A new high-performance chelation ion chromatographic system for the direct determination of trace transition metals in fuel ethanol
Jailson C.
Dias
a,
Lauro T.
Kubota
a,
Pavel N.
Nesterenko
*b,
Greg W.
Dicinoski
b and
Paul R.
Haddad
b
aDepartment of Analytical Chemistry, Institute of Chemistry, University of Campinas – UNICAMP, 13083-970, Campinas, SP, Brazil
bAustralian Centre for Research on Separation Science (ACROSS), School of Chemistry, University of Tasmania, Private Bag 75, Hobart, TAS 7001, Australia. E-mail: pavel.nesterenko@utas.edu.au; Fax: +61 3 6226 2858; Tel: +61 3 6226 2165
First published on 27th August 2010
Abstract
A new chelation ion chromatographic system for the determination of transition metals was developed. The isocratic separation of Fe2+, Fe3+ and Cu2+ simultaneously with Mn2+, Pb2+, Cd2+, Co2+, Zn2+ and Ni2+ was obtained on an iminodiacetic acid (IDA) functionalized silica column 150 × 4.0 mm i.d. with an optimized eluent composed of 2.5 mmol L−1 dipicolinic acid, 10 mmol L−1 HCl and 60% v/v of methanol at flow rate of 0.5 mL min−1. Post-column reaction with 4-(2-pyridylazo)resorcinol (PAR) was used for sensitive spectrophotometric detection at 510 nm of the separated metals at low ppb level. The applicability of the developed method to the analysis of fuel ethanol was demonstrated.
1 Introduction
After the world oil crisis in 1975, the Brazilian government launched the Ethanol Programme which pursued the intensive use of ethanol as a biofuel. Ethanol can be used either directly in the hydrated form or in an anhydrous form as a 20–25% additive to pure gasoline.1 At present, the production of ethanol in Brazil by fractional distillation of fermented sugarcane extracts reached 18.5 billion litres per year by using approximately half of the national sugarcane harvest. The substantial increases in the crude oil price in the decade before the financial crisis of 2008–2009 caused additional investments in the area of production of ethanol. It should be also noted that ethanol can be also used as a raw material for the synthesis of ethylene - an important precursor in industrial organic synthesis, so there are plans to increase the annual production of fuel ethanol up to 35 billions litres.The quality of fuel ethanol is carefully regulated by the National Agency of Petroleum, Natural Gas and Biofuels (ANP), the Brazilian Association for Technical Standards (ABNT) and the National Institute of Metrology using various physico-chemical parameters (density, acidity, conductivity, pH, and others) and presence of different organic and inorganic admixtures. Metal ions are considered as an important group of inorganic contaminants in fuel ethanol1,2 and their content in fuel ethanol is strictly controlled in accordance with ANP Resolution No. 36 dated 06/12/2005. Currently flame atomic absorption spectrometry (FAAS) is used for the determination of copper and iron (ABNT NBR 11331).3 According to ANP regulations the content of copper in anhydrous ethanol should be less than 0.07 mg kg−1 and iron less than 5 mg kg−1 in hydrated fuel ethanol (HFE). The contamination of HFE with iron can take place during production, transportation and storage and induce the formation of deposits during the explosion stage of fuel combustion, thus decreasing engine performance and ultimately resulting in damage to the engine.2,4,5 Traces of copper in ethanol can act as a catalyst for the oxidation of the gasoline, when it is mixed with alcohol.4,6,7 Therefore, the quality control of fuels is a matter of economic and environmental importance, since fuels outside of the quality specifications can bring direct prejudice to the consumer through the malfunction of the vehicle engine, increase in fuel consumption and maintenance costs.8,9
The analytical methods described in the literature for the determination of metal contaminants in fuel ethanol were developed mainly by Brazilian research groups and are generally based on using spectroscopic or electrochemical techniques.8,10–12 The use of ion chromatography for the separation of metal ions and their species has not been reported for the multielement analysis of fuel ethanol.
The majority of ion chromatographic systems developed for the separation of transition metal ions use strong acid cation-exchangers with sulfonic acid functional groups. The optimum selectivity is achieved by manipulation with effective charges of separated metal ions by using complexing eluents. On the other hand, the direct analysis/injection of organic solvents is often a challenge for ion chromatographic analysis, because of limited compatibility of polymer based cation-exchanger with organic solvents. The presence of organic solvent causes swelling of the polymer matrix, and possible loss of the layer of agglomerated particles resulting in significant changes in the properties of the ion exchanger. The other limitation is due to increased viscosity of alcohol-water mixtures and, therefore significant increases in the backpressure of the system. In view of this, only a few papers on application of ion chromatography (IC) for the separation/determination of cations in samples with high alcohol content have appeared in the open literature.13–15 Obrezkov et al.15 developed an IC method for the determination of several transition metals, including lead, in vodka (40% v/v of ethanol) and liqueur products. However, injections of aqueous solutions obtained after evaporation and dissolution of the residue were used. Other publications describing IC determination of alkali metal ions, magnesium and calcium in vodka employed either silica-based ion-exchangers with bonded sulfonic acid groups14 or a poly(butadiene-maleic acid) (PBDMA) coated silica Cation 1–2 column (Metrohm, Switzerland).16 The only separation of metals with direct injection of ethanol employs a PBDMA functionalized column Metrosep C2 (Metrohm, Switzerland) and has been reported for the determination of magnesium and calcium,17 but not for transition metals.
The use of silica-based columns and chelation as the key retention mechanism for the metal ions are less sensitive to injections of samples prepared in alcohol, so high-performance chelation ion chromatography (HPCIC18–22) represents a good alternative to IC for the simultaneous determination of metals in fuel ethanol. Moreover, HPCIC has also been used for the separation of Fe2+ and Fe3+ at low concentrations.19
Only the Brazilian standard specifications set a limit for the concentration of iron in fuel ethanol considering that deposits of this metal can be formed during the fuel explosion stage. Although it is only necessary to determine the total content of iron, it can be present in different species, such as Fe2+ and Fe3+, depending on the pH, the degree of corrosion of the distillation equipment and of the fuel reservoir tanks, the time of storage, etc. Therefore, the iron speciation may be useful tool to evaluate these factors.
In this work, a HPCIC system comprising a silica column functionalized with iminodiacetic acid (IDA) functional groups, an eluent containing dipicolinic acid (DPA) and photometric detection after post-column reaction with 4-(2-pyridylazo)resorcinol (PAR) was applied to the direct analysis of fuel ethanol samples. This system resulted in the simultaneous determination of up to nine metal ions (Fe2+, Fe3+ and Cu2+ simultaneously with Mn2+, Pb2+, Cd2+, Co2+, Zn2+ and Ni2+), including iron speciation, which is reported for the first time for this type of sample.
2 Experimental
2.1 Instrumentation
The determination of the metal ions was performed using Metrohm model 844 compact IC system (Metrohm GmbH, Switzerland) equipped with a post-column reactor and spectrophotometric detector. Chromatograms were recorded using the IC Net 2.3 software.IDA-Kromasil column 150 × 4.0 mm i.d., 5 μm particles (dp = 6 nm; 340 m2/g) column was obtained from JPP Chromatography Ltd, UK. The backpressure was monitored during all experiments to preserve the column and the chromatographic system.
All separations are obtained under isocratic conditions with 100 μL injected sample volumes. Photometric detection was carried out at 510 nm after PCR with 0.15 mmol L−1 PAR solution in 1 mol L−1 of ammonia delivered at flow rate of 0.36 mL min−1 (PVC peristaltic pump tube, 0.51 mm i.d.) if otherwise not stated. The volume of 2.5 m × 0.5 mm ID PCR reaction coil was 490 μL.
2.2 Reagents
High-purity reagents and deionized water from Milli-Q water purification system (Millipore, Bedford, MA, USA) were used for all preparations. The standard solutions of metal nitrates (1000 mg L−1 in diluted nitric acid) were purchased either from BDH (Poole, UK) (Cd2+, Co2+, Fe3+and Ni2+) or from Sigma-Aldrich (St. Louis, MO, USA) (Mn2+, Pb2+ and Zn2+) and used as stock solutions. Cu2+ and Fe2+ stock solutions were prepared from the corresponding sulfate salts (UNIVAR, Ajax Chemicals, Australia). The working standard solutions were prepared by dilution with pure ethanol (HPLC grade, Sigma-Aldrich).Dipicolinic acid (pyridine-2,6-dicarboxylic acid, 99%, Sigma-Aldrich), hydrochloric acid (fuming 37%, Sigma-Aldrich) and methanol (MeOH, analytical grade, Merck, Darmstadt, Germany) were used to prepare the elution systems. 4-(2-pyridylazo)resorcinol (PAR, Eastman Kodak, Rochester, NY, USA) and ammonium hydroxide solution (28%, Fluka, Glossop, UK) were used to prepare the post-column reagent. All eluents were filtered before use through a 0.22 μm Nylon membrane filter.
3 Results and discussion
In this work, nine metal ions were selected for analysis in fuel ethanol samples without any pretreatment. Fe2+, Fe3+ and Cu2+ were the primary targets and other metal ions including Mn2+, Pb2+, Cd2+, Co2+, Zn2+ and Ni2+ were checked as possible contaminants of fuel ethanol.Various liquid chromatographic methods can be used for the determination of transition metals including cation-exchange chromatography, anion-exchange chromatography of negatively charged metal complexes and reversed phase HPLC of metal chelates. However, HPCIC was chosen in the present work because both retention and separation selectivity of metal ions are more resistant to the presence of organic solvent in the samples. Although there are several types of chelating groups that can be used on stationary phases, silica bound IDA has shown excellent efficiency and separation selectivity for transition metal ions.18,23–27 A number of successful separations of transition metal using IDA-silica were published, but no isocratic separation for above mentioned set of nine metals has been reported.
The number of metal ions able to be separated on an IDA-silica column depends on type of eluent employed. The commonly used nitric acid – potassium nitrate mobile phase provides isocratic separation of Mn2+, Co2+, Cd2+, Zn2+ and Pb2+, but Ni2+, Cu2+ and Fe3+ have significantly greater retention. The retention of Fe2+ is close to the retention of Mn2+ so additional attenuation of separation selectivity is required. Two options for this are possible including gradient elution and the addition of complexing reagents to the eluent to enable complete separation of the nine metal ions of interest. Because of significant changes in the baseline response of PCR photometric detection during acid gradient elution the second option is preferable.
The main criteria for selection of complexing reagents include three specific criteria as follows: i) to allow the elution of Ni2+, Cu2+ and Fe3+ from IDA-silica column; ii) to preserve the retention of other metal ions and iii) adjustment of the separation selectivity for pair Mn2+/Fe2+. Jones and Nesterenko21 published a detailed study regarding the influence of different complexing reagents in the mobile phase on the separation selectivity of various metal elements separated by HPCIC using stationary phases based on IDA-silica. In this study, the authors showed that the metal complexation equilibrium between the ligand group of the eluent and the chelating group bound to the surface of the stationary phase represents a powerful way to control the selectivity of the chromatographic separation of certain metals. In particular, the retention of Pb2+ and Cd2+ on IDA functionalized adsorbents can be selectively controlled by changing the concentration of the chloride ion,20,21 while the retention all of Cu2+ Ni2+, and Fe3+ can be effectively controlled by addition of oxalic19,28,29 or dipicolinic (DPA)18,26,30 acids to the eluent. The aqueous solutions of the latter acid are more stable, so DPA based eluents were used in following experiments. Clearly, the high formation constants for the complexes of these metal ions with DPA in combination with low pKa of carboxylic groups (Table 1) provides high values for the conditional formation constants and strong elution power to this reagent, even at the low pH values of the corresponding eluents.
| Metal ion | IDA (pKa1 = 1.77, pKa2 = 2.98) | DPA (pKa1 = 2.07, pKa2 = 4.66) | ||
|---|---|---|---|---|
| logβ1 | logβ2 | logβ1 | logβ2 | |
| Mn2+ | 4.72 | 7.82 | 5.01 | 8.49 |
| Cd2+ | 5.71 | 10.10 | 6.36 | 10.90 |
| Fe2+ | 5.80 | 10.10 | 5.71 | 10.36 |
| Co2+ | 6.97 | 12.22 | 6.65 | 12.70 |
| Zn2+ | 7.15 | 12.40 | 6.35 | 11.88 |
| Pb2+ | 7.36 | 9.30 | 8.70 | 11.60 |
| Ni2+ | 8.30 | 14.60 | 6.95 | 13.50 |
| Cu2+ | 10.56 | 16.30 | 9.10 | 16.40 |
| Fe3+ | 10.72 | 19.15 | 10.91 | 17.13 |
3.1 Optimization of the separation
The key parameters effecting retention of metal ions on IDA-silica include concentration and pH of the eluent, organic solvent content and column temperature. So, these parameters were optimized initially only for the target metal ions (Fe2+, Fe3+ and Cu2+) and posteriorly more six other elements (Mn2+, Pb2+, Cd2+, Co2+, Zn2+ and Ni2+) were examined as possible interferents.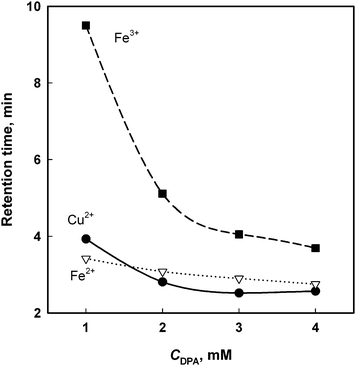 | ||
| Fig. 1 The effect of the concentration of DPA at constant pH 2.0 on the retention of Fe2+, Fe3+ and Cu2+. Eluent flow rate at 0.8 mL min−1. Room temperature (25 °C). | ||
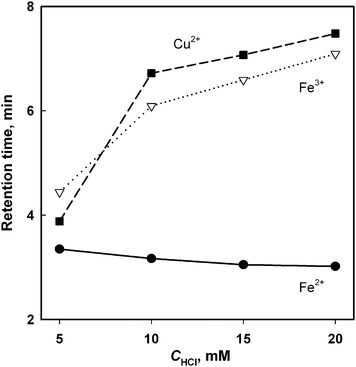
During the subsequent step of optimization of the medium acidity, the MeOH solvent was added to the eluent at 20% v/v in order to approximate the chromatographic conditions to alcoholic matrix of interest. Therefore, instead of adjusting the pH of the solution, the concentration of HCl was investigated thereafter.
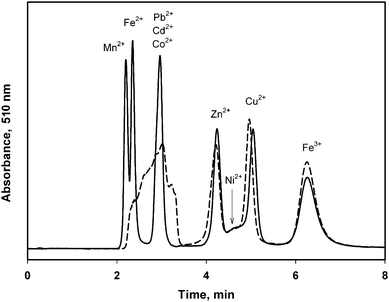 | ||
| Fig. 3 Separation of the mixture of nine metal cations injected as solution in the eluent (solid line) and in pure ethanol (dotted line). Eluent: 2.5 mmol L−1 DPA (pH 1.6, adjusted with HCl) and 5% vol. MeOH. Column temperature 25 °C. Sample volume 20 μL of solutions containing 6.7 μg mL−1 of each metal. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal-IDA complexes increase with the methanol solution content. As expected, the retention of Fe2+, Fe3+ and Cu2+ on IDA-silica increases with the addition of methanol to the eluent. The highest number of theoretical plates was obtained with an eluent containing 60% methanol.
1 metal-IDA complexes increase with the methanol solution content. As expected, the retention of Fe2+, Fe3+ and Cu2+ on IDA-silica increases with the addition of methanol to the eluent. The highest number of theoretical plates was obtained with an eluent containing 60% methanol.
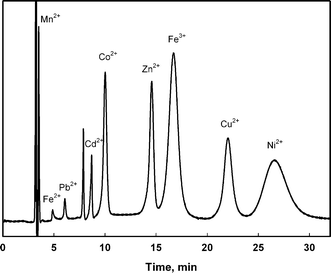 | ||
| Fig. 5 Chromatogram of nine metals under optimized conditions. Eluent: 2.5 mmol L−1 DPA, 10 mmol L−1 HCl and 60% MeOH, flow rate at 0.5 mL min−1. Column temperature 45 °C. PCR reagent flow rate at 0.82 mL min−1. Multielement standard solution with the following concentrations of each metal: 0.25 mg Mn2+ L−1, 3 mg Fe2+ L−1, 2 mg Pb2+ L−1, 1 mg Cd2+ L−1, 1 mg Co2+ L−1, 1 mg Zn2+ L−1, 1 mg Fe3+ L−1, 2 mg Cu2+ L−1, 5 mg Ni2+ L−1. | ||
3.2 Optimization of the detection
3.3 Analysis of fuel ethanol samples
After a univariate optimization process was complete, several experiments were carried out to establish the main analytical parameters for the validation of the developed methodology. Additionally, Zn2+ was included at this stage to evaluate its analytical parameters, since this metal has been observed at trace levels in some samples. Among the iron species, only Fe3+ was considered due to the continuous oxidation of Fe2+ in the sample.The limits of detection (LOD) and quantification (LOQ) were obtained for Zn2+, Fe3+ and Cu2+ ions using a signal-to-noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. These data are shown in Table 3, and were obtained via direct injection of fuel ethanol (without preconcentration) under the optimized chromatographic conditions. The LOD's were similar or lower than those observed by other authors.2,9,11,35,36Table 3 also details the optimum linear response range for each metal ion, with each analyte showing good linearity.
1, respectively. These data are shown in Table 3, and were obtained via direct injection of fuel ethanol (without preconcentration) under the optimized chromatographic conditions. The LOD's were similar or lower than those observed by other authors.2,9,11,35,36Table 3 also details the optimum linear response range for each metal ion, with each analyte showing good linearity.
| Parameter | Metal ion | ||
|---|---|---|---|
| Zn2+ | Fe3+ | Cu2+ | |
| Sensitivity (mAU sec L μmol−1) | 341 | 437 | 290 |
| Linear range (mg L−1) | 0.01–10 | 0.03–10 | 0.03–10 |
| Linearity (R2) | 0.9957 | 0.9996 | 0.9965 |
| LOD (μg L−1) | 2.0 | 8.9 | 7.4 |
| LOQ (μg L−1) | 6.8 | 29.6 | 24.8 |
| Precision (%RSD) | 3.6 | 3.0 | 3.1 |
| Recovery range (%) | 95–105 | 95–103 | 96–108 |
In order to determine the precision of the method, four replicate analysis of an ethanolic standard solution were performed. The percent relative standard deviations (%RSD) for all metals were within the range 3 to 3.6% (Table 3). The accuracy of the method was evaluated from recovery analysis through the preparation of spiked samples at three levels of concentration higher than the LOQ. The obtained values in all cases, shown in Table 3, were satisfactory from an analytical point of view, although some losses occurred during the experiments yielding results just below 100% for the lower concentrations. Finally, a sample of Brazilian fuel ethanol was analyzed to insure the applicability of the developed method. The obtained chromatogram is shown in Fig. 6.
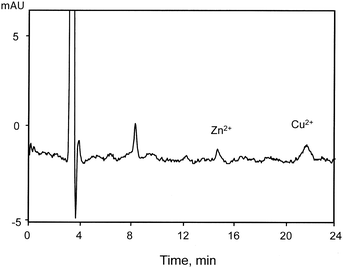 | ||
| Fig. 6 Chromatogram showing the separation of zinc and copper present in a Brazilian fuel ethanol sample in the chromatographic conditions previously optimized, similar to those described in Fig. 5. The estimated concentrations for each metal, in μg L−1, were: 3 for Zn2+ and 14 for Cu2+. | ||
Given the above, the DPA elution system, developed for the chromatographic separation of Fe2+, Fe3+ and Cu2+ in fuel ethanol, can be satisfactorily applied to identify and quantify these elements, although the iron speciation has been shown to be impracticable from an analytical point of view.
4 Conclusions
The described chromatographic conditions based on high-performance chelation ion chromatography with post-column reaction and photometric detection were well suited to the fast and accurate determination of trace metals in fuel ethanol via direct injection of the sample. Moreover, these chromatographic conditions can be easily applied for routine analysis in scientific laboratories or industries that have interest in analyzing these analytes in various alcohol-based samples, including alcoholic beverages.Acknowledgements
This work was supported by the IRGS grant of University of Tasmania, FAPESP (Process Number 2006/03960-1) and CNPq (Project Number 573672/2008-3).References
-
F. Rosillo-Calle, S. V. Bajay and H. Rothman, in Uso da biomassa para produção de energia na indústria brasileira, Editora da Unicamp, Campinas, 2005 Search PubMed
.
- M. F. de Oliveira, A. A. Saczk, L. L. Okumura, A. P. Fernandes, M. Moraes and N. R. Stradiotto, Anal. Bioanal. Chem., 2004, 380, 135–140
.
- Abnt Nbr 11331-Ethanol - Determination of iron and copper content - Flame atomic absorption spectrometric method. 2007.
- R. A. A. Munoz and L. Angnes, Microchem. J., 2004, 77, 157–162 CrossRef CAS
.
- P. M. Padilha, C. C. F. Padilha and J. C. Rocha, Quimica Analitica, 1999, 18, 299–303 Search PubMed
.
- K. J. B. Abd Karim, J. Y. Jin and T. Takeuchi, J. Chromatogr., A, 2003, 995, 153–160 CrossRef CAS
.
- D. B. Taylor and R. E. Synovec, Talanta, 1993, 40, 495–501 CrossRef CAS
.
- M. F. de Oliveira, A. A. Saczk, L. L. Okumura and N. R. Stradiotto, Energy Fuels, 2009, 23, 4852–4859 CrossRef
.
- L. S. G. Teixeira, J. F. Brasileiro, M. M. Borges, P. W. L. Cordeiro, S. A. N. Rocha and A. C. S. Costa, Quim. Nova, 2006, 29, 741–745 CAS
.
- R. M. Takeuchi, A. L. Santos, M. J. Medeiros and N. R. Stradiotto, Microchim. Acta, 2009, 164, 101–106 CrossRef CAS
.
- M. F. de Oliveira, V. R. Balbo, J. F. de Andrade, A. A. Saczk, L. L. Okumura and N. R. Stradiotto, Chem. Technol. Fuels Oils, 2008, 44, 430–434 Search PubMed
.
- C. D. Mattos, D. R. do Carmo, M. F. de Oliveira and N. R. Stradiotto, Int. J. Electrochem. Sci., 2008, 3, 338–345 Search PubMed
.
- J. G. Ibanez, A. Carreon-Alvarez, M. Barcena-Soto and N. Casillas, J. Food Compos. Anal., 2008, 21, 672–683 CrossRef CAS
.
- V. N. Arbuzov and S. A. Savchuk, J. Anal. Chem., 2002, 57, 428–433 CrossRef CAS
.
- O. N. Obrezkov, V. A. Tolkacheva, G. I. Zaikanova, V. A. Yamnikov, O. V. Krokhin, S. P. Zhukov and O. A. Shpigun, Industrial Laboratory, 2000, 66, 18–21 CAS
.
- J. Bruce, Analysis of alkali metal and alkaline earth metals in vodka using ion chromatography, International Laboratory News, 2002 Search PubMed
.
- I. C. Metrohm, Application Note C-97, Cations in ethanol used as biofuel, 2007 Search PubMed
.
- P. N. Nesterenko and O. A. Shpigun, Russ. J. Coord. Chem., 2002, 28, 726–735 CrossRef CAS
.
- P. N. Nesterenko and P. Jones, J. Sep. Sci., 2007, 30, 1773–1793 CrossRef CAS
.
- P. Jones and P. N. Nesterenko, J. Chromatogr., A, 1997, 789, 413–435 CrossRef CAS
.
- P. Jones and P. N. Nesterenko, J. Chromatogr., A, 2008, 1213, 45–49 CrossRef CAS
.
- P. R. Haddad, P. N. Nesterenko and W. Buchberger, J. Chromatogr., A, 2008, 1184, 456–473 CrossRef CAS
.
- P. N. Nesterenko and P. Jones, J. Chromatogr., A, 1997, 770, 129–135 CrossRef CAS
.
- W. Bashir and B. Paull, J. Chromatogr., A, 2001, 907, 191–200 CrossRef CAS
.
- W. Bashir and B. Paull, J. Chromatogr., A, 2002, 942, 73–82 CrossRef CAS
.
- G. Bonn, S. Reiffenstuhl and P. Jandik, J. Chromatogr., A, 1990, 499, 669–676 CrossRef CAS
.
- L. Barron, M. O'Toole, D. Diamond, P. N. Nesterenko and B. Paull, J. Chromatogr., A, 2008, 1213, 31–36 CrossRef CAS
.
- P. Nesterenko and P. Jones, J. Liq. Chromatogr. Relat. Technol., 1996, 19, 1033–1045 CrossRef CAS
.
- I. N. Voloschik, M. L. Litvina and B. A. Rudenko, J. Chromatogr., A, 1994, 671, 205–209 CrossRef CAS
.
- A. I. Elefterov, S. N. Nosal, P. N. Nesterenko and O. A. Shpigun, Analyst, 1994, 119, 1329–1332 RSC
.
- NIST Standard Reference Database 46-Version 8.0 for Windows, NIST Critically selected stability constants of metal complexes, 2004 Search PubMed.
- P. N. Nesterenko, M. A. Rybalko and B. Paull, Analyst, 2005, 130, 828–830 RSC
.
- R. A. Shalliker, H. J. Catchpoole, G. R. Dennis and G. Guiochon, J. Chromatogr., A, 2007, 1142, 48–55 CrossRef CAS
.
- T. B. Field and W. A. E. McBryde, Can. J. Chem., 1981, 59, 555–558 CrossRef CAS
.
- L. S. G. Teixeira, M. D. Bezerra, V. A. Lemos, H. C. dos Santos, D. S. de Jesus and A. C. S. Costa, Sep. Sci. Technol., 2005, 40, 2555–2565 CrossRef CAS
.
- J. E. da Silva, F. A. da Silva, M. F. Pimentel, R. S. Honorato, V. L. da Silva, B. S. M. Montenegro and A. N. Araujo, Talanta, 2006, 70, 522–526 CrossRef
.
| This journal is © The Royal Society of Chemistry 2010 |