One-pot preparation of dextran-capped gold nanoparticles at room temperature and colorimetric detection of dihydralazine sulfate in uric samples†
Yi
Wang
a,
Lei
Zhan
b and
Cheng Zhi
Huang
*ab
aEducation Ministry Key Laboratory on Luminescence and Real-Time Analysis, College of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715, PR China
bCollege of Pharmaceutical Sciences, Southwest University, Chongqing, 400715, PR China. E-mail: chengzhi@swu.edu.cn; Fax: (+86) 23 68866796; Tel: (+86) 23 68254659
First published on 13th October 2010
Abstract
We present a room-temperature strategy for the preparation of dextran-capped gold nanoparticles (AuNPs) in this contribution by employing dextran, which acts as both a reducing agent and a stabilizer, and thus well dispersed, uniform and biocompatible AuNPs could be successfully prepared in one pot. The investigations, including toxicological effect on cells and the capability of resisting aggregation in high ion medium, have demonstrated that the as-prepared AuNPs are biocompatible and stable even if in a medium of high ionic strength. In order to identify the practicability of the dextran-capped AuNPs, colorimetric detection of dihydralazine sulfate (DHZS) in uric samples based on the localized surface plasmon resonance (LSPR) of the AuNPs is made with the recovery in the range of 98.3–102.5%.
1. Introduction
Metal nanoparticles, especially gold nanoparticles (AuNPs), have attracted considerable attention owing to their interesting properties and potential technological applications in recent years. The unique optical and electronic properties of AuNPs, as well as their high chemical stability have made them be applied for various purposes such as biolabeling, DNA assays, crystal growth, self-assembly, catalysis, electron-transfer, and energy-transfer.1 For example, AuNPs, owing to the localized surface plasmon resonance (LSPR) absorption and scattering, can exhibit bright colors in the visible spectral range.2 Therefore, colorimetric detections of a variety of analytes based on AuNPs have been made including biomacromolecules,3,4 small molecules,5–7 and metal ions,8–10 taking advantage of their simplicity and rapidity but without the need for complicated apparatus.A lot of methods for preparing AuNPs with various shapes and different sizes have been reported up to now. In general, AuNPs are synthesized and coated with organic molecules to increase the stability in aqueous solution and to provide modifiable functional groups for conjugation chemistry. For instance, citrate-capped AuNPs, a kind of AuNPs extensively used in recent years, profit by their uniform particle sizes and good stability in aqueous solution. Nevertheless, they are generally prepared under the temperature of about 100 °C and need to be functionalized with other molecules for further conjugation and resist salt-induced aggregation.11 In addition, AuNPs synthesized in organic solvent or coated by non-biocompatible molecules are hard to be applied in biological systems due to their toxicity. Therefore, it is imperative to develop simple and controllable methods to prepare AuNPs with uniform size, excellent biocompatibility, good stability in aqueous solution, and a strong capability of withstanding salt-induced aggregation. In this case, biocompatible polymers are good options for preparing and surface coating the AuNPs, especially for applications with biological uses.
Stated thus, we developed a room-temperature strategy for preparation of biocompatible polysaccharide-capped AuNPs using dextran wherein it acts as both a reducing agent and a stabilizer (Scheme 1). The as-prepared AuNPs are well dispersed, uniform, and stable in the medium of high ionic strength, making them promising candidates for fabricating novel nanoarchitectures and being applied in the assays of complex samples. Furthermore, in order to display the practicability of the as-prepared dextran-capped AuNPs, a colorimetric method for the assay of dihydralazine sulfate (DHZS), a kind of well-known vasodilator drug, is proposed based on the change of LSPR of the AuNPs under dispersed and aggregated states. Compared with previous methods for the determination of DHZS such as spectrophotometry,12,13 chemiluminescence spectrometry,14–16 liquid chromatography,17 and HPLC-MS technique,18 the present one has the advantages of simplicity and speediness, avoiding the use of complicated apparatus and complex pretreatment.
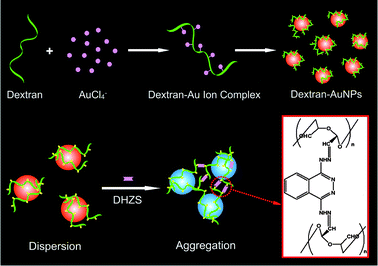 | ||
| Scheme 1 Schematic illustration for the preparation of dextran-capped AuNPs and their aggregation process mediated by DHZS. | ||
2.1. Materials
Dextran-40 was commercially purchased from BBI (Kitchener, Canada) with high purity, and used as both a reducing agent and a stabilizer for the preparation of AuNPs. The reagents including hydrogen tetrachloroaurate(III) tetrahydrate (HAuCl4·4H2O, Sinopharm Group Chemical Regent Co., Ltd., Shanghai, China), trisodium citrate (Na3C6H5O7·2H2O, Chemical Reagent Co., Shanghai, China), sodium borohydride (NaBH4, Huanwei Fine Chemical Co., Ltd., Tianjin, China), cetyltrimethylammonium bromide (CTAB, Sinopharm Group Chemical Regent Co., Ltd., Shanghai, China), L-ascorbic acid (L-AA, Chuandong Chemical Group Co., Ltd., Chongqing, China) were commercially available to prepare citrite- and CTAB-capped AuNPs.Cell Counting Kit-8 (CCK-8) containing WST-8 reagent was commercially purchased from Beyotime Institute of Biotechnology (Jiangsu, China) for evaluating the cell growth inhibition of AuNPs. Dihydralazine sulfate was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Tris-HCl buffer (pH 8.6, 0.05 mol L−1) and NaCl (0.5 mol L−1) solutions were used to adjust the acidity and ionic strength. All reagents used were of analytical grade without further purification, and Mili-Q purified water (18.2 MΩ) was used throughout the experiments. All glassware were cleaned by aqua regia solution (HCl/HNO3 in volume = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and subsequently rinsed with a copious amount of Mili-Q purified water.
1) and subsequently rinsed with a copious amount of Mili-Q purified water.
2.2. Apparatus
The absorption spectra of AuNPs were measured with a UV-3600 UV-Visible-NIR spectrophotometer (Shimadzu, Japan). The scanning electron microscope (SEM) images of AuNPs were captured with a Hitachi S-4800 scanning electron microscope (Tokyo, Japan). An Olympus BX51 microscope (Tokyo, Japan) was used to record the dark-field scattering images of the AuNPs. Dynamic light scattering (DLS) measurements were performed using a Zatasizer Nano-ZS90 System (Malvern Inc.). The cell livabilities were measured by Mode 680 Microplate Reader (BIO-RAD, USA). Photographs of the AuNPs suspension used for colorimetric detection were recorded by an Olympus E-510 digital camera. SZCL-3A digital controlled magnetic stirrers (Henan, China) which could control the temperature precisely were used to prepare the AuNPs.2.3. Preparation of dextran-capped AuNPs
Dextran-capped AuNPs were prepared at room temperature (25 °C) using dextran as both a reducing agent and a stabilizer. Firstly, 47.0 mL of Mili-Q purified water, 10.0 mg of dextran, and 2.0 mL of 1.0% (w/w) HAuCl4 were mixed in a flask with ultrasonic irradiation. Then, 1.0 mL of 1.0 mol L−1 NaOH was quickly added to the solution under vigorous magnetic stirring to adjust the pH to 11, and the color of solution changed to deep red within 12–16 h.2.4. Preparation of citrate- and CTAB-capped AuNPs
Citrate- and CTAB-capped AuNPs were prepared according to previous references 11 and 19 and were analyzed in parallel as a control of dextran-capped AuNPs. The details for the preparation are shown in the Supporting Information.†2.5. Procedures of DHZS determination
Typically, 50 μL of 0.05 mol L−1 Tris-HCl buffer, 90 μL of 0.5 mol L−1 NaCl and an appropriate volume of DHZS solution (1.0 × 10−4 mol L−1) were successively added into a 1.5 mL plastic vial. The mixture was diluted to 300 μL with Mili-Q purified water and vortexed, followed by addition of 200 μL AuNPs (6.0 nmol L−1) with thorough mixing. After 20 min, absorption spectra, DLS data and photographs of AuNPs were directly recorded, and the specimen preparation of SEM and dark-field scattering imaging were carried out.3. Results and discussion
3.1. Characterization of dextran-capped AuNPs
The solution of as-prepared dextran-capped AuNPs is wine red, and has a characteristic LSPR absorption band of AuNPs at 520 nm (λmax) with narrow peak half-width (Fig. S1 in the Supporting Information),† suggesting that the diameter (d) of the dextran-capped AuNPs is about 15.0 nm, as calculated by eqn (1).20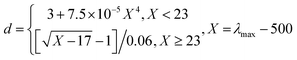 | (1) |
And it is also easy to obtain the concentration of this kind of AuNP (6.0 nmol L−1) by means of Lambert–Beer's law, wherein the value of ε used is 2.7 × 108 L mol−1 cm−1.21
The obtained AuNPs are highly uniform and dispersed as displayed by the SEM image of dextran-capped AuNPs and the histogram for their size distributions shown in Fig. 1. By counting 375 NPs in the region of the whole SEM image, it could be calculated that the average particle size of AuNPs is 13.6 ± 1.4 nm (Fig. 1, insert histogram), which is consistent with the calculated results showed in eqn (1) according to the absorption spectra of the AuNPs solution.
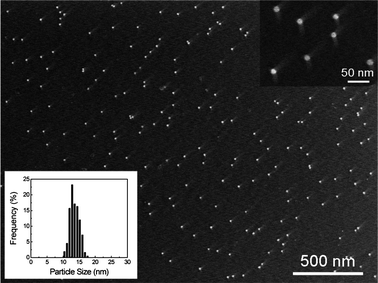 | ||
| Fig. 1 SEM image of dextran-capped AuNPs and histogram of their size distributions. The average particle size is 13.6 ± 1.4 nm and the total number of particles counted for the histogram is 375. | ||
In addition, it was found that these dextran-capped AuNPs could be stable in aqueous solution under 4 °C for at least 6 months without conglomeration.
3.2. Biocompatibility of the dextran-capped AuNPs
To evaluate the biocompatibility of dextran-capped AuNPs, the toxicological effect against mammalian cells was investigated. HeLa and lung cancer (A549) cells were applied for the investigation, and citrate- (13 nm) and CTAB-coated (25 nm) AuNPs were analyzed in parallel as a control of dextran-capped AuNPs. The concentrations of these three kinds of AuNPs were calculated according to the absorption spectra, respectively.21 Firstly, a series of concentrations (0.004–0.08 nmol L−1) of the NPs were incubated with cells in 96-well plates for 24 h. Then, the cells were washed three times by phosphate buffer, and WST-8 was added to the cells and incubated with them for 0.5 h. Finally, the data were recorded by the Mode 680 Microplate Reader, and the cell livabilities were calculated by the absorbance ratio of sample cells to control cells.Previous reports have confirmed that citrate-capped AuNPs are biocompatible, while CTAB can restrain the growth of cells.22,23 As shown in Fig. 2A, the livabilities of HeLa cells which had been treated with citrate-capped AuNPs were above 100% under the used concentrations of NPs after 24 h exposure. On the contrary, CTAB-capped AuNPs made the cell livabilities fall to 70–80%. Compared with the previous two kinds of AuNPs, dextran-capped AuNPs displayed similar biocompatibility to citrate-capped AuNPs. Similarly, for lung cancer cells (A549), the dextran- and citrate-capped AuNPs revealed excellent biocompatibility, while the cell livabilities of CTAB-capped AuNPs were 40–65% (Fig. 2B).
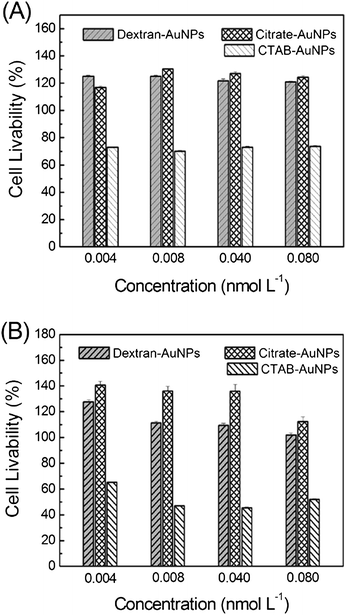 | ||
| Fig. 2 Cell growth inhibition assays of dextran-capped AuNPs: (A) HeLa cells; (B) lung cancer cells (A549). Citrate-and CTAB-capped AuNPs were analyzed in parallel as a control. | ||
Recently, toxicological studies have demonstrated that the toxicity of NPs to cells is derived from their physical and chemical properties such as particle size, morphology, crystal structure and composition, surface chemistry, and so on.24 In the present experiment, although the size of these three kinds of AuNPs used is not completely equivalent, the maximum difference among them is the surface chemistry, which coated with dextran, citrate, and CTAB, respectively. Therefore, it is considered that surface chemistry is the primary contributor to the toxicity of these NPs to cells. Dextran, which acts as both a reducing agent and a stabilizer with unexceptionable biocompatibility, makes the as-prepared AuNPs have excellent biocompatibility. Overall, the initial results reported here are promising, but additional toxicological assays are needed to understand the full effect of these kinds of AuNPs on human health.
In order to make sure whether the obtained dextran-capped AuNPs can be further applied for analytical purposes without surface modification, we have investigated their capability of withstanding high ionic strength-induced aggregation, which is a very important performance in bioanalysis and bioimaging. As Fig. 3 shows, the citrate-capped AuNPs aggregated when the concentration of NaCl exceeds 20 mmol L−1, in which the color changes remarkably from red to blue. On the contrary, the dextran-capped AuNPs still retain the initial color of wine red even when the concentration of NaCl was up to 100 mmol L−1. The results suggested that the present dextran-capped AuNPs might be directly applied in biological and environmental systems without complex surface modifications.
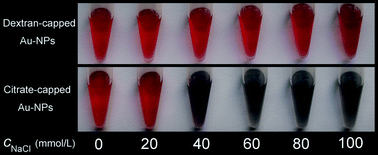 | ||
| Fig. 3 Investigations of dextran-capped AuNPs withstand salt-induced aggregation. Citrate-capped AuNPs were analyzed in parallel as a control. | ||
3.3. DHZS-induced aggregation of dextran-capped AuNPs
In order to show the practicability of the biocompatible dextran-capped AuNPs, we further applied them for drug assay. DHZS, which contains two hydrazine groups, is a well-known vasodilator drug with antihypertensive properties (the molecular structure of DHZS is shown in Fig. 4 insert picture). With the addition of DHZS, aggregation of dextran-capped AuNPs occurred remarkably.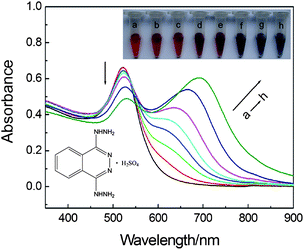 | ||
| Fig. 4 UV-vis absorption spectra of dextran-capped AuNPs in the presence of DHZS. Concentrations: AuNPs, 2.5 nmol L−1; DHZS (in the order of Curves a–h, μmol L−1): 0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0; NaCl, 90 mmol L−1; pH, 8.6. The inserted pictures from left to right at the top displays the color change corresponding to Curves a–h, while the inserted picture in the bottom is the molecular structure of DHZS. | ||
Fig. 4 displays the LSPR absorption spectral and solution color changes of dextran-capped AuNPs in the presence of different concentrations of DHZS. With the increase of DHZS, the characteristic LSPR absorption band at 520 nm decreased gradually, and a new absorption band appeared at a longer wavelength and red-shifted gradually in the region of 600–800 nm. The spectral change suggested that strong plasmon coupling of AuNPs occurred, resulting in a lowering energy of the LSPR compared with that of a noninteracting AuNP.25,26 Moreover, the color of solution changed correspondingly from wine red to dark red, purplish red, purple, bluish purple, and finally blue. Therefore, a semi-quantitative colorimetric method for the assay of DHZS could be proposed according to the relationship between AuNPs solution colors and the concentrations of DHZS.
To investigate the effect of DHZS on the morphology and degree of dispersity of dextran-capped AuNPs, SEM images of AuNPs in the absence and presence of DHZS were also recorded (Fig. 5a–c). As the SEM images show, AuNPs were well dispersed in the absence of DHZS (Fig. 5a). With the addition of 2.0 μmol L−1 of DHZS, the AuNP aggregates could be clearly identified (Fig. 5b). Some of the aggregates were up to 1 μm in size. Furthermore, the degree of aggregation of the AuNPs gets largely increased when DHZS was up to 5.0 μmol L−1 (Fig. 5c).
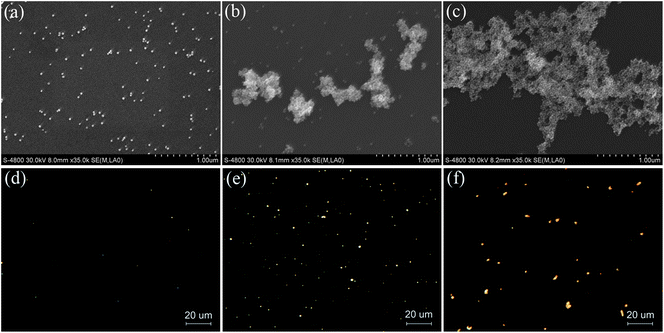 | ||
| Fig. 5 SEM images and dark-field scattering images of dextran-capped AuNPs in the absence and presence of DHZS. Concentrations: AuNPs, 2.5 nmol L−1; NaCl, 90 mmol L−1; DHZS (μmol L−1): (a, d) 0; (b, e) 2.0; (c, f) 5.0; pH, 8.6. | ||
It is known that the LSPR properties of AuNPs are related to their size, shape, surrounding environmental medium, and the space between NPs. Previous reports have confirmed that the scattered light of single spherical AuNP with a diameter less than 50 nm is green and relatively weak.27 In the present work, the scattered light of dextran-capped AuNPs could hardly be observed in the dark-field scattering imaging microscope owing to their small size (13.6 ± 1.4 nm) (Fig. 5d). With the addition of 2.0 μmol L−1 of DHZS, beautiful orange scattered light was observed (Fig. 5e). However, the color of scattered light changed to tangerine when the concentration of DHZS increased to 5.0 μmol L−1, and the intensity of the light also largely increased (Fig. 5f). The obvious color and intensity changes of the scattered light should be ascribed to the decrease of spacing between individual NPs, resulting in the increase of plasmon coupling.
In order to make sure whether the aggregation of dextran-capped AuNPs actually occurred in the solution in the presence of DHZS, an investigation of DLS was carried out (Fig. 6). It was found that the average hydrodynamic diameter (AHD) of dispersed dextran-capped AuNPs was 37.4 nm, which was relatively larger than that measured by SEM imaging (13.6 ± 1.4 nm) (Fig. 1). Nevertheless, the AHD of AuNPs which coexisted with 2.0 μmol L−1 of DHZS was 51.9 nm, and further increased to 113.3 nm when the concentration of DHZS was up to 5.0 μmol L−1. The DLS data clearly revealed the different size distributions of dextran-capped AuNPs after adding different concentrations of DHZS, indicating that the aggregation of AuNPs assuredly occurred in the solution but not because of solvent drying during specimen preparation of SEM and dark-field scattering imaging.28,29
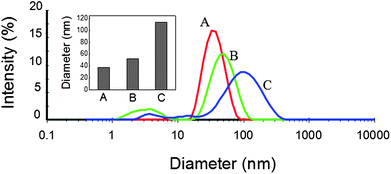 | ||
| Fig. 6 DLS measurements of dextran-capped AuNPs in the absence and presence of DHZS. Concentrations: AuNPs, 2.5 nmol L−1; NaCl, 90 mmol L−1; DHZS (μmol L−1): (A) 0; (B) 2.0; (C) 5.0; pH, 8.6. | ||
Overall, absorption spectra, SEM imaging, dark-field scattering imaging and DLS investigations all demonstrated that DHZS could induce the aggregation of dextran-capped AuNPs, and the degree of aggregation increased accompanying the increase of DHZS. Previously, it has been reported that the hydroxyl groups of dextran could be oxidated to aldehyde, forming a new kind of biomacromolecules, polyaldehyde dextran.30,31 In the present experiment, no any other reducing agent except dextran was used in the reaction system. Therefore, dextran acted as an excellent reducing agent for deoxidizing HAuCl4 to gold crystals, and synchronously, the hydroxyl groups of dextran were oxidated effectively to be aldehyde groups. The formed new biomacromolecule with an extensive number of aldehyde groups could adsorb on the surfaces of AuNPs, which was consistent with the previous reports.32,33
In addition, it is easy to form hydrazone by the reaction of aldehyde and hydrazine groups at room temperature.34 Thus, the two hydrazine groups of DHZS might react with the aldehyde groups which were coated on the surfaces of different AuNPs, respectively. This reaction made DHZS molecules act as bridges among AuNPs, resulting in the decrease of their spacing and further inducing their aggregations. Then, the near-field plasmon coupling occurred when the spacing between AuNPs was smaller than 2.5 times that of their diameters,35 and lead to the LSPR absorption and scattering properties of the NPs to be changed. A hypothesized process for the preparation of dextran-capped AuNPs and their aggregation mediated by DHZS is presented as Scheme 1.
3.4. Optimization of the determination of DHZS
We have also optimized the experimental conditions for the sake of successful visual detection of DHZS, including the pH of solution and the concentration of electrolyte. It was found that the absorption of the solution at 520 and 650 nm were related to the quantities of dispersed and aggregated AuNPs, respectively. Thus, we used the ratio of the values of absorption, A650/A520, to express the molar ratio of aggregated and dispersed AuNPs. Investigations showed that the values of A650/A520 were low and stable as pH ranged from 7.2 to 9.0 in the absence of DHZS (Fig. 7), suggesting AuNPs were well dispersed. However, in the presence of DHZS, the values of A650/A520 largely increased, and we chose the pH 8.6 as the most appropriate working condition.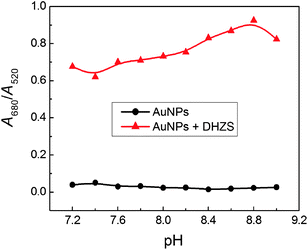 | ||
| Fig. 7 Effect of pH on the aggregation of dextran-capped AuNPs in the absence and presence of DHZS. Concentrations: AuNPs, 2.5 nmol L−1; NaCl, 90 mmol L−1; DHZS, 4.0 μmol L−1. | ||
The ionic strength is another factor affecting the aggregation of AuNPs. Fig. 8 showed that the values of A650/A520 were low and stable as the concentrations of NaCl were less than 100 mmol L−1 in the absence of DHZS, while the values started to increase as the addition of 50 mmol L−1 of NaCl with addition of 4.0 μmol L−1 DHZS. The main reason is that high ionic strength could reduce the electrostatic repulsive force between AuNPs and further accelerate the DHZS-induced aggregation of AuNPs. The maximum value of A650/A520 was obtained when the addition of 90 mmol L−1 of NaCl.
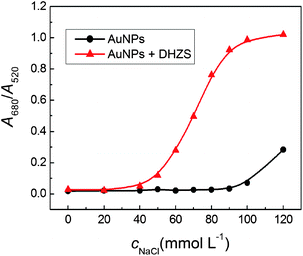 | ||
| Fig. 8 Effect of ionic strength on the aggregation of dextran-capped AuNPs in the absence and presence of DHZS. Concentrations: AuNPs, 2.5 nmol L−1; DHZS, 4.0 μmol L−1; pH, 8.6. | ||
3.5. Availability of the analytical method
Under the optimum conditions, a good linear relationship existed between the values of A650/A520 of AuNPs and the concentrations of DHZS in the range of 0.5–6.0 μmol L−1. The standard regression equation was ΔA680/520 = 0.206c − 0.125 with a correlation coefficient of 0.9962, and the limit of determination (3σ) was 33 nmol L−1. The excellent precision of this method was also validated with the relative standard deviation of 1.5% (cDHZS = 4.0 μmol L−1, n = 14).The selectivity of this method was investigated in the presence of 4.0 μmol L−1 DHZS following the general procedure by premixing with potential interfering substances such as common metal ions, sugars, amino acids, and pharmaceuticals (the data were given in Table S1 in the Supporting Information).† The absorption spectra were used to monitor the aggregation of AuNPs. It was found that common saccharides (glucose, lactose, sucrose, fructose, and maltose), amino acids (glycin, L-serine, and L-glutamic acid), organic acids (citric acid and oxalic acid), low-valent metal ions (K+ and Na+), and carbamide could be allowed at very high concentrations (50–100 times than DHZS). While, common high-valent metal ions such as Mg2+, Ca2+, Zn2+, Cu2+ and Al3+ could coexist at relatively low concentrations (5–20 times than DHZS). The main reason is that the higher positive charge of the high-valent metal ions might reduce the electrostatic repulsive force facilely between the AuNPs and accelerate their aggregations. In addition, hydrazine sulfate and the drug isoniazid, which contains hydrazine groups, could also coexist at very high concentration (100 times than DHZS).
To demonstrate the feasibility of this method, it was then applied to detect uric samples added with trace DHZS. The uric samples were pretreated by centrifugation (10000 rpm, 10 min) and diluted by 100 times. The results revealed no effect on the responses of the dextran-capped AuNPs when the addition of diluted uric samples, and the recoveries for the determination were between 98.3%–102.5% under the optimal conditions (Table 1), indicating that this method is reliable and practical.
4. Conclusions
In summary, we have successfully developed a simple, one-pot, controllable, and totally green strategy for the preparation of biocompatible AuNPs by means of dextran as both a reducing agent and a stabilizer at room temperature. The characterization and property investigations of dextran-capped AuNPs have confirmed that the as-prepared NPs were well dispersed, uniform, stabilized, biocompatible, and could withstand higher salt concentration than that of generally used citrate-capped AuNPs. In addition, a colorimetric method for the determination of vasodilator drug, DHZS, was proposed based on the change of LSPR of this kind of AuNPs. SEM imaging, dark-field imaging, absorption spectra, and DLS data all have demonstrated that the aggregation of AuNPs occurred in the presence of DHZS. Moreover, the excellent properties of the as-prepared dextran-capped AuNPs make them promising candidates for fabricating novel nanoarchitectures and as probes for future applications in pharmaceutical, biological, and environmental analysis.Acknowledgements
All authors are grateful to the financial supports from the National Natural Science Foundation of China (NSFC 21035005) and the Postgraduate Science and Technology Innovation Program of Southwest China University (Grant ky2009004).References
- R. Sardar, A. M. Funston, P. Mulvaney and R. W. Murray, Langmuir, 2009, 25, 13840–13851 CrossRef CAS.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- H. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS.
- J. Wang, L. Wang, X. Liu, Z. Liang, S. Song, W. Li, G. Li and C. Fan, Adv. Mater., 2007, 19, 3943–3946 CrossRef CAS.
- J. Zhang, L. Wang, D. Pan, S. Song, F. Y. C. Boey, H. Zhang and C. Fan, Small, 2008, 4, 1196–1200 CrossRef CAS.
- S.-J. Chen, Y.-F. Huang, C.-C. Huang, K.-H. Lee, Z.-H. Lin and H.-T. Chang, Biosens. Bioelectron., 2008, 23, 1749–1753 CrossRef CAS.
- J.-S. Lee, M. S. Han and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 46, 4093–4096 CrossRef CAS.
- Y. Zhou, S. Wang, K. Zhang and X. Jiang, Angew. Chem., Int. Ed., 2008, 47, 7454–7456 CrossRef CAS.
- J. M. Slocik, J. S. J. Zabinski, D. M. Phillips and R. R. Naik, Small, 2008, 4, 548–551 CrossRef CAS.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735–743 CrossRef CAS.
- C. A. Georgiou, M. A. Koupparis and T. P. Hadjiioannou, Talanta, 1991, 38, 689–696 CrossRef CAS.
- G. P. Miralles, R. García-Domenech, J. M. Vinuesa and J. M. Buigues, J. Pharm. Biomed. Anal., 1993, 11, 647–650 CrossRef CAS.
- X.-F. Yang and H. Li, Talanta, 2004, 64, 478–483 CrossRef CAS.
- H. Yao, X. Yang and H. Li, Microchim. Acta, 2006, 153, 171–178 CrossRef CAS.
- X.-F. Yang, Anal. Chim. Acta, 2008, 616, 190–195 CrossRef CAS.
- C. Laugel, P. Chaminade, A. Baillet and D. Ferrier, J. Chromatogr., A, 1994, 686, 344–349 CrossRef CAS.
- D. G. Mascher, W. Tscherwenka and H. J. Mascher, J. Pharm. Biomed. Anal., 2007, 43, 631–645 CrossRef CAS.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648–8649 CrossRef CAS.
- N. G. Khlebtsov, Anal. Chem., 2008, 80, 6620–6625 CrossRef CAS.
- H. D. Hill and C. A. Mirkin, Nat. Protoc., 2006, 1, 324–336 Search PubMed.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721–1730 CrossRef CAS.
- A. M. Alkilany, P. K. Nagaria, C. R. Hexel, T. J. Shaw, C. J. Murphy and M. D. Wyatt, Small, 2009, 5, 701–708 CrossRef CAS.
- S. M. Hussain, L. K. Braydich-Stolle, A. M. Schrand, R. C. Murdock, K. O. Yu, D. M. Mattie, J. J. Schlager and M. Terrones, Adv. Mater., 2009, 21, 1549–1559 CrossRef CAS.
- J. Wang, Y. F. Li, C. Z. Huang and T. Wu, Anal. Chim. Acta, 2008, 626, 37–43 CrossRef CAS.
- M. Gluodenis and C. A. Foss, J. Phys. Chem. B, 2002, 106, 9484–9489 CrossRef CAS.
- J. Yguerabide and E. E. Yguerabide, Anal. Biochem., 1998, 262, 157–176 CrossRef CAS.
- Y. Wang, Y. F. Li and C. Z. Huang, J. Phys. Chem. C, 2009, 113, 4315–4320 CrossRef CAS.
- Y. Wang, Y. F. Li, J. Wang, Y. Sang and C. Z. Huang, Chem. Commun., 2010, 46, 1332–1334 RSC.
- T. Azzam, H. Eliyahu, L. Shapira, M. Linial, Y. Barenholz and A. J. Domb, J. Med. Chem., 2002, 45, 1817–1824 CrossRef CAS.
- Y. Wang, L. Q. Chen, Y. F. Li, X. J. Zhao, L. Peng and C. Z. Huang, Nanotechnology, 2010, 21, 305601 CrossRef.
- Y. Ma, N. Li, C. Yang and X. Yang, Anal. Bioanal. Chem., 2005, 382, 1044–1048 CrossRef CAS.
- S. Nath, C. Kaittanis, A. Tinkham and J. M. Perez, Anal. Chem., 2008, 80, 1033–1038 CrossRef CAS.
- H. Zhang, C. Smanmoo, T. Kabashima, J. Lu and M. Kai, Angew. Chem., Int. Ed., 2007, 46, 8226–8229 CrossRef CAS.
- K.-H. Su, Q.-H. Wei, X. Zhang, J. J. Mock, D. R. Smith and S. Schultz, Nano Lett., 2003, 3, 1087–1090 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of citrate- and CTAB-capped AuNPs; Fig. S1, UV-vis absorption spectra and solution color of dextran-capped AuNPs and Table S1, Tolerance of foreign substances. See DOI: 10.1039/c0ay00470g |
| This journal is © The Royal Society of Chemistry 2010 |
