Thionine/nanogold multilayer film for electrochemical immunoassay of alpha-fetoprotein in human serum using biofunctional double-codified gold nanoparticles
Biling
Su
a,
Juan
Tang
a,
Huafeng
Chen
a,
Jianxin
Huang
b,
Guonan
Chen
a and
Dianping
Tang
*a
aKey Laboratory of Analysis and Detection for Food Safety (MOE and Fujian Province), Department of Chemistry, Fuzhou University, Fuzhou, 350108, China. E-mail: dianping.tang@fzu.edu.cn; dianping.tang@hotmail.com; Fax: +86-591-22866135; Tel: +86-591-22866125
bClinical Laboratory and Medical Diagnostics Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, China
First published on 30th September 2010
Abstract
A simple and sensitive electrochemical immunoassay method was developed for the detection of alpha-fetoprotein (AFP) in human serum. The immunoassay is based on the use of an AFP-functionalized thionine/nanogold multilayer film ({Thi/AuNP}n) on a single-walled carbon nanotubes (CNT)-coated gold electrode and double-codified gold nanoparticles with horseradish peroxidase-labelled anti-AFP antibodies (HRP-anti-AFP-AuNPs). With a competitive immunoassay format, the assay was performed using HRP-anti-AFP-AuNP as trace and H2O2 as enzyme substrate. The electrochemical behavior of the multilayer {Thi/AuNP}n films was studied. The maximal signal was obtained at n = 3 (i.e. {Thi/AuNP}3). Under optimal conditions, the obtained electrochemical responses were proportional to the AFP levels in the sample. The dynamic range for AFP quantification was 0.25–45 ng mL−1. Both the intra- and inter-assay coefficients of variation were less than 9.5%. The proposed assay had a detection limit of 0.05 ng mL−1, which was 20-fold vs. 10-fold lower than that obtained using monolayer Thi/AuNP film vs. conventional HRP-labelled anti-AFP antibodies. Importantly, no significant differences at the 0.05 confidence level were encountered in the analysis of clinical serum samples between the proposed immunoassay and the commercially available Roche 2010 Electrochemiluminescent (ECL) Automatic Analyzer for AFP determination.
Introduction
Immunoassays have long been widely used in a variety of applications including medical diagnostics, pharmaceutical analysis, environmental analysis, food safety testing, and for basic scientific investigations, because of their simplicity, sensitivity, and specificity.1,2 Most commercially available immunoassays have been based on the catalysis of enzyme-labelled secondary antibodies toward appropriate substrates to yield chromogenic or chemiluminescent products.3,4 However, the sensitivity of these immunoassays is still limited, which restricts their usefulness for the early diagnosis of disease. Recently, great attention has been focused on signal amplification using bionanoparticle labels5,6 or multienzyme labels,7 and the use of DNA as an amplified signal reporter.8 In the DNA-based immunoassays, the signal was usually amplified by the polymerase chain reaction (PCR) after target recognition of the capture antibody. In the bio-barcode immunoassay the signal was pre-amplified by using nanoparticles with a high ratio of DNA to capture the antibody. Thus, their application is restricted due to the complex detection procedures or conjugation processes.9Nanotechnology is multidisciplinary and interdisciplinary and covers diverse fields including chemistry, physics, material science, engineering, biology, and even medicine.10,11 It provides excitingly new possibilities for advanced development of new analytical methods and instruments for bioanalytical and biotechnological applications.12 Currently, a vast library of nanostructures has been synthesized and documented, with a wide variety of properties and application.13 Hauch and co-authors reviewed nanotechnology diagnostics for infectious diseases prevalent in developing countries.14 Liu and Lin summarized recent advances in nanomaterial labels in electrochemical immunosensors and immunoassays.15 These nanolabels mainly consisted of nanogold, nanosilver, nanosilica, semiconductor nanoparticles, carbon nanotubes and so on. In the past, we have also synthesized magnetic nanogold microspheres,16 nanogold hollow microspheres17 and enzyme-doped silica nanoparticles18 for the labelling of biomolecules. We subsequently found that these large-sized microspheres/nanoparticles were not especially suitable for the labelling of secondary antibodies in sandwich-type electrochemical immunoassays. This might be due to the fact that the antigen-antibody binding force is very weak, and that the bionanolabels could not firmly pull over the electrode via the binding force. Therefore, homogeneous nanoparticles with high conductivity and good biocompatibility are preferable.
Gold nanoparticles (AuNPs), as a class of nanomaterial, have many unique properties and have been widely used for analytical and biomedical purposes.19,20 In the early 1970’s, the Hayatt group utilized gold colloids as electron-dense probes in immunocytochemistry.21 With surface modification, AuNPs can bind with biomolecules including peptides, enzymes, antibodies and DNA. Gonzalez-Garcia22 and Dequaire,23 employed gold nanoparticles as electrochemical labels for the voltammetric monitoring of protein interaction. Gold nanoparticles with high volume-to-surface ratio and strong surface free energy enhanced the sensitivity of electrochemical immunoassays.
Another key factor influencing the sensitivity of electrochemical immunoassays is the method used to fabricate the immunosensor.24 Encapsulation, absorption, covalent binding and self-assembly techniques have been used for the construction of immunosensors.25 A major limitation of encapsulation is the additional diffusion barrier resulting from the entrapped materials, whereas one of the problems commonly associated with covalent binding is the decrease in protein bioactivity when the proteins are exposed to reactive groups and harsh reaction conditions.26 Recently, layer-by-layer (LBL) construction of organic multiple films by the alternating adsorption technique has been receiving increased attention.27 The LBL technique is especially suitable for film production in the nanometre range with vertical organization of different sandwich-like layers.28 Iler reported a LBL pioneering work for oppositely charged colloidal particles.29 The simplicity and universality of the LBL technique combined with the uniform distribution of nanoparticle films make its perspectives particularly attractive for the construction of electrochemical immunosensors. Carbon nanotubes with unique structure and mechanical and electronic properties make them an ideal electrode material for the construction of electrochemical sensing devices, especially single-walled carbon nanotubes (SWNTs). The reason might be the fact that the band gap of SWNTs can vary from zero to about 2 eV and that their electrical conductivity can show metallic or semiconducting behavior, whereas multi-walled carbon nanotube (MWNTs) are zero-gap metals. SWNTs as an important variety of carbon nanotubes could exhibit electric properties that are not shared by the MWNT variants.
It is well known that AFP is an important tumor marker with an average concentration of 10 ng mL−1 in healthy human serum. The serum AFP concentration rises greatly in patients with liver cancer. So, there is a need for the development of validated analytical methods for the rapid and cost effective screening of AFP on a large scale and at low concentration levels. Herein, we introduce a simple and sensitive electrochemical immunoassay for the detection of AFP in human serum by combining the advantage of a self-assembly {Thi/AuNP}n multiplayer film with the signal amplification of gold nanolabels. The aim of this study is to further verify the application of the self-assembly LBL technique and bionanolabels in clinical immunoassays.
Experimental
Chemical and instruments
HRP-labelled monoclonal mouse anti-AFP antibodies (HRP-anti-AFP, clone 1G7) and AFP standards were purchased from Biocell Biotechnol. Co. Ltd. (Zhengzhou, China). Thionin acetate salt (Thi, Dye content ≥85%), bovine serum albumin (BSA, lyophilized powder, ∼66 kDa), and sodium citrate tribasic hydrate were obtained from Sigma-Aldrich. HAuCl4·4H2O was purchased from Sinopharm Chem. Re. Co., Ltd. (Shanghai, China). Single-walled carbon nanotubes (CNTs, chemical vapour deposition (CVD) method, purity ≥98%, diameter 5–10 nm, and length 1–2 μm) were supplied by Shenzhen Nanoport Co. Ltd. (Shenzhen, China). Deionized and distilled water was used throughout the study. 0.1 M acetic acid-buffered saline (ABS) at various pHs were prepared by mixing the stock solutions of 0.1 M HAc and 0.1 M NaAc, and 0.1 M KCl was added as the supporting electrolyte. A 0.08 mol L−1 phosphate-buffered saline (PBS, pH 7.4) solution was prepared by dissolving 12.2 g K2HPO4, 1.36 g KH2PO4, and 8.5 g NaCl in 1000 mL deionized water. All other chemicals used were of analytical reagent grade and were used without further purification. Clinical serum samples were a gift from Fujian Provincial Hospital, China.Electrochemical measurements were carried out with an Electrochemical Quartz Crystal Microbalance CHI 430A (China). Electrochemical impedance spectroscopy (EIS) was performed in pH 7.4 PBS containing 10 mM Fe(CN)64−/3− at a bias potential of 0.17 V on a CHI 604D Electrochemical Workstation (China). The alternative voltage was 5 mV with a frequency range of 10−1–105 Hz. The electrochemical cell consisted of the immunosensing working electrode, a platinum wire counter electrode, and an Ag/AgCl reference electrode. Ultraviolet-vis absorption (UV-vis) spectra were recorded with an 1102 UV-vis spectrophotometer (Techcomp, China). The size of the gold colloids was characterized using Tecnai G2 F20 transmission electron microscopy (TEM, USA). Scanning electron microscopic (SEM) images were obtained using a Philips XL30E SEM (Philips-FEI, The Netherlands). Clinical serum samples were assayed using an Elecsys 2010 Electrochemiluminescent Automatic Analyzer (Roche, Switzerland).
Synthesis of gold colloids
16 nm gold colloids were synthesized using our previously published protocol.30 All the glassware used in the following procedures was cleaned in a bath of K2Cr2O7–H2SO4, rinsed thoroughly in doubly-distilled water and dried in air. Briefly, 1 mL of 1.0 wt% HAuCl4 solution was initially added to 99 mL of distilled water, and then 2.5 mL of 1.0 wt% sodium citrate was quickly dropped into the boiling solution. The mixture was boiled for about 10 min until the color became claret. The gold colloids thus obtained were cooled at room temperature (RT), and stored at 4 °C when not in use.Conjugation of AuNPs with HRP-anti-AFP (HRP-anti-AFP-AuNP)
The HRP-anti-AFP-AuNP conjugates were prepared according to a previously published paper with some modifications.31 Briefly, 100 mL of gold colloids (C[Au] = 24 mM) was initially adjusted to pH 9.0–9.5 using Na2CO3 followed by the addition of 1.5 mL of HRP-anti-AFP (0.5 mg mL−1). The mixture was shaken gently for 5 min, and transferred to a refrigerator for further reaction (overnight). The suspension was centrifuged at 4 °C for 30 min at 13500g. The purified HRP-anti-AFP-AuNP conjugates were stored in 5 mL of pH 7.4 PBS containing 1.0 wt% BSA at 4 °C until use.Preparation of the electrochemical immunosensor
A gold electrode (Au, 4 nm in diameter) was polished repeatedly with 1.0 and 0.3 μm alumina slurry, followed by successive sonication in bi-distilled water and ethanol for 5 min and drying in air. Prior to the experiment, the Au electrode was cleaned with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2SO4 and H2O2 for 10 min, and then continuously scanned within a potential range of 0 to 1.5 V in freshly prepared deoxygenated 0.5 M H2SO4 until a voltammogram characteristic of the cleaned gold electrode was established.
1 mixture of H2SO4 and H2O2 for 10 min, and then continuously scanned within a potential range of 0 to 1.5 V in freshly prepared deoxygenated 0.5 M H2SO4 until a voltammogram characteristic of the cleaned gold electrode was established.
Next, the shortened CNTs were prepared by continuously sonicating the CNTs in a solution containing H2SO4 and HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for 4 h.32 Afterwards, 5 mg of CNTs was dissolved in 2 mL of 0.5 mg mL−1 BSA solution, and the mixture was stirred for 2 h at RT. Following that, 5 μL of the BSA-CNTs was dropped onto the electrode surface, and dried at RT. After washing with distilled water, the multilayer films were grown by alternately dipping the BSA-CNT/Au electrode into the negatively charged AuNPs and the positively charged thionine for 20 min, respectively (note: the films were carefully washed with water after each dipping step and then dried with N2 gas). The sequence was repeated to obtain the desired number of layers. The multilayer {AuNP/Thi}n film-modified electrode was then immersed in 200 ng mL−1 AFP for 6 h at 4 °C to prepare the immunosensor. Finally, the immunosensor was incubated in 1.0 wt% BSA for 60 min at room temperature to eliminate the non-specific binding effect and block the remaining active groups, it was then stored at 4 °C until use. The fabrication process of the electrochemical immunosensor is illustrated in Scheme 1.
1, v/v) for 4 h.32 Afterwards, 5 mg of CNTs was dissolved in 2 mL of 0.5 mg mL−1 BSA solution, and the mixture was stirred for 2 h at RT. Following that, 5 μL of the BSA-CNTs was dropped onto the electrode surface, and dried at RT. After washing with distilled water, the multilayer films were grown by alternately dipping the BSA-CNT/Au electrode into the negatively charged AuNPs and the positively charged thionine for 20 min, respectively (note: the films were carefully washed with water after each dipping step and then dried with N2 gas). The sequence was repeated to obtain the desired number of layers. The multilayer {AuNP/Thi}n film-modified electrode was then immersed in 200 ng mL−1 AFP for 6 h at 4 °C to prepare the immunosensor. Finally, the immunosensor was incubated in 1.0 wt% BSA for 60 min at room temperature to eliminate the non-specific binding effect and block the remaining active groups, it was then stored at 4 °C until use. The fabrication process of the electrochemical immunosensor is illustrated in Scheme 1.
 | ||
| Scheme 1 Structure of the multilayer {Thi/AuNP}n film-modified electrochemical immunosensor and measurement principle. | ||
Analytical procedure
For the electrochemical measurement, the content of AFP was assayed using a competitive-type immunoassay format with HRP-anti-AFP-AuNP as trace and H2O2 as enzyme substrate. Prior to the experiment, the incubation solution was prepared by mixing 5 μL of AFP standards with different concentrations of serum samples with 5 μL of HRP-anti-AFP-AuNP. As shown in Scheme 1, the incubation solution was dropped onto the electrochemical immunosensor and incubated for 30 min at RT, and then washed carefully with pH 7.4 PBS to obtain an HRP-anti-AFP-AFP immunocomplex-modified electrode. Following that, the differential pulse voltammetric (DPV) measurements were carried out in pH 5.5 ABS containing 5.0 mM H2O2 from −400 to 0 mV at a pulse amplitude of 50 mV and width of 50 ms.Datum analysis
Analyses were carried out in triplicate. All experiments were performed at RT (25 ± 1.0 °C). Standard curves were obtained by plotting mean DPV peak currents against target analyte concentrations unless specified otherwise. A statistical data analysis was performed using SAS ver. 9.0 and SPSS ver. 9.0 software. Comparisons between dependent variables were determined using analysis of variance (ANOVA), Duncan multiple range test, correlation analysis and multiple regression analysis. Results are expressed as mean value ± standard deviation (SD) of three determinations and statistical significance was defined at P ≤ 0.05.Results and discussion
Characterization of the immunosensing interface and nanolabels
For the successful development of a new analytical method, an important precondition is to verify the successful establishment of the method. In this research, two critical issues were investigated: (i) the successful fabrication of the immunosensing interface, and (ii) the successful synthesis of the HRP-anti-AFP-AuNP nanolabels. First of all, we used the SEM technique to investigate the immunosensing interfaces at various steps. Fig. 1 shows the SEM images of BSA-CNTs, AuNP/BSA-CNTs, {Thi/AuNP}3/BSA-CNTs and AFP/AuNP/{Thi/AuNP}3/BSA-CNTs on the aluminium foil surface. Fig. 1a displays an homogeneous dispersion of carbon nanotubes on the surface. When the first layer of gold nanoparticles was assembled onto the BSA-CNT surface via the nanogold-protein interaction, an image of the nanoparticles was clearly observed (Fig. 1b). But, the dispersion of the nanoparticles was scattered. Dense nanoparticles could be achieved on the BSA-CNT surface after the formation of three layers {Thi/AuNP}3 (Fig. 1c). The compact nanoparticles could provide more room for the binding of AFP, and enhance the recognition of HRP-anti-AFP to the binding sites of AFP. | ||
| Fig. 1 SEM images of (a) BSA-CNT-, (b) Thi/AuNP/BSA-CNT-, and (c) {Thi/AuNP}3/BSA-CNT-modified aluminium foil surface. | ||
Fig. 2a shows the UV-vis absorption spectra of the as-prepared gold colloids. The absorption peak was 520 nm. The average size of the nanoparticles was 16 nm, which was confirmed by TEM (Fig. 2d). Fig. 2c displays the UV-vis absorption spectrum of HRP-anti-AFP-AuNPs. Two absorption peaks at 520 and 280 nm could be observed. The peak at 280 nm was mainly derived from the labelled HRP-anti-AFP protein (Fig. 2b). This shows that the HRP-labelled anti-AFP antibodies could be bound to the AuNP surface. The successful synthesis of HRP-anti-AFP-AuNPs was the basis for the amplification of the electrochemical signal.
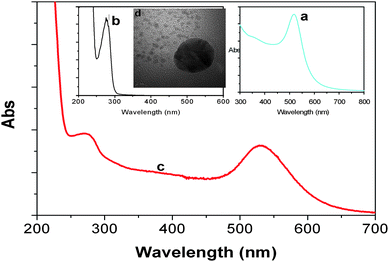 | ||
| Fig. 2 UV-vis absorption spectra of (a) gold colloids, (b) HRP-anti-AFP, and (c) HRP-anti-AFP-AuNP. Inset: (d) HRTEM image of gold nanoparticle. | ||
Cyclic voltammetric characterization of multilayer films
The electrochemical activity of the multilayer {Thi/AuNP}n films was monitored by using cyclic voltammetry to acquire information about reagent immobilization, activity and stability. At the first layer, gold nanoparticles can be easily immobilized on the BSA surface via the –SH group and the remaining –NH2 group of BSA, while thionine in the protonated form (Thi+) could be adsorbed onto the negatively charged gold nanoparticles via π–π stacking interaction to make layered, ordered, and electroactive multilayer films. Fig. 3A shows the cyclic voltammograms of variously modified electrodes at 50 mV s−1 in pH 5.5 ABS. Almost no peak was observed for BSA-CNTs/Au (Fig. 3a) and AuNP/BSA-CNTs/Au (Fig. 3b). Compared with BSA-CNTs/Au, the background currents at AuNP/BSA-CNTs/Au were increased, which suggested that gold nanoparticles favor the electron transfer. When thionine molecules were assembled onto the electrode, a pair of well-defined cyclic voltammograms, characteristic of a diffusion-limited redox process, was obtained (Fig. 3c), which indicated that thionine as a electron mediator could be immobilized on the electrode via the opposite-charged absorption technique. | ||
| Fig. 3 (A) Cyclic voltammograms of (a) BSA-CNT/Au, (b) AuNP/BSA-CNT/Au and (c) Thi/AuNP/BSA-CNT/Au; (B) cyclic voltammograms of {Thi/AuNP}n/BSA-CNT/Au with n = 1, 2, 3, and 4 in pH 5.5 ABS. Inset of (B): the relationship between the layer number (n) and anodic peak current (ipa). | ||
Fig. 3B shows the cyclic voltammograms of multilayer {Thi/AuNP}n films with various layers. The peak currents increased regularly with an increase in the{Thi/AuNP} layer number, which was in accordance with the principle of LBL methods. Moreover, the cathodic peaks (ipc) exhibited a linear relationship to some extent with the layer number (n): ipa (μA) = 29.46 × n + 58.5 (R2 = 0.967, n ≤ 4). To further verify that the preparation of the electrochemical immunosensor was an adsorption-limited process, the cyclic voltammograms of the AFP/{Thi/AuNP}3/BSA-CNT-modifed electrode at various scan rates were investigated in pH 5.5 ABS (data not shown). It was found that the peak current increased with increasing scan rate, while the ΔEp expands slowly. Moreover, the peak currents rose linearly with the scan rate, v, not with v1/2, indicating that the redox reaction is an adsorption-limited reaction.
Additionally, the stability of the multilayer films was also investigated. The {Thi/AuNP}3/BSA-CNT-modifed electrode was stored dry at 4 °C over pH 7.4 PBS, and the cyclic voltammograms were measured periodically. The peak potentials were essentially unchanged after four weeks, while the peak current decreased less than 9% compared with the initial current. The results indicated that the electrostatic interaction between thionine and gold nanoparticles was very strong, and that AFP molecules could be firmly immobilized on the electrode surface.
EIS characterization of multilayer {Thi/AuNP}n films
To further monitor the electrochemical properties of the multilayer films-modified electrode, we utilized electrochemical impedance spectroscopy (EIS) to investigate the variously modified electrodes. A typical EIS usually consists of four parameters: (i) the resistance of the electrolyte solution (Rs), (ii) the lipid bilayer capactance, Cdl; (iii) the charge transfer resistance, Rct; and (iv) the Warburg element (Zw) derived from the diffusion property of the applied redox probe. Rs and Zw are not affected by chemical transformations occurring at the electrode surface, while Cdl and Rct depend on the dielectric and insulating features at the electrode/electrolyte interface. In measurement, the complex impedance can be presented as the sum of the real (Zre) and imaginary (Zim) components that originate mainly from the resistance and capacitance of the cell. The semicircle diameter of EIS equals the Rct, which controls the electron transfer kinetics of the redox-probe at the electrode interface. The value varies at the variously modified electrodes.First, the formation process of the multilayer films on the BSA-CNT/Au was characterized by using EIS. Fig. 4 shows the EIS of the {Thi/AuNP}n/BSA-CNT/Au with various layers. As shown in Fig. 4a, the BSA-CNT-modified gold electrode displayed high resistance (Rct = 682 Ω). The resistances decreased with the increment of assembled {Thi/AuNP}n layer number (Fig. 4b–e). This may be because the anometre-sized gold colloids and the redox activity of thionine play an important role similar to a conducting wire or electron-conduction tunnel, which makes it easier for the electron transfer to take place. Moreover, the resistances exhibited a linear relationship with the layer number (n): Rct (Ω) = 85.5 × n − 713.2 (R2 = 0.993, n ≤ 4). Additionally, we also demonstrated the advantage of the doped CNT using EIS. The inset of Fig. 4 displays the EIS of the BSA/Au and BSA-CNT/Au. With the addition of CNTs into the BSA, the resistance decreased compared with that of BSA/Au (Fig. 4f). These results were in accordance with those obtained from cyclic voltammetry. On the basis of the results from cyclic voltammetry and EIS, we may conclude that multilayer {Thi/AuNP}n films could be formed on the BSA-CNT/Au surface.
 | ||
| Fig. 4 Electrochemical impedance spectra of (a) BSA-CNT/Au, (b–e) {Thi/AuNP}n/BSA-CNT/Au with n = 1, 2, 3, 4, and (f) BSA/Au. Inset: the relationship between the layer number (n) and the impedance (Rct). | ||
Optimization of multilayer films (n value) and assay conditions
As can be seen from the experimental results mentioned above, the electrochemical behavior of the immunosensor was different at various {Thi/AuNP}n-modified electrodes. Further, we investigated the effect of multilayer films on the peak currents of the immunosensor toward AFP standards. The assay was based on the shift in current before and after reaction with AFP standards. As shown in Fig. 5a, the optimal change in current occurred at n = 3 (i.e. {Thi/AuNP}3). This may be because the number of gold nanoparticles immobilized on the surface increases with an increase in the layer number, and that at n = 3 the nanoparticles almost covered the entire surface of the electrode. Although {Thi/AuNP} could still be assembled on the surface at n > 3, a large number of gold nanoparticles densely packed on the surface, and increased the steric hindrance of AFP accessing to gold nanoparticles. So, {Thi/AuNP}3 was chosen for the preparation of the immunosensor. | ||
| Fig. 5 Effects of (a) {Thi/AuNP}n layer number, (b) pH of ABS, (c) incubation time and (d) concentration of H2O2 on the currents of the immunoassay toward 10 ng mL−1 AFP. | ||
The pH value of the assay solution greatly affects the bioactivity of the enzyme. Fig. 5b displays the effect of pH value of ABS on the DPV peak current of the immunosensor after reacted with 10 ng mL−1 AFP. The optimal response was obtained at pH = 5.5. At this condition, we also investigated the effect of incubation time on the response of the immunosensor (Fig. 5c). A high current response was achieved after the antigen-antibody interaction for 30 min. Longer incubation time did not obviously improve the response. In addition, the concentration of H2O2 in the detection cell should be optimized to adequately release the bioelectrocatalytic reaction of the conjugated enzyme. As seen in Fig. 5d, the DPV peak current of the immunosensor increased with an increase in H2O2 concentration after an immunoreaction with HRP-anti-AFP in the absence of AFP, and levelled off at C[H2O2] = 5.0 mM. Therefore, pH 5.5 ABS, 30 min incubation time and 5.0 mM H2O2 were used for the determination of AFP in the following experiments.
Analytical performance
Under optimal conditions, the sensitivity and dynamic range of the electrochemical immunoassay was first assessed using HRP-anti-AFP-AuNP as trace and H2O2 as enzyme substrate with a competitive-type immunoassay format. The DPV peak currents decreased with an increase in AFP concentration in the sample, and the calibration plots exhibited a good linear relationship between the peak currents and AFP concentrations in the range of 0.25 to 45 ng mL−1 (Fig. 6a).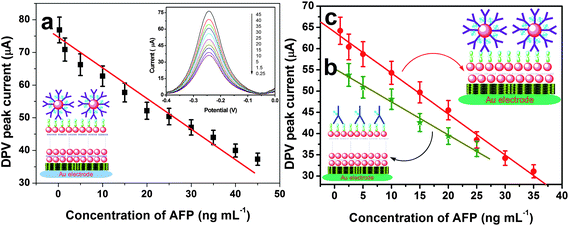 | ||
| Fig. 6 Calibration curves of (a) the {Thi/AuNP}3-modified immunosensor with HRP-anti-AFP-AuNP, (b) the {Thi/AuNP}3-modified immunosensor with HRP-anti-AFP, and (c) the {Thi/AuNP}1-modified immunosensor with HRP-anti-AFP-AuNP toward AFP standards. | ||
For monolayer {Thi/AuNP} film-based immunosensor (Fig. 6b):
2.5–25 ng mL−1; LOD: 1.0 ng mL−1 (note: using HRP-anti-AFP-AuNP as trace);
For traditional HRP-labelled anti-AFP antibodies (Fig. 6c):
1.0–35 ng mL−1; LOD: 0.5 ng mL−1 (note: using {Thi/AuNP}3-based immunosensor).
From the results obtained, the LOD value of the proposed immunoassay was found to be 20-fold vs. 10-fold lower than that obtained by using monolayer {Thi/AuNP} film vs. a conventional HRP-anti-AFP antibodies.
The precision of the electrochemical immunoassay was evaluated by calculating the intra- and inter-batch variation coefficients (CVs). Experimental results indicated that the CVs of the assays using HRP-anti-AFP-AuNP from the same batch were 5.4, 7.7 and 6.3% at 1.0, 10 and 35 ng mL−1 AFP levels, respectively, while the CVs of the assays using HRP-anti-AFP-AuNP from different batches were 8.9, 7.3 and 9.3% at the above-mentioned analyte concentrations. The electrode-to-electrode reproducibility was investigated using HRP-anti-AFP-AuNP from the same batch for the determination of 10 ng mL−1 AFP (as an example). The CV value was 9.8% for 5 electrodes. The electrochemical immunoassay exhibited satisfactory stability. In fact, as much as 90% of the initial DPV response was preserved after storage of the immunosensor and HRP-anti-AFP-AuNP at 4 °C for 25 days.
Additionally, the selectivity of the electrochemical immunoassay was studied by using other biomarkers, such as prostate-specific antigen (PSA), human chorionic gonadotropin (HCG), carcinoembryonic antigen (CEA), glucose, L-cysteine, and BSA. The results are listed in Table 1. The interference degree of variability between lineage-different biomarkers was 3.0–5.6%. Thus, the selectivity of the proposed immunoassay is acceptable.
| Crossing reagenta | Assay time and detected concentrations of AFP/ng mL−1 | RSD (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 3 | 5 | ||
| a Containing 10 ng mL−1 AFP and 10 ng mL−1 (or U mL−1) of interfering agents; and the concentrations were calculated according to the calibration curve. | ||||||
| AFP | 62.7 | 66.1 | 61.3 | 65.3 | 59.1 | 4.1 |
| AFP + PSA | 63.4 | 55.3 | 60.5 | 57.9 | 58.4 | 4.6 |
| AFP + HCG | 56.6 | 62.5 | 54.6 | 55.6 | 57.9 | 4.8 |
| AFP + CEA | 59.2 | 61.4 | 63.2 | 55.1 | 59.6 | 4.5 |
| AFP + BSA | 61.4 | 55.3 | 63.1 | 63.2 | 56.6 | 5.6 |
| AFP + glucose | 63.7 | 67.2 | 64.3 | 66.8 | 62.1 | 3.0 |
| AFP + cysteine | 60.1 | 63.3 | 65.4 | 64.2 | 66.9 | 3.6 |
Analysis of human serum samples and interlaboratory validation
To monitor the trueness and applicability of the developed immunoassay for testing real samples, human serum specimens were assessed using the electrochemical immunoassay and a commercially available Electrochemiluminescent enzyme-linked immunoassay (ECL-ELIA) as a reference method (note: an appreciable dilution was preferable when the concentration of AFP was too high). Comparison of the experimental results obtained by the proposed immunoassay with those of the ECL-ELIA was performed via the use of a regression method (Fig. 7). The regression line was fitted to y = (1.06 ± 0.17)x − (2.2 ± 5.6) (R2 = 0.990) where x stands for the AFP concentrations estimated with the immunoassay and y stands for those of the reference value. No significant differences at the 0.05 significance level were encountered between the optimum values of intercept and slope and experimental data, thereby revealing a good agreement between both analytical methods. | ||
| Fig. 7 Comparison of assayed results for human serum samples using the proposed immunoassay and the ECL-ELIA reference method. | ||
Comparison of analytical performance with other AFP immunoassays
To highlight the merits of the developed analytical method, we compared the analytical performance of the proposed immunoassay with those of other AFP immunosensors or immunoassays.33–43 The comparable items mainly consist of the dynamic range, LOD, and labelled method involving secondary antibodies (Table 2). As shown in Table 2, the working range and LOD of the developed electrochemical immunoassay is acceptable. More importantly, the sensitivity and LOD of the developed immunoassay could be obviously improved by using multilayer {Thi/AuNP}3 films as immunosensing probe and double-codified gold nanolabels, compared with our previous reports which used an encapsulation method and a monolayer nanoparticle technique.33,44 Some possible explanations might be attributed to the results: (i) the multilayer {Thi/AuNP}3 films could increase the amount of thionine molecules on the electrode, and improve the cyclic voltammetric characteristics of the immunosensor since the electrochemical behavior of the immunosensor mainly derives from the assembled thionine; (ii) the doped multilayer nanogold nanoparticles could increase the surface coverage of the electrode, and amplify the immobilized amount of biomolecules due to the pinhole of the multilayer films; (iii) the doped gold nanoparticles might serve as an intervening “spacer” matrix to extend the immobilized biomolecules away from the substrate matrix in the mobile phase, resulting in binding sites more accessible to antigens; and (iv) the double-codified gold nanolabels contain many HRP-anti-AFP molecules on each nanoparticle surface due to the high surface-to-volume ratio of nanogold particles, and thus enhance the catalytic reduction of H2O2.| Immunoassay and immunosensor type | Labels | Dynamic range/ng mL−1 | LOD/ng mL−1 | Refs. |
|---|---|---|---|---|
| Amperometry | HRP-anti-AFP-irAuNP | 0.02–4.0 | 0.01 | 33 |
| Electrochemiluminescence | anti-AFP-SiO2 | 0.05–20 | 0.035 | 34 |
| Microchip-based ELISA | HRP-anti-AFP | 0.001–0.1 | 0.001 | 35 |
| Channeling sensor | ALP-anti-AFP | 1.0–150 | 0.8 | 36 |
| Photochemistry | — | 0.05–50 | 0.04 | 37 |
| Chemiluminescence | HRP-anti-AFP | 1.0–80 | 0.1 | 38 |
| Potentiometry | — | 4.9–158.5 | 1.6 | 39 |
| Quartz crystal microbalance | — | 15.3–600 | — | 40 |
| Surface plasmon resonance | — | 50–500 | — | 41 |
| Sandwich-type immunosensor | HRP-anti-AFP-CNP | 0.05–6 | 0.02 | 42 |
| Sandwich-type immunosensor | HRP-anti-AFP-AuNP | 0.008–0.3 | 0.005 | 43 |
| Amperometry | HRP-anti-AFP-AuNP | 0.25–40 | 0.05 | This work |
Conclusions
In summary, we have demonstrated the potential of a versatile and sensitive electrochemical immunoassay for the determination of AFP (as a model analyte) in disease diagnostics using thionine/nanogold LBL self-assembly multilayer films as immunosensing probes and double-codified gold nanoparticles for signal amplification. Experimental results suggested that the LBL self-assembly technique was suitable for the preparation of stable and ordered multilayer films containing electrochemically active organic dyes and inorganic gold nanoparticles. The electrochemical behaviour of the multilayer film-based immunoassay was studied in detail. Given the greater sensitivity of the developed immunoassay coupled with good reproducibility, selectivity and stability, the LBL self-assembly technique and double-codified nanolabels might hold promise in immuno-/biosensors. To fully assess the application potential and the added value of the electrochemical immunoassay, future research should focus on other target analytes and sample types.Acknowledgements
Financial support from the High-Qualified Talent Funding of FZU (XRC-0929) is gratefully acknowledged. Further, we thank the National Basic Research Program of China (2010CB732403), the NSFC (20877019, 20735002), the Key NSF of Fujian Province (D0520001), the Key Program of Science and Technology Department of Fujian Province (2007Y0026), and NTU-MOE Academic Research Funds (RG65/08) for financial support.References
- C. Lin, J. Wang, H. Wu and G. Lee, J. Assoc. Lab. Autom., 2010, 15, 253 CrossRef CAS.
- J. Hantash, M. Smidt and R. Bowsher, Anal. Methods, 2009, 1, 144 RSC.
- H. Azzazy and M. Mansour, Clin. Chim. Acta, 2009, 403, 1 CrossRef CAS.
- A. Wu, Clin. Chim. Acta, 2006, 369, 119 CrossRef CAS.
- J. Durner, Angew. Chem., Int. Ed., 2009, 49, 1026 CrossRef.
- J. Wang, Electroanalysis, 2007, 19, 769 CrossRef CAS.
- A. De La Escosura-Muniz and A. Merkoci, Expert Opin. Med. Diagn., 2010, 4, 21 Search PubMed.
- C. Chan, Y. Cheung, R. Renneberg and M. Seydack, Adv. Biochem. Engin./Biotechnol., 2007, 109, 123 Search PubMed.
- J. Das, M. Aziz and H. Yang, J. Am. Chem. Soc., 2006, 128, 16022 CrossRef CAS.
- P. Li, Q. Zhang and W. Zhang, TrAC, Trends Anal. Chem., 2009, 28, 1115 CrossRef CAS.
- G. Liu, J. Wang, H. Wu and Y. Lin, Electroanalysis, 2007, 19, 777 CrossRef CAS.
- D. Knopp, D. Tang and R. Niessner, Anal. Chim. Acta, 2009, 647, 14 CrossRef CAS.
- R. Costi, A. Saunders and U. Banin, Angew. Chem., Int. Ed., 2010, 49, 4878 CrossRef CAS.
- T. Hauck, S. Giri, Y. Gao and W. Chan, Adv. Drug Delivery Rev., 2010, 62, 438 CrossRef CAS.
- G. Liu and Y. Lin, Talanta, 2007, 74, 308 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, Anal. Chem., 2008, 80, 1582 CrossRef CAS.
- D. Tang and J. Ren, Anal. Chem., 2008, 80, 8064 CrossRef CAS.
- D. Tang, B. Su, J. Tang, J. Ren and G. Chen, Anal. Chem., 2010, 82, 1527 CrossRef CAS.
- Z. Lin, J. Sauceda-Friebe, J. Lin, R. Niessner and D. Knopp, Anal. Methods, 2010, 2, 824 RSC; M. Mashhadizadeh and H. Khani, Anal. Methods, 2010, 2, 24 RSC.
- Y. Lin, C. Liu and H. Chang, Anal. Methods, 2009, 1, 14 RSC.
- M. Hayatt (Ed.), Colloidal gold-principles, methods and applications. Academic Press, San Diego, 1989 Search PubMed.
- M. Gonzalez-Garcia, C. Fernandez-Sanchez and A. Costa-Garcia, Biosens. Bioelectron., 2000, 15, 315 CrossRef CAS.
- M. Dequaire, C. Degrand and B. Limoges, Anal. Chem., 2000, 72, 5521 CrossRef CAS.
- D. Kampouris, R. Kadara, N. Jeekinson and C. Bank, Anal. Methods, 2009, 1, 25 RSC.
- B. Privett, H. Jae and M. Schoenfisch, Anal. Chem., 2008, 80, 4499 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, J. Phys. Chem. B, 2006, 110, 11640 CrossRef CAS.
- L. Del Mercato, P. Rivera-Gil, A. Abbasi, M. Ochs, C. Ganas, I. Zins, C. Sonnichsen and W. Parak, Nanoscale, 2010, 2, 458 RSC.
- L. Wang, F. Tang, K. Ozawa and G. Lu, Int. J. Surface Sci. Engin., 2009, 3, 44 Search PubMed.
- P. Iler, J. Colloid Sci., 1966, 21, 569 Search PubMed.
- R. Yuan, D. Tang, Y. Chai, X. Zhong, Y. Liu and J. Dai, Langmuir, 2004, 20, 7240 CrossRef.
- D. Li, S. Wei, H. Yang, Y. Li and A. Deng, Biosens. Bioelectron., 2009, 24, 2277 CrossRef CAS.
- J. Zhang, J. Lei, C. Xu, L. Ding and H. Ju, Anal. Chem., 2010, 82, 1117 CrossRef CAS.
- J. Tang, B. Su, D. Tang and G. Chen, Biosens. Bioelectron., 2010, 25, 2657 CrossRef CAS.
- J. Qian, Z. Zhou, X. Cao and S. Liu, Anal. Chim. Acta, 2010, 665, 32 CrossRef CAS.
- Y. Liu, H. Wang, J. Huang, J. Yang, B. Liu and P. Yang, Anal. Chim. Acta, 2009, 650, 77 CrossRef CAS.
- J. Lin, C. He and S. Zhang, Anal. Chim. Acta, 2009, 643, 90 CrossRef CAS.
- G. Wang, J. Xu, H. Chen and S. Fu, Biosens. Bioelectron., 2009, 25, 791 CrossRef CAS.
- Z. Yang, Z. Fu, F. Yan, H. Liu and H. Ju, Biosens. Bioelectron., 2008, 24, 35 CrossRef CAS.
- L. Zhou, R. Yuan and Y. Chai, Electroanalysis, 2007, 19, 1131 CrossRef CAS.
- Y. Ding, J. Liu, H. Wang, G. Shen and R. Yu, Biomaterials, 2007, 28, 2147 CrossRef CAS.
- Y. Teramura and H. Iwata, Anal. Biochem., 2007, 365, 201 CrossRef CAS.
- D. Du, Z. Zou, Y. Shin, J. Wang, H. Wu, M. Engelhard, J. Liu, I. Aksay and Y. Lin, Anal. Chem., 2010, 82, 2989 CrossRef CAS.
- X. Yang, Y. Guo, S. Bi and S. Zhang, Biosens. Bioelectron., 2009, 24, 2707 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, Biotechnol. Lett., 2006, 28, 559 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
