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10552–10557 CrossRef CAS.
- B. X. Wang, S. H. Yang, G. H. Chen, Y. Wu, Y. Hou and G. W. Xu, J. Sep. Sci., 2008, 31, 721–726 CrossRef CAS.
- L. J. Xie, Y. B. Ying and T. J. Ying, Chemom. Intell. Lab. Syst., 2009, 97, 141–145 CrossRef CAS.
- Y. N. Li, H. L. Wu, J. F. Nie, S. F. Li, Y. J. Yu, S. R. Zhang and R. Q. Yu, Anal. Methods, 2009, 1, 115–122 RSC.
- L. Zhang, H. L. Guan and Z. K. He, Sci. China Chem., 2010, 53, 245–249 Search PubMed.
- J. M. Guo, Y. Y. Xin and X. B. Yin, J. Agric. Food Chem., 2010, 58, 6556–6561 CrossRef CAS.
- R. A. de Toledo and C. M. P. Vaz, Microchem. J., 2007, 86, 161–165 CrossRef CAS.
- K. B. Wu, Y. Y. Sun and S. S. Hu, Sens. Actuators, B, 2003, 96, 658–662 CrossRef.
- X. Y. Zhang, X. M. Liu, W. L. Liu, M. Yang and Z. Q. Zhang, Chem. J. Chin. Univ.-Chin., 2010, 31, 33–37 Search PubMed.
- X. J. Qu, J. Zhou and J. G. Gao, Anal. Sci., 2005, 21, 1223–1226 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
