A simple cyanide test kit for water and fruit juices†
Manita
Untang
,
Juwadee
Shiowatana
and
Atitaya
Siripinyanond
*
Department of Chemistry and Center for Innovation in Chemistry, Faculty of Science, Mahidol University, Rama VI Rd., Bangkok, 10400, Thailand. E-mail: scasp@mahidol.ac.th; Fax: +66-2-354-7151; Tel: +66-2-201-5129
First published on 24th September 2010
Abstract
A sensitive, selective, rapid, and user-friendly field test kit was developed for the determination of cyanide. The method was based on a reaction to generate hydrogen cyanide from the solution containing cyanide by reaction with sulfuric acid in a specially designed reaction bottle. The gaseous hydrogen cyanide diffused and was collected on a paper strip impregnated with p-nitrobenzaldehyde and o-dinitrobenzene in sodium hydroxide. Hydrogen cyanide reacted with p-nitrobenzaldehyde to form an intermediate p-cyanobenzadehyde cyanohydrin, which reacted with o-dinitrobenzene to give a purple compound, the dianion of o-nitrophenyl hydroxylamine. The intensity of the purple color depended on the concentration of cyanide in the test solution. The developed field test kit could be used for the detection of cyanide as low as 0.01 mg l−1 within 10 min. The cyanide field test kit was applied to determine cyanide concentrations in natural water and commercial fruit juices with good accuracy as validated by using the APHA/AWWA/WEF standard method 4500-CN−.
Introduction
Water quality monitoring is becoming an increasingly important component of national activities. Cyanides can occur naturally or come from anthropogenic processes.1 Cyanides in water come mainly from industrial processes such as organic chemical industries, iron and steel plants or manufacturers, and publicly owned wastewater treatment facilities. Furthermore, cyanides are also found in a number of foods and plants.1 At least 2,650 species of plants and micro-organisms have been shown to contain one or more of nearly twenty compounds capable of producing cyanide, including agriculturally important species such as cassava, flax, sorghum, alfalfa, bamboo, peach, pear, cherry, plum, corn, potato, cotton, almond, and beans.2 Other cyanide sources include vehicle exhaust, releases from certain chemical industries, burning of municipal waste, and the use of cyanide-containing pesticides.3 These activities have resulted in significant amounts of cyanide being introduced into the environment. Therefore, the study of cyanide in the environment is becoming increasingly important for pollution monitoring.Owing to the health risks from cyanide related compounds, the limits for cyanide in drinking water have been set as 0.2 mg l−1 by the Environmental Protection Agency (EPA)4 and 0.1 mg l−1 by the Ministry of Natural Resources and Environment of Thailand.5 Analytical monitoring is a useful preventive approach in water quality management. The prevention of poor health effects is only possible if the water testing results can be obtained almost instantly in the field with reasonable accuracy.
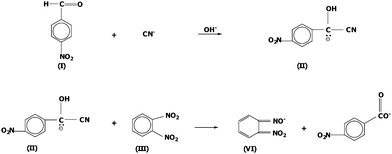
This work aims to develop a sensitive, selective, rapid, low-cost, and user-friendly field test kit for the monitoring of cyanide in water. The method was based on Guilbault and Kramer,6 a reaction of hydrogen cyanide with p-nitrobenzaldehyde (I) to form an intermediate cyanohydrin (II) which reacts with o-dinitrobenzene (III) to give a purple compound (IV) as illustrated in Fig. 1. Furthermore, in order to demonstrate the applicability of the developed field test kit to complex matrices, several commercial fruit juices were also tested and the developed method was validated with the APHA/AWWA/WEF standard method 4500-CN−.
Experimental
A specially designed reaction bottle
A specially designed reaction bottle, shown in Fig. 2, was used for containment of the test solution. The design of this reaction bottle was similar to what has been used as a reaction bottle for arsenic tests, which has already been patented.7 This reaction bottle was designed to allow the containment of a water sample to react with required reagents for vapor generation. The screw cap of the reaction bottle was furnished with a polyethylene ring and a silicone ring to allow the paper strip (0.8 × 4.1 cm filter paper, no. 3, Whatman, Maidstone, England) containing the cyanide detection reagent to be inserted through as shown in Fig. 2. The description of each part of the device set-up for cyanide testing is as follows: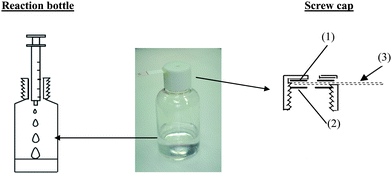 | ||
| Fig. 2 Reaction bottle for cyanide test kit: 1) silicone ring; 2) polyethylene ring; and 3) paper strip, for hydrogen cyanide gas trapping. | ||
The polyvinylchloride reaction bottle (capacity 60 ml) was used to contain the water sample, and to allow the reaction to generate hydrogen cyanide gas. The polypropylene screw cap (OD 22.5 mm and ID 20.4 mm) for closing the reaction bottle was furnished with a polyethylene ring (OD 19.2 mm) and a silicone ring (OD 19.2 mm). All the rings were holed in the center to allow the passage of hydrogen cyanide vapor.
Chemicals
All reagents used were of analytical grade. p-Nitrobenzaldehyde and o-dinitrobenzene were purchased from Sigma Aldrich (St. Louis, MO, USA). Ethyleneglycol monomethyl ether (methyl cellosolve) was from Sigma Aldrich. Sodium hydroxide and sulfuric acid were from Labscan (Bangkok, Thailand). A mixed reagent containing p-nitrobenzaldehyde (3.0 × 10−1 mol l−1) and o-dinitrobenzene (3.0 × 10−1 mol l−1) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was prepared in 10 ml of methyl cellosolve. An aqueous sodium hydroxide solution was prepared by dissolving sodium hydroxide pellets in 1,000 ml of deionized water. Sulfuric acid was diluted in deionized water to obtain a concentration of 1.0 mol l−1 before use. A standard solution of cyanide was prepared by weighing an appropriate amount of potassium cyanide and dissolving it in a sodium hydroxide solution of 0.1 mol l−1, and kept in a refrigerator until use. Magnesium chloride was purchased from Merck (Darmstadt, Germany) and it was prepared by dissolving 50 g in 100 ml of deionized water to obtain a concentration of 2.5 mol l−1.
1 was prepared in 10 ml of methyl cellosolve. An aqueous sodium hydroxide solution was prepared by dissolving sodium hydroxide pellets in 1,000 ml of deionized water. Sulfuric acid was diluted in deionized water to obtain a concentration of 1.0 mol l−1 before use. A standard solution of cyanide was prepared by weighing an appropriate amount of potassium cyanide and dissolving it in a sodium hydroxide solution of 0.1 mol l−1, and kept in a refrigerator until use. Magnesium chloride was purchased from Merck (Darmstadt, Germany) and it was prepared by dissolving 50 g in 100 ml of deionized water to obtain a concentration of 2.5 mol l−1.
Samples
Two natural water samples were collected from two canals in Bangkok (Saen Saep canal and Chak Phra canal). Eight commercial fruit juice samples consisting of apple, apricot, cherry berry, mulberry, and prune were bought from a local supermarket.Colorimetric determination of cyanide in solution
The color development of cyanide in solution exploits the reaction between cyanide and p-nitrobenzaldehyde with o-dinitrobenzene in basic solution, as reported by Guilbault and Kramer.6 One drop of a mixture of reagent containing 3.0 × 10−1 mol l−1 of p-nitrobenzaldehyde and o-dinitrobenzene each, and one drop of 7.5 × 10−1 mol l−1 of sodium hydroxide solution were added into a 5 ml solution containing various concentrations of cyanide standard solution. The color was recorded at a reaction time of 10 min.Colorimetric determination of cyanide on a paper strip after converting the free cyanide into the vapor form
To perform the test, a paper strip was prepared by immersing the paper into a solution containing a mixture of 3.0 × 10−1 mol l−1 of p-nitrobenzaldehyde and o-dinitrobenzene. Then, 7.5 × 10−1 mol l−1 sodium hydroxide solution was dropped onto the paper. This paper was then inserted into a slot on the bottle cap and was ready for use. A syringe was used to transfer a 20 ml test solution into the specially designed reaction bottle. Then, 1.5 ml of 1.0 mol l−1 sulfuric acid was added into the 20 ml test solution. The bottle was then capped immediately. After 10 min of reaction time, the cap was loosened. Then, the paper strip was taken out for color reading.Construction of the visual color scales for cyanide determination
A standard color scale for cyanide determination was constructed for the visual determination of cyanide by comparing the intensity of the colors developed on the paper strip with the cyanide standard solution in concentrations ranging from 10–200 μg l−1. The RGB (red, green, blue) color model with the digital 8-bit per channel notation was used to represent the color. The RGB model describes how much of each of the colors (red, green, blue) is included by which the value can vary from 0 to 255 with the 8-bit per channel notation. If all color components are zero, the color is black. If all color components are 255, the color is white.Validation of the developed cyanide field test kit
To validate the developed cyanide field test kit, the cyanide determination results obtained from the developed method were compared with those from the APHA/AWWA/WEF standard method 4500-CN−.8 The test solution of 250 ml was added into a 500 ml three-necked boiling flask: one neck to allow a slow stream of air to enter the flask; another one to connect to the air inlet tube for addition of sulfuric acid; and the center one to connect to a condenser. The condenser was connected onto the boiling flask, which was further joined with an absorbing tube containing 10.00 ml of 0.2 mol l−1 sodium hydroxide. Before distillation, a slow stream of air was adjusted so that approximately two bubbles of air per second entered the boiling flask through the air inlet tube. Then, 25 ml of 18 N sulfuric acid was slowly added through the air inlet tube which was rinsed with distilled water. Then, the air was flowed to mix the flask contents for 3 min. Subsequently, 10 ml of magnesium chloride was poured into the air inlet and washed down with a stream of water. The heat was then turned on to boil the solution under reflux for one hour. Then, the heat was turned off and the airflow was continued for at least 15 min. After cooling the boiling flask, the absorber was disconnected and the airflow was terminated. The solution was drained from the absorber into a 50.00 ml volumetric flask and the volume was adjusted to the mark with distilled water. The distillate obtained was subjected to colorimetric determination of cyanide using the procedure described above.Results and discussion
Cyanide detection strategy
The colorimetric determination of cyanide in solution is based on the reaction between cyanide and p-nitrobenzaldehyde with o-dinitrobenzene in basic solution to generate the purple color of the dianion of o-nitrophenyl hydroxylamine.9 This reaction product showed a strong purple color compound and therefore could be detected visually by eye with a lowest detectable concentration of 1 mg l−1 cyanide. With a higher concentration of cyanide, the purple color was more intense as illustrated in Table 1 and the color was represented by the RGB (red, green, blue) color model. Nonetheless, the lowest detectable concentration of cyanide (2 mg l−1) was higher than the maximum allowable concentration of cyanide in water (0.1 mg l−1). Therefore, the cyanide test method was further improved for its detection sensitivity.| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Concentration/mg l−1 (for 5 ml sample) | 1 | 2 | 5 | 10 | 20 | 30 | 50 |
| Color scale |

|

|

|

|

|

|

|
| RGB value, R | 255 | 255 | 243 | 205 | 178 | 150 | 135 |
| RGB value, G | 255 | 235 | 213 | 102 | 57 | 50 | 43 |
| RGB value, B | 255 | 255 | 243 | 205 | 181 | 150 | 137 |
In order to improve the sensitivity of the method and also to avoid any potential interferences, the strategy was to separate cyanide from any other interfering ions by converting cyanide to gaseous hydrogen cyanide upon addition of sulfuric acid to the test solution in a specially designed reaction bottle,7 as shown in Fig. 2. Then the gaseous hydrogen cyanide diffused and was collected on a paper inserted in a slot on the bottle cap. Before the test, this paper strip was impregnated with p-nitrobenzaldehyde and o-dinitrobenzene in sodium hydroxide to allow the reaction with the generated hydrogen cyanide, resulting in a purple color of the dianion of o-nitrophenyl hydroxylamine. The intensity of the purple color depended on the concentration of cyanide in the test solution.
To perform the test, a paper strip was prepared by immersing the paper into a solution containing a mixture of suitable concentrations of p-nitrobenzaldehyde and o-dinitrobenzene. Then, a sodium hydroxide solution of suitable concentration was dropped onto the paper. This paper was then inserted into a slot on the bottle cap and was ready for use. A syringe was used to transfer a 20 ml portion of test solution into a reaction bottle. Then, an appropriate volume/concentration of sulfuric acid was added into the 20 ml test solution. The bottle was then capped immediately. After a suitable reaction time, the cap was loosened. Then, the paper strip was taken out for color reading.
Standard color scale for cyanide detection
Parameters affecting the test sensitivity were examined including the concentrations of sulfuric acid, p-nitrobenzaldehyde and o-dinitrobenzene, sodium hydroxide, as well as the reaction time. With the optimum conditions: 1.0 mol l−1 of sulfuric acid, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of p-nitrobenzaldehyde and o-dinitrobenzene with concentrations of 3.0 × 10−1 mol l−1 each, 7.5 × 10−1 mol l−1 of sodium hydroxide, and 10 min reaction time; the standard color scale for cyanide determination on a paper strip was constructed as illustrated in Table 2. The color scale discriminates the following μg l−1 ranges of cyanide concentration: 0–10; 10–20; 20–50; 50–100; 100–200; and >200. The RGB (red, green, blue) color model was used to represent the color scale obtained from each cyanide concentration range as summarized in Table 2. With the developed method, the lowest detectable concentration of cyanide was 10 μg l−1.
1 ratio of p-nitrobenzaldehyde and o-dinitrobenzene with concentrations of 3.0 × 10−1 mol l−1 each, 7.5 × 10−1 mol l−1 of sodium hydroxide, and 10 min reaction time; the standard color scale for cyanide determination on a paper strip was constructed as illustrated in Table 2. The color scale discriminates the following μg l−1 ranges of cyanide concentration: 0–10; 10–20; 20–50; 50–100; 100–200; and >200. The RGB (red, green, blue) color model was used to represent the color scale obtained from each cyanide concentration range as summarized in Table 2. With the developed method, the lowest detectable concentration of cyanide was 10 μg l−1.
| Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Concentration/μg l−1 (for 20 ml sample) | 0 | 10 | 20 | 50 | 100 | 200 |
| Color scale |

|

|

|

|

|

|
| RGB value, R | 255 | 245 | 220 | 180 | 150 | 110 |
| RGB value, G | 255 | 230 | 200 | 160 | 130 | 90 |
| RGB value, B | 255 | 255 | 230 | 190 | 170 | 140 |
Application of the developed test kit for the determination of the available cyanide concentration in natural water and commercial fruit juices
In order to demonstrate the applicability of the developed cyanide test kit, two types of samples were tested including water samples and fruit juices. Two surface water samples were collected from canals, and analyzed for their cyanide concentrations using the proposed method. Furthermore, a total of eight commercial fruit juice samples was analyzed. Cyanide was not found in a detectable level (<10 μg l−1) in water samples and several fruit juices, however, it was found in three fruit juice samples including apricot (50 μg l−1), plum (50 μg l−1), and prune (10 μg l−1) as summarized in Table 3. To check for the recovery of the test method, 10 and 50 μg l−1 of standard cyanide were added into the samples with the analysis results shown in Table 3. For all samples tested, the recovery was satisfactory.| Number | Sample | Concentration found/μg l−1 | Sample + 10 μg l−1 cyanide | Sample + 50 μg l−1 cyanide |
|---|---|---|---|---|
| a Note N.D. = not detectable | ||||
| Natural water | ||||
| 1 | Chak Phra canal | N.D. | 10 | 50 |
| 2 | Saen Saep canal | N.D. | 10 | 50 |
| Commercial fruit juice | ||||
| 3 | 100% Apple | N.D. | 10 | 50 |
| 4 | Apricot | 50 | 50 | 100 |
| 5 | 100% Cherry berry | N.D. | 10 | 50 |
| 6 | Mulberry | N.D. | 10 | 50 |
| 7 | Peach | N.D. | 10 | 50 |
| 8 | Plum yellow | 50 | 50 | 100 |
| 9 | Prune brand A | 10 | 20 | 50 |
| 10 | Prune brand B | N.D. | 10 | 50 |
Validation of the developed cyanide test kit by comparison with the APHA/AWWA/WEF standard method 4500-CN−
To validate the cyanide test kit, the APHA/AWWA/WEF standard method 4500-CN− for the determination of water and wastewater was used.8 The results obtained from the developed cyanide field test kit were compared with those obtained from the APHA/AWWA/WEF standard method. The accuracy of the standard method was checked by performing cyanide distillation for standard solutions (5, 25, and 50 mg l−1) and the results are shown in Table 4 which indicate good recovery. A further two commercial fruit juices (apricot and plum yellow samples) were subjected to cyanide determination by the standard method. The concentrations of cyanide in commercial fruit juice samples were very low, and were not detectable (<10 μg l−1). Therefore, a standard cyanide of 25 mg l−1 was spiked into the samples. The distillates were then subjected to colorimetric detection of cyanide and the results are summarized in Table 4.| Sample | Concentration found/mg l−1 |
|---|---|
| 5 mg l−1 cyanide standard | 4.9 |
| 25 mg l−1 cyanide standard | 24.4 |
| 50 mg l−1 cyanide standard | 49.3 |
| Apricot sample | <2 |
| Apricot sample + 25 mg l−1 cyanide | 26.2 |
| Plum yellow sample | <2 |
| Plum yellow sample + 25 mg l−1 cyanide | 24.1 |
As can be seen from Table 4, good recoveries were obtained for the cyanide spiked apricot and plum yellow samples. To further confirm that the developed cyanide test kit is free from matrix interference, pure apricot and plum yellow juices were subjected to the cyanide distillation method and the distillates were analyzed for cyanide content using the developed cyanide field test kit. The concentrations of cyanide found in the distillates were in good agreement with those observed without using the distillation method, suggesting that with the developed cyanide field test kit, the distillation step to separate cyanide from complex matrices is not necessary. The developed cyanide field test kit provides good accuracy and can handle complex matrices without any prior pretreatment step.
Conclusions
The specially designed reaction bottle was found effective to facilitate a non instrumental low cost semi-quantitative method for the determination of cyanide in water and fruit juice samples. The developed cyanide field test kit can handle complex matrices and allows the detection of cyanide as low as 0.01 mg l−1 within 10 min. Although the method was purposely developed for field testing in Thailand with a normal temperature of approximately 30 °C, the method could also be applied for field testing in any other countries where the temperature may be higher or lower than this (please see ESI†). Nonetheless, the standard color scale must be constructed at the same temperature as for the field use. Owing to its rapidity, user-friendliness, and low-cost, the method is appropriate for field detection of cyanide. The method may be applied to wastewater in mining or industrial areas as a screening test for free cyanide content. Moreover, the specially designed reaction bottle may be applied to other analytes which could be converted into the vapor form, such as ammonia, arsenic, mercury, sulfide, etc.Acknowledgements
We are grateful for the research supports from the East Asia and Pacific Regional Office of the United Nations Children's Fund (UNICEF) and the Center for Innovation in Chemistry: Postgraduate Education and Research Program in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education. Thanks are also due to Miss Aujchara Pathomchaiamporn for checking the effect of temperature on the color formation in the developed cyanide field test kit.References
- Cyanide in Water and Soil: Chemistry, Risk, and Management. ed. D. A. Dzombak, R. S. Ghosh, and G. M. Wong-Chong. Taylor & Francis Group: Boca Raton, FL. 2006 Search PubMed.
- M. R. Haque and J. H. Bradbury, Food Chem., 2002, 77, 107–114 CrossRef.
- S. K. Dubey and D. S. Holmes, World J. Microbiol. Biotechnol., 1995, 11, 257–265 CrossRef CAS.
- C. K. Zacharis, P. D. Tzanavaras, A. N. Voulgaropoulos and B. Karlberg, Talanta, 2009, 77, 1620–1626 CrossRef CAS.
- Ministry of natural resources and environment; Pollution control department, Thailand, http://www.wepa-db.net/policies/law/thailand/std_drinking.htm.
- G. G. Guilbault and D. N. Kramer, Anal. Chem., 1966, 38, 834–836 CrossRef CAS.
- J. Shiowatana, S. Lanwongsa, O. Kitjabuncha, and S. Suwanchot, Patent No.19341 Jan 2549, Thailand.
- APHA/AWWA/WEF Method 4500-CN: Cyanide in standard methods for the examination of water and wastewater. 20th ed. L. S. Clesceri, A. E. Greenberg and A. D. Eaton, Washington D C: American Public Health Association American Water Works Association and Water Environment Federation, 1998 Search PubMed.
- A. Favero, J. Dibbern and M. Tubino, Anal. Sci., 2003, 19, 1139–1143 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Effect of temperature on the color scale and the RGB values obtained for cyanide detection. See DOI: 10.1039/c0ay00458h |
| This journal is © The Royal Society of Chemistry 2010 |