Nanocomposites of sub-10 nm SnO2 nanoparticles and MWCNTs for detection of aldrin and DDT
Fan-Li
Meng†
a,
Yong
Jia†
ab,
Jin-Yun
Liu
a,
Min-Qiang
Li
a,
Yu-Feng
Sun
ac,
Jin-Huai
Liu
*a and
Xing-Jiu
Huang
*a
aResearch Center for Biomimetic Functional Materials and Sensing Devices, Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei, 230031, China. E-mail: xingjiuhuang@iim.ac.cn; jhliu@iim.ac.cn
bDepartment of Pharmacy, Anhui University of Traditional Chinese Medicine, Hefei, 230031, China
cDepartment of Mechanical Engineering, Anhui University of Technology and Science, Wuhu, 241000, China
First published on 29th September 2010
Abstract
The preparation of materials with size and porosity in the nanometre range is of technological importance for a wide range of sensing applications. Here, the ultrasensitive detection of Persistent Organic Pollutants (POPs), i.e., Aldrin and DDT, has been investigated using a sub-10 nm SnO2/MWCNT nanocomposite-based gas sensor. The features of prepared nanocomposites are carefully studied using SEM, TEM, and Raman spectroscopy. The sensing material shows a very attractive improved sensitivity compared to a conventional SnO2 sensor. One major advantage of this sensing material is the stable attachment between SnO2 nanoparticles and carbon nanotubes. Accordingly, facile preparation, high sensitivity, long-term stability, and nanoscale effects in this composite can create novel avenues and applications for fabricating important POPs sensors.
1. Introduction
Persistent Organic Pollutants (POPs), are a group of very stable compounds (they can build up in humans and animals over time) that includes dioxins and pesticides such as chlordane, mirex, DDT, and aldrin that persist in the environment, bioaccumulate through the food chain, and pose a risk of causing adverse effects to human health and the environment.1 They may accumulate in animals and plants at the ends of the food chain, and have been linked to a range of human health problems. Their usage has been strictly restricted by the “Stockholm convention on POPs”, which was enacted during the United Nations Conference in 2001.2 However, DDT is still in use for malaria vector control in some tropical countries as recommended by the World Health Organization.3 Therefore, it is of significant importance to detect POPs for both human health and the environment before they enter the environment during manufacture and disposal.Instrumental analytical methods, such as gas chromatography, gas chromatography/mass spectrometry, high performance liquid chromatography, and optical testing methods have been proposed for the detection of POPs.4–8 However, most of these techniques require a complicated and tedious process and are time-consuming. Despite the many advantages, a number of challenges need to be addressed in electrochemical assays. One major constraint is their limited capacity for combination with immunoassay.6,7 In addition, the operability of electrochemical methods needs to be improved owing to the low water solubility of POPs. There is currently a pressing need for simple, robust and highly sensitive strategies for the detection of POPs in different environments.
Semiconductor gas sensors are predominantly solid-state gas detecting devices for domestic, commercial and industrial application, which have many advantages such as low cost, ease of production, compact size and simple measuring electronics. However, the performance of such sensors is significantly influenced by the morphology and structure of the sensing materials, resulting in a great obstacle for gas sensors based on bulk materials or thin films to achieve highly-sensitive properties. It becomes a serious problem for further exploring novel applications of gas sensors. In the past few years, many efforts have been devoted to improve their selectivity and sensitivity.9–14 Sakai and co-workers found that the porous structure of the sensing film plays a critical role in the performance of the sensor because it defines the rate of gas diffusion.15 Xu and co-workers found that the particle size affects the sensitivity of the sensor.16 When the particle size of SnO2 is close to or less than 10 nm the sensitivity of the sensor will increase by a large scale. Very recently, we found that porous SnO2 nanotubes exhibited high sensitivity and good reversibility toward some organic gases, such as ethanol and acetone.17 In addition to the morphology of the sensing materials,18–20 it has been demonstrated that the p-n junction plays an important role during the gas sensing.21 p-n junction controlled gas sensors mostly exhibit high sensitivity. However, little research was done to improve the sensitivity of semiconductor gas sensor to POPs.
In the present paper, we addressed the aforementioned issues by implementing sub-10 nm SnO2 nanoparticles along with multi-walled carbon nanotubes (MWCNTs) to form nanocomposites that can accurately detect aldrin (1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-1,4,5,8-dimethanonaphthalene) and DDT (1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane) at extremely low concentrations (see Fig. 1 for structures). Three factors, i.e. gas diffusion, particle size and p-n junction, are considered to improve the sensitivity to POPs. MWCNTs can be regarded as the framework and the SnO2 particles are grown along them. Highly porous 3D structured SnO2/MWCNT nanocomposites enhance the ability of gas diffusion. The attachment of sub-10 nm SnO2 particles to the MWCNTs results in p-n junction formation between carbon nanotubes and localized SnO2 nanoparticles. The preliminary measurements show that the sensor based on SnO2/MWCNT nanocomposites are highly sensitive to POPs. This kind of nanocomposite can potentially allow a quick and easy way to detect POP molecules.
2. Experimental
2.1. Materials
MWCNTs with approximate diameter 20–30 nm were purchased from Shenzhen Nanotech Port Co. Ltd. DDT and aldrin were supplied by the Institute of Agro-Environmental Protection, Ministry of Agriculture, China. Other chemicals were analytical grade and used without further purification as purchased from China National Medicine Group Shanghai Chemical Reagent Company.2.2. Preparation of SnO2/MWCNT nanocomposites and gas sensor
The raw MWCNTs were firstly purified by heat treatment at 350 °C for 2 h to remove amorphous carbon and acidification treatment at 120 °C for 12 h to remove catalyst. The SnO2/MWCNT nanocomposites were synthesized following a previous literature procedure.17 Briefly, 1 g of tin(II) chloride (SnCl2·2H2O) was dissolved in 40 mL of distilled H2O, and then 0.25 mL of HCl (38%) was added. Subsequently, 10 mg of the as-treated MWCNTs was dispersed in the above solution. The mixture was sonicated for 20 min and then stirred at room temperature for 2 h. The as-prepared wet SnO2/MWCNT nanocomposites were directly coated on the outer surface of a ceramic tube substrate and dried in air. The dried nanocomposites were calcined at 350 °C for 2 h and then heated at 650 °C for 2 h under Ar protection.The structure of the sensor is shown in Fig. 2. The as-prepared SnO2/MWCNT nanocomposites as sensing materials were coated on the ceramic tube with a pair of pre-prepared gold electrodes. Each gold electrode was symmetrically pasted with a pair of gold wires, respectively. A piece of nichrome wire about 32 Ω as heating wire was placed in the interior of the ceramic tube. The temperature of the sensor can be controlled by modulating the heating voltage.
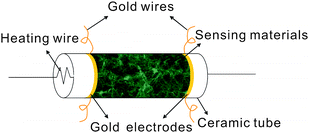 | ||
| Fig. 2 The structure of the gas sensor based on the SnO2/MWCNT nanocomposites. | ||
2.3. Measurement system
The measurement system for Aldrin and DDT detection was mainly composed of three parts, i.e., gas carrier, gasification and detection chambers, as illustrated in Fig. 3. Two kinds of sensor, traditional SnO2 sensor and SnO2/MWCNT nanocomposite-based sensor, were housed in the detection chamber and each sensor was connected in series with a constant resistance, respectively. The constant resistance was connected to a data acquisition device (Art USB2005, China) which was connected to a computer. The voltage across the constant resistance was acquired by the data acquisition device and then transmitted, stored and real-time displayed on the computer. The measuring process was as follows: firstly, liquid sample was injected into the gasification chamber by a microsyringe and then transformed into vapor by heating. Under a temperature of 200 °C, Aldrin and DDT solution, including solvent ligroine, were gasified without decomposition. Secondly, the as-formed vapor was transferred into the detection chamber by a constant air flow which served as the gas carrier. Once the sample gas contacted with gas-sensing nanomaterials, a responding voltage signal would be acquired and transmitted to the computer. Finally, exhaust gases were collected by a solution at the end of the system. | ||
| Fig. 3 Schematic showing SnO2/MWCNT nanocomposite-based gas sensing system. | ||
2.4. Characterization
Field emission scanning electron microscope images (FESEM) were obtained with a FEI Sirion 200 FEG scanning electron microscope (American). Transmission electron microscopy (TEM) was carried out using a JEOL JEM-2010 transmission electron microscope (Japan). The Raman scattering measurements were performed using a DXR Smart Raman Spectrometer and the 532 nm line of an argon laser.3. Results and discussion
3.1. Morphological characterization of SnO2/MWCNTs nanocomposites
Fig. 4 shows the comparison of the purified MWCNTs with SnO2/MWCNT nanocomposites. The image indicates that the MWCNTs are highly entangled (Fig. 4a) and no residue can be observed. The surface of the nanotubes was smooth. As shown in Fig. 4b, the SnO2/MWCNT nanocomposites become thicker than the original MWCNTs, suggesting that the SnO2 nanoparticles uniformly packed on the MWCNTs. Further evidence can be obtained from TEM images, as will be discussed in the following section. Highly porous 3D structured SnO2/MWCNT nanocomposites can be seen from Fig. 4b. MWCNTs can be regarded as the framework and the SnO2 particles are grown along them, which may enhance the ability of gas to diffuse into and out of the body of the sensing film. | ||
| Fig. 4 FESEM images of (a) the purified MWCNTs and (b) the SnO2/MWCNT nanocomposites. | ||
Raman spectroscopy can provide unique information about vibrational and electronic properties of the material.22,23. Fig. 5 shows the Raman spectra of the raw and treated MWCNTs, which display two strong bands, viz. the so-called disorder mode (ca. 1290 cm−1) (D-band) and tangential mode (ca. 1590 cm−1) (G-band). The G-band corresponds to the characteristic A1g, E1g and E2g modes of graphene sheet.24 The D-band is related to resonance-enhanced scattering of an electron via phonon emission by a defect that breaks the basic symmetry of the graphene plane,25 which can be used to diagnose the disruptions in the hexagonal framework of the CNTs, or indicate the sample purity, such as by identification of amorphous carbon impurities. In the spectra, the intensity ratio of the D-band to the G-band (ID/IG) decreased after the MWCNTs were heat-treated at 350 °C for 2 h, indicating that the amorphous carbons were partly removed. After the heat-treated MWCNTs were acidified, ID/IG increased which suggested that the defects of the MWCNTs increased. In addition, it is interesting to note that the intensity of the D-band is substantially increased after acidification, indicating that the formation of defect sites with sp3-hybridized carbon atoms in the framework of the nanotubes is due to the addition of functional groups, such as –COOH and –OH, etc.
 | ||
| Fig. 5 Raman spectra of MWCNTs under different conditions. Experimental conditions: 532 nm laser wavelength; 10 mW laser power; 10 s exposure × 10 accumulations. | ||
TEM studies confirmed the success of the attachment of SnO2 nanoparticles to MWCNTs, as shown in Fig. 6. Well-dispersed SnO2 nanoparticles decorate the walls and ends of the nanotubes quite uniformly (Fig. 6a). The sub-10 nm size is apparent at high magnification (Fig. 6b). The SnO2 nanoparticles are attached through a well-defined scheme that involves interaction of the sites on the nanotube walls decorated by carboxyl functional groups. It is also noted that the interaction between the SnO2 nanoparticles and nanotubes is quite strong, because thorough washing does not remove them. As a control, raw MWCNT (without any treatments) was mixed with tin(II) chloride solution, and a few SnO2 nanoparticles were found on the nanotubes (Fig. 6c). This indicates that carboxyl or hydroxyl etc. functional groups play a key role in the attachment; acting as a bridge to connect SnO2 nanoparticles with MWCNTs. As can be seen in Fig. 6d, the temperature of heat treatment affects the synthesis of SnO2/MWCNT nanocomposites.
 | ||
| Fig. 6 TEM images of the SnO2/MWCNT nanocomposites. (a) Purified MWCNT decorated with SnO2 nanoparticles (heat treatment at 650 °C). (b) High magnified image of panel a. (c) Raw MWCNT decorated with SnO2 nanoparticles (heat treatment at 650 °C). (d) Purified MWCNT decorated with SnO2 nanoparticles (heat treatment at 350 °C). | ||
3.2. Sensing properties towards Aldrin and DDT
The sensor was placed abreast with a reference traditional SnO2 sensor in the test chamber. POPs (DDT and aldrin) were dissolved in ligroine at a concentration of 1.0 × 10−3 g L−1 for detection. For comparison, the same volume of pure ligroine which served as the solvent in POPs solutions was also detected. The real-time response curves of both sensors to 1 μL POPs solutions and pure ligroine are shown in Fig. 7a. As can be seen, the measured voltage of the SnO2 sensor increases rapidly after the injection of sample and the response amplitudes are nearly equal to both pure ligroine and POPs solutions, indicating that the traditional SnO2 sensor is seldom sensitive to POPs. In contrast, the SnO2/MWCNT nanocomposite-based sensor responded a little to pure ligroine, yet responded obviously to both DDT and aldrin solutions. The results illustrate that the structure of the SnO2/MWCNT nanocomposites enhances the sensitivity and selectivity of SnO2 material to POPs. 1 ng DDT and Aldrin also gave a sharp response. This sensor can detect these POPs with a lower concentration limit of about 0.5 ng. Besides, the recovery rate of the SnO2/MWCNT nanocomposite-based sensor is obviously faster than that of the traditional SnO2 sensor, which may be attributed to the highly porous 3D structure. From Fig. 7b, it can be seen that the response of the SnO2/MWCNT nanocomposite-based sensor linearly increases from 1 to 100 μg mL−1. | ||
| Fig. 7 (a) Real-time response of the traditional SnO2 sensor and SnO2/MWCNT nanocomposite-based sensor to the vapours of 1 μL pure ligroine and 1 μL POPs ligroine solution (1.0 × 10−3 g L−1). (b) Sensitivity of the SnO2/MWCNT nanocomposite-based sensor upon exposure to DDT and Aldrin at concentrations from 1 to 100 μg mL−1. | ||
3.3. Sensing mechanism
MWCNTs were uniformly coated with 5–10 nm SnO2 nanoparticles. According to previous reports, when the size of the SnO2 particle decreases to less than 10 nm, the sensitivity of the sensor increases remarkably because of their small size effect. Below this size, the oxygen depletion layer will occupy the whole particle and there are few mobile charge carriers in it.26 In the body of the SnO2/MWCNT nanocomposites, it can be modeled that SnO2 nanoparticles are connected to each other and the MWCNTs are dispersed in them. It is widely accepted that in an air environment, O2 is adsorbed on the surface of the SnO2 nanoparticles and it will trap and react with the electrons from the SnO2 materials to produce negative oxygen ions, such as O2−, O−, and O2−.13,27,28 The presence of chemisorbed oxygen anions generate an electron depletion layer around the surface of the SnO2 nanoparticles. According to the reported results, the thickness of the depletion layer (L) of SnO2 in air is about 3 nm.29–33 The mean diameter (D) of the SnO2 particle is approximately equal to or slightly larger than 2L. Therefore, there are very few mobile charge carriers in the inner layer of the SnO2 particles, which can lead to large changes in conductivity. It is concluded that SnO2 nanoparticles 5–10 nm in size are very sensitive. On the other hand, MWCNTs also act as a p-type semiconductor in air,34,35 whereas SnO2 behaves as an n-type semiconductor. In this case, the p-n junctions are formed at the interfaces between SnO2 nanoparticles and carbon nanotubes. A large number of p-n junctions can further improve the sensitivity of the sensor.36Carbon nanotubes, as a one dimensional material, provide advantages in transmitting charge carriers.37 In the sensing film, there are only the highly sensitive depletion layers of SnO2 and p-n junctions and the carbon nanotubes. There are few mobile charge carriers, which leads to a highly sensitive system. From Fig. 7a, it can be seen that the traditional SnO2 sensors are highly sensitive to pure ligroine, but they are not sensitive to POPs. However, POPs molecules process higher chemical activity than ligroine molecules. Although the amount of POPs molecules is small, the SnO2/MWCNT sensor responded obviously to both DDT and aldrin solutions, since very few electrons can cause a large conductivity change.
In order to investigate the selectivity of the SnO2/MWCNT nanocomposite-based sensor, some usual solvents, e.g., hexane, benzene, acetone, ethanol and water etc. have been selected for comparison (Fig. 8). The present results reveal that the sensitivities to ligroine, hexane and benzene are sufficiently lower than those of DDT and Aldrin. The sensor is highly responsive to other solvents. Therefore, ligroine, hexane and benzene are appropriate solvents for the extraction of POPs from soil or vegetables. The variations in sensitivity of the SnO2/MWCNT nanocomposite-based sensor upon exposure to 1 μg mL−1 Aldrin at different working temperatures was also investigated and is shown in Fig. 9. The maximal sensitivity could be observed at 350 °C.
 | ||
| Fig. 8 Selectivity of the SnO2/MWCNT nanocomposite-based sensor to other chemicals compared to 1 μg mL−1 DDT and Aldrin. | ||
 | ||
| Fig. 9 Sensitivity of the SnO2/MWCNT nanocomposite-based sensor upon exposure to 1 μg mL−1 Aldrin at different working temperatures. | ||
4. Conclusion
Although SnO2/MWCNT nanocomposites have been widely synthesized,38–40 the detection of Persistent Organic Pollutants (POPs), such as Aldrin and DDT, at low concentrations using sub-10 nm SnO2/MWCNT nanocomposite-based gas sensors is studied for the first time in the present paper. A sharp response and low limiting concentration (1 ng) is observed for both Aldrin and DDT, suggesting potential application for analytical methods. The sensing mechanism is carefully investigated. The high sensitivity can be attributed to highly porous 3D structured SnO2/MWCNT nanocomposites (which enhance the ability of gas diffusion), the size of sub-10 nm SnO2 particles, and p-n junction formation between carbon nanotubes and SnO2 nanoparticles. The preliminary results make SnO2/MWCNT nanocomposites attractive for the purpose of POPs gas sensors.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 90923033, 60604022), the National Basic Research Program of China (Grant No. 2007CB936603), the National High Technology Research and Development Program of China (Grant No. 2007AA022005) and the Anhui Provincial Natural Science Foundation (090412036). X.-J. H especially thanks the One Hundred Person Project of the Chinese Academy of Sciences, China. We also thank Yingxian Wang and Lin Ni for their useful suggestions and technical support.References
- J. Ruzzin, R. Petersen, E. Meugnier, L. Madsen, E.-J. Lock, H. Lillefosse, T. Ma, S. Pesenti, S. B. Sonne, T. T. Marstrand, M. K. Malde, Z.-Y. Du, C. Chavey, L. Fajas, A.-K. Lundebye, C. L. Brand, H. Vidal, K. Kristiansen and L. Froyland, Environ. Health Perspect., 2010, 118, 465–471 CAS.
- G. Karlaganis, R. Marioni, I. Sieber and A. Weber, Environ. Sci. Pollut. Res., 2001, 8, 216–221 CrossRef CAS.
- G. Zhang, A. Parker, A. House, B. X. Mai, X. D. Li, Y. H. Kang and Z. S. Wang, Environ. Sci. Technol., 2002, 36, 3671–3677 CrossRef CAS.
- J. F. Focant, J. W. Cochran, J. M. D. Dimandja, E. DePauw, A. Sjodin, W. E. Turner and D. G. Patterson, Analyst, 2004, 129, 331–336 RSC.
- K. Turner, S. F. Xu, P. Pasini, S. Deo, L. Bachas and S. Daunert, Anal. Chem., 2007, 79, 5740–5745 CrossRef CAS.
- H. Y. Chen and H. S. Zhuang, Anal. Bioanal. Chem., 2009, 394, 1205–1211 CrossRef CAS.
- S. Laschi, M. Mascini, G. Scortichini, M. Franek and M. Mascini, J. Agric. Food Chem., 2003, 51, 1816–1822 CrossRef CAS.
- Y. Yang and G. Meng, J. Appl. Phys., 2010, 107, 044315–044319 CrossRef.
- N. Yamazoe, G. Sakai and K. Shimanoe, Catal. Surv. Asia, 2003, 7, 63–75 CrossRef CAS.
- J. H. Liu, X. J. Huang, G. Ye, W. Liu, Z. Jiao, W. L. Chao, Z. B. Zhou and Z. L. Yu, Sensors, 2003, 3, 110–118 CrossRef CAS.
- X. J. Huang, Y. F. Sun, F. L. Meng and J. H. Liu, Sens. Actuators, B, 2004, 102, 235–240 CrossRef.
- X. J. Huang, L. C. Wang, Y. F. Sun, F. L. Meng and J. H. Liu, Sens. Actuators, B, 2004, 99, 330–335 CrossRef.
- X. J. Huang, F. L. Meng, Z. X. Pi, W. H. Xu and J. H. Liu, Sens. Actuators, B, 2004, 99, 444–450 CrossRef.
- X. J. Huang, J. H. Liu, D. L. Shao, Z. X. Pi and Z. L. Yu, Sens. Actuators, B, 2003, 96, 630–635 CrossRef.
- G. Sakai, N. Matsunaga, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2001, 80, 125–131 CrossRef.
- C. N. Xu, J. Tamaki, N. Miura and N. Yamazoe, Sens. Actuators, B, 1991, 3, 147–155 CrossRef.
- Y. Jia, L. F. He, Z. Guo, X. Chen, F. L. Meng, T. Luo, M. Q. Li and J. H. Liu, J. Phys. Chem. C, 2009, 113, 9581–9587 CrossRef CAS.
- X. H. Kong and Y. D. Li, Sens. Actuators, B, 2005, 105, 449–453 CrossRef.
- Y. Tan, C. C. Li, Y. Wang, J. F. Tang and X. C. Ouyang, Thin Solid Films, 2008, 516, 7840–7843 CrossRef CAS.
- H. Z. Wang, J. B. Liang, H. Fan, B. J. Xi, M. F. Zhang, S. L. Xiong, Y. C. Zhu and Y. T. Qian, J. Solid State Chem., 2008, 181, 122–129 CrossRef CAS.
- X. Y. Xue, L. L. Xing, Y. J. Chen, S. L. Shi, Y. G. Wang and T. H. Wang, J. Phys. Chem. C, 2008, 112, 12157–12160 CrossRef CAS.
- X. J. Huang, H. S. Im, D. H. Lee, H. S. Kim and Y. K. Choi, J. Phys. Chem. C, 2007, 111, 1200–1206 CrossRef CAS.
- X. J. Huang, H. S. Im, O. Yarimaga, J. H. Kim, D. H. Lee, H. S. Kim and Y. K. Choi, J. Phys. Chem. B, 2006, 110, 21850–21856 CrossRef CAS.
- M. H. Liu, Y. L. Yang, T. Zhu and Z. F. Liu, Carbon, 2005, 43, 1470–1478 CrossRef CAS.
- M. S. Strano, C. A. Dyke, M. L. Usrey, P. W. Barone, M. J. Allen, H. W. Shan, C. Kittrell, R. H. Hauge, J. M. Tour and R. E. Smalley, Science, 2003, 301, 1519–1522 CrossRef CAS.
- A. Rothschild and Y. Komem, J. Appl. Phys., 2004, 95, 6374–6380 CrossRef CAS.
- P. G. Harrison and M. J. Willett, Nature, 1988, 332, 337–339 CrossRef.
- J. Y. Liu, Z. Guo, F. L. Meng, Y. Jia and J. H. Liu, J. Phys. Chem. C, 2008, 112, 6119–6125 CrossRef CAS.
- X. M. Yin, C. C. Li, M. Zhang, Q. Y. Hao, S. Liu, Q. H. Li, L. B. Chen and T. H. Wang, Nanotechnology, 2009, 20, 455503–455507 CrossRef.
- V. D. Nguyen, V. H. Nguyen, T. H. Pham, D. C. Nguyen, M. Thamilselvan and J. Yi, Phys. E., 2008, 41, 258–263 CrossRef.
- H. Ogawa, M. Nishikawa and A. Abe, J. Appl. Phys., 1982, 53, 4448–4455 CrossRef CAS.
- T. Zhang, L. Liu, Q. Qi, S. C. Li and G. Y. Lu, Sens. Actuators, B, 2009, 139, 287–291 CrossRef.
- H. T. Feng, R. F. Zhuo, J. T. Chen, D. Yan, J. J. Feng, H. J. Li, S. Cheng and P. X. Yan, Phys. E., 2009, 41, 1640–1644 CrossRef CAS.
- V. Derycke, R. Martel, J. Appenzeller and P. Avouris, Appl. Phys. Lett., 2002, 80, 2773–2775 CrossRef CAS.
- Y. Li, H. C. Wang, X. H. Cao, M. Y. Yuan and M. J. Yang, Nanotechnology, 2008, 19, 015503–015507 CrossRef.
- L. F. He, Y. Jia, F. L. Meng, M. Q. Li and J. H. Liu, J. Mater. Sci., 2009, 44, 4326–4333 CrossRef CAS.
- I. D. Kim, A. Rothschild, B. H. Lee, D. Y. Kim, S. M. Jo and H. L. Tuller, Nano Lett., 2006, 6, 2009–2013 CrossRef CAS.
- W. Q. Han and A. Zettl, Nano Lett., 2003, 3, 681–683 CrossRef CAS.
- N. D. Hoa, N. V. Quy and D. Kim, Sens. Actuators, B, 2009, 142, 253–259 CrossRef.
- R. Leghrib, R. Pavelko, A. Felten, A. Vasiliev, C. Cane, I. Gracia, J. J. Pireaux and E. Llobet, Sens. Actuat. B-Chem., 145, pp. 411–416 Search PubMed.
Footnote |
| † These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |

