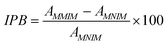High selectivity chemiluminescence sensor for determination of puerarin in diet foods/weight loss promoters based on novel rhodanine and monodisperse molecularly imprinted microspheres
Lei
Ge
a,
Ping
Dai
b,
Shenguang
Ge
a and
Jinghua
Yu
*a
aSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan, 250022, China. E-mail: jndxgelei@gmail.com; Tel: +86-531-82767161
bChina Building Materials Academy, China Building Material Test and Certification Center, Beijing, 100024, China
First published on 10th September 2010
Abstract
Puerarin-monodisperse molecularly imprinted microspheres were prepared by suspension polymerization. The binding property was investigated by calculating imprinting-induced binding promotion. Using monodisperse microspheres as the recognition material, rhodanine as the chemiluminescence probe, a novel chemiluminescence sensor was developed for the determination of puerarin in diet foods/weight loss promoters. The flow path was designed to obtain a continuous chemiluminescence signal. Due to the combination of special morphology of the monodisperse microspheres and excellent properties of the novel chemiluminescence system, this sensor displayed good selectivity and sensitivity. Under optimum conditions, the CL intensity has a linear relationship versus the concentration of puerarin, the linear range is 1 × 10−7 – 3 × 10−5 g mL−1 with a low detection limit of 3.4 × 10−8 g mL−1. The proposed method showed satisfactory application in the determination of puerarin in diet foods/weight loss promoters.
1. Introduction
Diet foods/weight loss promotion is a very popular phenomenon all over the world because it enables fitness without undergoing treatment with appetite controlling drugs. It is prescribed that no functional drug can be added in diet foods/weight loss promoters. However, in some regions, drugs that can make people lose weight are added to diet foods/weight loss promoters by manufacturers to gain profits, which is illegal and dangerous to health. For example take puerarin, an isoflavones compound, which is the effective component of kudzu and has been applied in the treatment of heart attack.1 Recent reports have shown that puerarin can be fatal by inducing erythrolysis with misuse.2 Therefore long term use of puerarin may seriously harm people's health, however such drugs can be added to diet foods/weight loss promoters because they help achieve weight loss. Hence, it is of great importance to monitor the content of puerarin in the diet foods/weight loss promoters on the market. High performance liquid chromatography (HPLC) has been reported for the detection of puerarin.3–5 HPLC can provide good sensitivity and accuracy, but it requires complicated pretreatment and expensive instruments.Chemiluminescence has been widely used for the detection of pharmaceuticals because of its simple operation and sensitivity.6–8 Reports of the determination of puerarin by chemiluminescence have been published.9–13 However, chemiluminescence suffers from poor selectivity and in some circumstances a screening agent has to be used to erase interferences from coexisting substances.14 In this paper, for the first time, molecularly imprinted microspheres have been introduced to improve the selectivity of chemiluminescence in detecting puerarin. Molecularly imprinted polymers (MIPs) are increasingly recognised as highly target-specific polymeric molecular recognition materials with a broad range of potential applications in separation sciences, catalysis, molecular sensing and drug delivery.15 Traditionally MIPs are created by imprinting template molecules in a polymer matrix, but this method suffered from many disadvantages such as small binding capacity, incomplete template removal, slow mass transfer, and irregular materials shape. Due to the high cross-linking nature of molecularly imprinted polymers, templates located in the interior area of a bulk matrix are quite difficult to extract.16–18 Moreover, in generated recognition sites in the interior of a matrix surface, the target species cannot access the empty recognition sites encased within the rigid matrix.19,20 In contrast, monodisperse molecularly imprinted microspheres (MMIMs), nano-imprinted materials, have a small dimension with extremely high surface to volume ratio, so that in this case most of the recognition sites are situated at the surface and in proximity to the microsphere's surface. Therefore, nano-imprinted MMIMs are expected to show excellent improvement to binding efficiency.
Rhodanine is an established chromogenic reagent which has been used to determine novel metals in spectrophotometry.21 Recent research has found that novel rhodanine derivations could be synthesized by substituting different groups to the rhodanine matrix,22 and they were investigated to show excellent fluorescent performance in determination of metal ions by spectrofluorimetry.23,24 In the present work, –SO3H was substituted to the rhodanine matrix, and for the first time 3-o-nitrylphenyl-5-(4′-methyl-2′-sulfono phenylazo) rhodanine (M2NRASP) was synthesized, its chemiluminescent properties were found to be excellent. We found this derivative CL system had a highly sensitive CL response for the determination of puerarin in diet foods/weight loss promoters.
In this work, the puerarin-MMIMs were prepared with puerarin as template, methacrylic acid (MAA) as functional monomer and ethylene glycol dimethacrylate (EGDMA) as cross-linker. Scanning electronic microscopy was carried out to describe the morphology, and the binding characteristic of the imprinted polymer to puerarin was evaluated by imprinting-induced promotion of binding (IPB).25 The recognition part of this sensor is the MMIMs column. A novel rapid and simple MMIMs-CL sensor is established for detection of puerarin in diet foods/weight loss promoters. The CL sensor displayed high selectivity and high sensitivity to puerarin. The proposed method was applied in the determination of puerarin in diet foods/weight loss promoters with satisfactory results.
2. Material and methods
2.1 Apparatus and manifold
The IFFM-D CL analyzer (Xi'an Remex Electronic Instrument High-Tech Ltd., China) was equipped with a novel flow path designed in our lab and a detection system. Peristaltic pumps were used to deliver all solutions and PTFE tubing (0.8 mm i.d.) was used to connect the flow system. The flow cell was a coil of glass tube that was positioned in front of the detection window of the photomultiplier tube (PMT). The CL signal was treated with a personal computer. Elemental analysis was carried out with a Perkin-Elmer (America) Model 2400II CHNS/O element analysis meter. Nicolet 380 infrared spectrum meter (Thermo Electron Corporation, America) was used to attribute chemicals. The SEM images were taken by scanning electronic microscope (Hitachi, Japan). UV absorption spectras were measured on a UV-3101 spectrophotometer (Shimadzu, Japan).All chemicals were of analytical reagent grade or above. The doubly distilled-deionized water used throughout was obtained by SYZ-550 quartz sub-boil high-purified water distiller (Jiang Su Jin Tan, Jiang Su, China).
The 3-o-nitrylphenyl-5-(4′-methyl-2′-sulfonylphenylazo) rhodanine (M2NRASP) was synthesized as per the method described below. A standard solution of puerarin (1.0 × 10−4 g mL−1) was prepared by dissolving 0.0100 g puerarin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) with water and diluting to 100 mL with water. A 1.0 × 10−6 g mL−1 standard solution of puerarin was prepared by diluting this stock solution with water. A stock solution containing 2.0 × 10−4 mol L−1 3-o-nitrylphenyl-5-(4′-methyl-2′-sulfonylphenylazo) rhodanine (M2NRASP) was prepared by dissolving 0.0090 g of M2NRASP with 100 mL ethanol. A stock solution containing 5.0 × 10−3 mol L−1 potassium permanganate was prepared by dissolving 0.0790 g potassium permanganate (Shanghai Chemical Reagent Company, Shanghai, China) with water and diluting to 100 mL with water. A standard solution of potassium permanganate was obtained by dilution of the stock solution with water. A 5.0 mol L−1 stock solution of hydrochloric acid was prepared by diluting 62.5 mL concentrated hydrochloric acid (Shanghai Chemical Reagent Company, Shanghai, China) with 437.5 mL water to give a final total volume of 500 mL.
Methacrylic acid (MAA), 2, 2′-azobis-(2-methylpropinitrile) (AIBN) and polyvinyl alcohol were obtained from Shanghai Chemical Reagent Company, Shanghai, China. EGDMA was obtained from Alfa Aaser. MAA, EGDMA and AIBN were recrystallized before use to remove polymerization inhibitor.
2.2 Synthesis of new type rhodanine
Novel 3-o-nitrylphenyl-5-(4′-methyl-2′-sulfonylphenylazo) rhodanine (M2NRASP) has been synthesized for the first time by the authors.In a 250 mL conical flask 35 mL ammonia (1.0 mol) and 15 mL carbon disulfide (0.2 mol) were added, and 17.0 g o-nitroaniline (0.2 mol) was added in later while stirring. The mixture was stirred for 10 h and then pump filtrated, dithio-p-nitrobenzene amine formate ammonium was obtained as (a) 18.8 g chloroactic acid which was neutralized to pH 7.0 with 8.0 g sodium hydroxide in 20 mL water. Afterwards (a) was put in the neutralized solution, and 60 mL concentrated hydrochloric acid was added, heated to boiling and at once filtrated, a straw yellow solid 3-o-nitrophenyl rhodanine (b) was obtained.
The diazonium salt was obtained by mixing 0.01 mol o-aminobenzenesulfonic acid with 10.0 mL sodium (VNaOH : VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and adding 0.01 mol sodium nitrite to the mixture in an ice bath.
10), and adding 0.01 mol sodium nitrite to the mixture in an ice bath.
In the ice bath, 0.01 mol of (b) was added into 10 mL ammonia, followed by injection of the diazonium salt, to make sure that the solution had pH 7–9. After one hour of stirring, the mixture was filtrated, and the filtrate was acidified with concentrated hydrochloric acid (at room temperature), the precipitate was collected. The 3-o-nitrylphenyl-5-(4′-methyl-2′-sulfonylphenylazo) rhodanine was obtained by filtration and drying.
Data from the infrared spectrogram (KBr discs, cm−1) is shown as follows:
υ
OH = 3446; υN = N = 1456; υC = S = 1265; υAr–C–N = 1265; υo, p, p-substituted = 826; υS = O = 1492; υbenzene ring(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) = 1549, 1629, 1498; υC–N = 1022; υNO2 = 1386.
C) = 1549, 1629, 1498; υC–N = 1022; υNO2 = 1386.
Elemental analysis of M2NRASP gave a composition (%) of: C (38.02%) H (3.51%) N (8.54%) S (13.58%), which is in good agreement with the theoretical composition of M2NRASP: C (48.33%) H (3.46%) N (8.97%) S (13.40%).
2.3 Preparation of puerarin-MMIMs
Typically, 0.2000 g polyvinyl alcohol (PVA) and 40 mL water were added in a round bottom flask and the mixture was heated to 90 °C to dissolve the PVA, then cooled to room temperature. 1 mmol of puerarin was dissolved in 10 mL methylbenzene. Methacrylic acid (4 mmol) was added to the solution, which was poured into the flask with PVA. Then 20 mmol EGDMA and 30 mg AIBN were added and the whole mixture was poured into a 50 mL flask. After purging with nitrogen for 15 min the mixture was sealed under vacuum. The polymerization reaction was performed at 65 °C for 24 h under stirring. The mixture was centrifuged to obtain puerarin-MMIMs. The monodisperse non-molecularly imprinted microspheres (MNIMs) were prepared in the same way without the addition of puerarin. The schematic representation of MMIMs synthesis is shown in Fig. 1.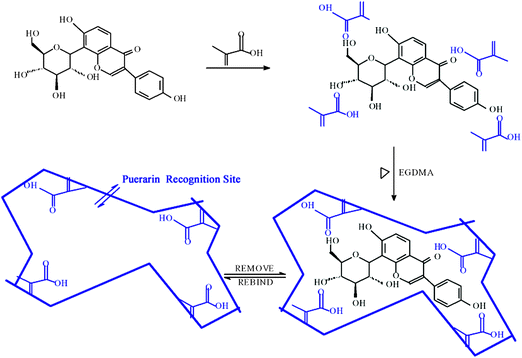 | ||
| Fig. 1 Schematic representation of MMIMs synthesis. | ||
2.4 Recognition experiments (imprinting-induced promotion of binding)
IPB was introduced in our experiments to assess the specific recognition ability of the MMIMs to puerarin. Puerarin was removed from MMIMs by washing with 50 mL of acetic acid/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) with SoxHlet extraction machine for 24 h before recognition experiments. The microspheres were dried to a constant weight at 60 °C under vacuum. Then 10.0 mg of MMIMs was respectively mixed with 5.0 mL puerarin, calycosin and genistin standard solution with the same concentration of 1.0 × 10−4 g mL−1 in a 10 mL conical flask and oscillated for 12 h at room temperature. After centrifuging at 3000 rpm for 5 min and filtration, the concentration of free molecules in the supernatant was detected by UV spectrophotometry at 249 nm, 208 nm and 242 nm. The amount of molecules bound to the microspheres was calculated by subtracting the concentration of free molecules from their initial concentration. The data obtained was used for the IPB calculation.
9, v/v) with SoxHlet extraction machine for 24 h before recognition experiments. The microspheres were dried to a constant weight at 60 °C under vacuum. Then 10.0 mg of MMIMs was respectively mixed with 5.0 mL puerarin, calycosin and genistin standard solution with the same concentration of 1.0 × 10−4 g mL−1 in a 10 mL conical flask and oscillated for 12 h at room temperature. After centrifuging at 3000 rpm for 5 min and filtration, the concentration of free molecules in the supernatant was detected by UV spectrophotometry at 249 nm, 208 nm and 242 nm. The amount of molecules bound to the microspheres was calculated by subtracting the concentration of free molecules from their initial concentration. The data obtained was used for the IPB calculation.Where AMMIM is the adsorption of the target molecule by the molecularly imprinted polymer, and AMNIM is the adsorption of the target molecule by the corresponding non-molecularly imprinted polymer. For the molecularly imprinted polymer to its target molecule, the greater the IPB, the better the specific recognition ability is; while for the molecularly imprinted polymer to the non-target molecules with similar structure, the larger the IPB, the worse the specific recognition ability is. That is to say, for one kind of molecularly imprinted polymer, the bigger the difference of IPB among the different molecules, the better is the selectivity of the polymer to its target molecule.
2.5 Preparation of MMIMs column
A polymethyl methacrylate (PMMA) module was drilled through to obtain a “Y” shape flow path (Fig. 2), through which three reactants were injected simultaneously. As shown in Fig. 2, one of its branches, named Branch-C, was filled with 10.0 mg of puerarin-MMIMs and plugged with glass wool at both ends. Branch-C was connected to the flow cell in front of the detection window of the photomultiplier tube (PMT), from which the CL signal was detected directly. It was used as the recognition part of the CL-sensor. The merger stream of potassium permanganate, HCl and M2NRASP passed through Branch-A of MMIMs column and reacted with the adsorbed puerarin to inhibit CL. During this procedure the affinity binding between MMIMs and puerarin was destroyed and the cavities of binding sites were left on the MMIMs column. When the CL blank signal was stable, water carrier was pumped through Branch-B of the column to clean the MMIMs, preparing for the next concentration determination. The concentration of puerarin was quantified via decrease of CL intensity, which was obtained by subtracting the blank CL intensity from that of the puerarin standard solution or sample. | ||
| Fig. 2 Schematic diagram of the experiment pathway (a) M2NRASP; (b) potassium permanganate solution; (c) hydrochloric acid; (d) water; (e) sample solution; P1, P2-peristaltic pump; S-switch valve; (A) Branch-A; (B) Branch-B; (C) Branch-C; (D) detector. | ||
2.6 Principle of FI-MMIMs-CL sensor for determination of puerarin
A schematic diagram for the FI-MMIMs-CL sensor is shown in Fig. 2. The determination could be carried out in 4 steps:Step 1 Adsorption of puerarin. Pump 1 was stopped and switch value was connected to (e). Pump 2 delivered puerarin solution to flow through the MMIMs column and puerarin in the sample solution was selectively adsorbed on the microspheres by specific recognition. The optimum flow rate of pump 2 was 1.5 mL min−1.
Step 2 Removal of other substances except puerarin. In this step pump 1 was stopped and the switch value connected to (d). Pump 2 delivered water through the MMIMs column. The other substances in solution adsorbed by non-specific interaction were washed off the microspheres and only puerarin was left in the recognition cavities.
Step 3 Chemiluminescence responses. Pump 2 was stopped. The merged stream of potassium permanganate, M2NRASP and HCl was delivered through the MMIMs column by pump 1. The optimum flow rate of pump 1 was 1.5 mL min−1. Puerarin adsorbed on the microspheres reacted with the three above to inhibit CL.
Step 4 Cleaning the MMIMs column. In this step pump 1 was stopped and the switch value was connected to (d), water was pumped through the MMIMs column by pump 2 to clean the residue from CL reaction in step 3 on the microspheres for further determination.
3. Results and discussion
3.1 Binding characteristics of MMIMs
Morphology of puerarin-MMIMs was obtained by scanning electronic microscope. Fig. 3 indicated that the MMIMs had a good monodispersity with size less than 1 μm, so that they possessed extremely high surface to volume ratio, and most of the effective recognition sites were at the surface and in the proximity of the microspheres' surface. This can increase the number of effective recognition sites remarkably. Therefore, MMIMs are expected to improve the recognition capacity, binding efficiency, and sites accessibility of imprinted materials. | ||
| Fig. 3 Scanning electron micrograph for puerarin-MMIMs. | ||
The specific recognition characteristics of MMIMs were investigated by recognition experiments. In the presence of 10.0 mg MMIMs, the recognition experiments were carried out in puerarin, calycosin and genistin standard solutions with the same concentration of 1.0 × 10−4 μg mL−1. The IPB of puerarin, calycosin and genistin was 12, 1.6 and 0.58, respectively. The IPB of puerarin was much more than that of the structurally similar calycosin and genistin, which indicated that the puerarin-MMIMs had strong specific recognition ability for puerarin.
3.2 Kinetic characteristics of CL reactions
Kinetic characteristics of the CL reactions were investigated. The CL kinetic curves of the system are shown in Fig. 4. When HCl and KMnO4 was injected into the flow path, the CL intensity is very much lower (curve 1). A sharp CL intensity could be detected when M2NRASP was injected into the merged solution mentioned above (curve 3), and with the addition of puerarin, the CL intensity decreased (curve 4). It can be found from Fig. 4 that as the amount of puerarin increased, the CL intensity decreased continually (curve 5). Hence, the KMnO4–HCl–M2NRASP–puerarin CL system can be used to determine puerarin quantitatively. | ||
| Fig. 4 The CL kinetic curves of the system 1: HCl + KMnO4 2: HCl + KMnO4 + puerarin (1.0 μg mL−1) 3: M2NRASP + KMnO4 + HCl 4: M2NRASP + KMnO4 + HCl + puerarin (1.0 μg mL−1) 5: M2NRASP + KMnO4 + HCl + puerarin (10.0 μg mL−1). | ||
3.3 Optimisation of analytical procedures
To obtain steady and strong relative CL, a series of experiments were done to optimize the experimental conditions.3.4 Optimum of chemiluminescence conditions
Pump speed is an important factor which effects the sensitivity of the method. The effect of pump speed was investigated in the range of 0.5–3.0 mL min−1, and both pump 1 speed and pump 2 speed were chosen to be 1.5 mL min−1 at which level the CL signal was stable and strong. The following conditions were optimized in order to obtain the greatest sensitivity by conducting a series of experiments using 1.0 × 10−6 g mL−1 puerarin standard solution.To obtain the strongest CL intensity, HCl, H2SO4 and NaOH were investigated as a medium to study the effect of KMnO4, Ce(SO4)2, H2O2 and K3[Fe(CN)6] on CL. It was found that in potassium permanganate–HCl medium, the strongest relative CL signal was detected. The CL reaction took place in acidic medium, the acidity was adjusted by changing concentration of HCl. In the range of 0.5–2.0 mol L1, the effect of different concentrations of HCl to CL was studied and it was found that the relative CL intensity was strong and steady when the concentration of HCl was 1.5 mol L−1. Hence, acidity of the system was chosen to be 1.5 mol L−1 of HCl. M2NRASP was chemiluminescent reagent, its concentration had a great effect on CL intensity. The effect of M2NRASP was studied in the range of 1.0 × 10−5 mol L−1 – 4.0 × 10−4 mol L−1. 2.0 × 10−5 mol L−1 was selected to be the proper concentration of M2NRASP at which the relative CL intensity was strong. Potassium permanganate was an oxidant in the CL system, and the concentration of oxidant was a great factor in CL. The effect of potassium permanganate was examined in the range of 1.0 × 10−4 mol L−1 – 5.0 × 10−4 mol L−1and 3.0 × 10−4 mol L−1 of potassium permanganate was selected to get the maximum relative CL intensity.
3.5 Stability of the proposed MMIMs-CL sensor
The repeatability of this MMIMs-CL sensor was investigated, the concentration of puerarin was 1.0 × 10−6 mol L−1, the pump rate of main pump and deputy pump was 15 mL min−1. The life time of this MMIMs-CL sensor was investigated at room temperature in air. Under the conditions mentioned above, this MMIMs-CL sensor can be used for 400 continuous repeated measurements with the relative standard deviation of CL signal intensity less than 5% (Fig. 5a). After 400 continuous repeated measurements, the intensity of the relative CL signal decreased remarkably, probably because of oxidation damage to the MMIMs. When the MMIMs-CL sensor was stored in air at 4 °C for 200 days, it remained about 98% of its initial CL intensity (Fig. 5b). | ||
| Fig. 5 The repeatability and life time of the MMIMs-CL sensor. | ||
3.6 Possible mechanism
The chemiluminescence spectra of HCl + KMnO4, HCl + KMnO4 + puerarin and M2NRASP + KMnO4 + HCl are shown in Fig. 4 under the same conditions. It can be seen that the CL emission may be due to the oxidation of M2NRASP. Therefore, in order to find out the evidence, the fluorescence spectra of M2NRASP, M2NRASP + HCl, M2NRASP + KMnO4 and M2NRASP + KMnO4 + HCl are shown in Fig. 6. The red shift of the excited wavelength and the slight blue shift of the emission wavelength proved that M2NRASP was oxidised by acidic potassium permanganate. | ||
| Fig. 6 Fluorescence spectrum of the system 1,1′: M2NRASP; 2,2′: M2NRASP + HCl; 3,3′: M2NRASP + KMnO4; 4,4′: M2NRASP + KMnO4 + HCl. | ||
Transformation between the carbonyl group and the enol form in M2NRASP is known to occur under weakly acidic conditions (Fig. 7). This ensures that M2NRASP is coplanar and conjugated, permitting a strong energy emission.24 In the proposed method, all solutions were in 1.5 mol L−1 HCl, which ensured strong and steady emission by M2NRASP. Under optimum conditions, the CL mechanism can be proposed as comprising oxidation of M2NRASP by potassium permanganate accompanied by CL emission. On the introduction of puerarin into the mixture excess potassium permanganate reacted with puerarin and released energy. Therefore, the CL emission produced by the direct oxidation of M2NRASP decreased. The released energy was absorbed partly by M2NRASP and the oxygen ion was excited to an oxygen free radical. The oxygen free radical of high energy level returned to the ground state and at the same time a conjugated and coplanar ring-structure was formed so that the CL emission could be measured. The total CL emission decreased due to the non-radiant energy loss in the energy transfer process, thus, the total CL emission was quenched.
 | ||
| Fig. 7 Possible mechanism. | ||
The mechanism can be expressed simply as:
| Potassium permanganate + M2NRASP → product + hυ |
| Potassium permanganate + puerarin → product + E |
| M2NRASP + E → M2NRASP* |
| M2NRASP* → M2NRASP + hυ’ |
3.7 Analytical performance
The proposed CL sensor was studied for linearity, precision, and sensitivity. Under the optimum conditions, a linear relationship between puerarin concentration and relative CL intensity was obtained over the range of 1.0 × 10−7 to 3.0 × 10−5 g mL−1 with a regression equation of ΔI = 145.75 + 33.71 cpuerarin (cpuerarin/10−6 g mL−1) (r = 0.9994). The low detection limit of puerarin was found to be 3.4 × 10−8 g mL−1.3.8 Study of selectivity
It is known that the intensity of chemiluminescence is affected by the coexistence of other chemicals. And it is also known that CL has a deficiency in selectivity. In this work, the MMIMs column was connected to the detection flow and the effect of coexisting substances was investigated with and without the puerarin-MMIMs, respectively. The maximum tolerable concentration ratios of coexisting substances in the determination of 1.0 × 10−6 g mL−1 puerarin are shown in Table 1 (the R.S.D is below ±5%), where the tolerance fold is defined as the maximum concentration of coexisting substances that produced a difference between positive and negative controls.| Coexisting substances | Without MMIMs | With MMIMs | Coexisting substances | Without MMIMs | With MMIMs |
|---|---|---|---|---|---|
| calycosin | 100 | 1000 | genistin | 100 | 1000 |
| sucrose | 50 | 1000 | Fe3+ | 50 | 1000 |
| fructose | 10 | 1000 | Ca2+ | 50 | 500 |
| cyclodextrin | 30 | 1000 | Sn2+ | 5 | 500 |
| starch | 20 | 1000 | Zn2+ | 5 | 500 |
| L-phenylalanine | 20 | 500 | Mg2+ | 5 | 500 |
| DL-maleic acid | 10 | 500 | L-methionine | 1 | 800 |
| sibutramine | 5 | 500 | L-histidine | 10 | 500 |
| Sb2+ | 10 | 100 | L-proline | 10 | 500 |
| Al3+ | 5 | 100 | L-lysine | 10 | 100 |
Table 1 indicated that the coexisting substances interfered greatly in the determination of puerarin without MMIMs, whereas the interferences were erased when the MMIMs column was used, the deficiency of CL was overcome and a high selectivity sensor for determination of puerarin was prepared.
4. Applications
In order to evaluate the applicability and reliability of the proposed sensor, puerarin levels in three samples was determined. Three kinds of diet foods/weight loss promoters were purchased from a pharmacy; 300 mg of each were ground and soaked with 100 mL water, then the mixtures were blended adequately and centrifuged to obtain the supernatant for detection. The blank experiment was carried out at the same time. As can be seen in Table 2, the results obtained are in agreement with those obtained by the official method5 such as HPLC. The recoveries of added puerarin can be quantitatively determined, and t-tests assume there are no significant differences between recovery efficiency and 100% at confidence level of 95%. It can be concluded from Table 2 that the proposed method has excellent analytical performance.| Sample | Puerarin concentration/mg g−1 | ||||
|---|---|---|---|---|---|
| Proposed sensor | Chromatography | ||||
| Added | Founda ± S.D. | R.S.D.% | Recovery (%) | Founda ± S.D. | |
| a Average of eleven measurements. | |||||
| 1 | — | 4.47 ± 0.08 | 1.7 | — | 4.52 ± 0.5 |
| 10.00 | 14.62 ± 0.14 | 1.0 | 101.5 | 14.41 ± 0.2 | |
| 2 | — | 12.87 ± 0.13 | 1.0 | — | 12.85 ± 0.3 |
| 10.00 | 22.74 ± 0.18 | 0.8 | 98.7 | 22.70 ± 0.2 | |
| 3 | — | 24.47 ± 0.24 | 0.9 | — | 24.58 ± 0.1 |
| 10.00 | 34.76 ± 0.26 | 0.7 | 102.9 | 34.71 ± 0.3 | |
5. Conclusions
A novel MMIMs-CL sensor with puerarin-MMIMs recognition was developed for detection of puerarin, and it greatly improved the selectivity of the chemiluminescence method. The quality of diet foods/weight loss promoters and some other foods can be successfully monitored. Novel rhodanine derivation M2NRASP was first applied in foods analysis by chemiluminescence, which widened the potential applications of the new reagent.Acknowledgements
This work was financially supported by National Natural Science Foundation of People's Republic of China (No. 50972050); Natural Science Research Foundation of Shandong Province, China (Y2007B07); Postgraduate Innovation Program of Shandong Province (SDYY08030).References
- Z. Ye and C. Jiang, Clinical observation of puerain in treating unstable angina pectoris, Clin. Med., 2008, 28(6), 3 Search PubMed.
- D. Yao and X. Ding, Discussions in pharmacological action and the development in clinical application of puerarin, Chinese J. Clin. Pharmacol. Therapeut., 2008, 13(4), 468 Search PubMed.
- L. Huang, C. Huang and J. Yang, To Determinate the Contents of Puerarin in Jiangzhijianfei Tablets by HPLC, Chinese J. Medical Guide, 2008, 10(3), 452 Search PubMed.
- H. Luo, H. Feng and X. Luo, Content Determination of Paeoniflorin in Xinkeshu Capsules by HPLC, Guiding J. Tradit. Chinese Med. Pharm., 2008, 14(7), 95 Search PubMed.
- C. Yu, Y. Cheng and F. Chen, Document analysis of hemolysis reaction of puerarin injection, Herald Med., 2007, 26(3), 320 Search PubMed.
- F. Nie and J. Lu, Determination of ketotifen by using calcein as chemiluminescence reagent, Anal. Chim. Acta, 2007, 592(2), 168 CrossRef.
- Y. Li, W. Niu and J. Lu, Sensitive determination of phenothiazines in pharmaceutical preparation and biological fluid by flow injection chemiluminescence method using luminol-KMnO4 system, Talanta, 2007, 71(3), 1124 CrossRef CAS.
- W. Niu, N. Feng, F. Nie and J. Lu, Investigating the post-chemiluminescence behavior of phenothiazine medications in the luminol-potassium ferricyanide system: molecular imprinting-post-chemiluminescence method for the determination of chlorpromazine hydrochloride, Anal. Bioanal. Chem., 2006, 385(1), 153 CrossRef CAS.
- H. Yao, X. Yang and H. Li, Investigation of Chemiluminescence Behavior of Flavonoids with Cerium (IV)-Rhodamine B System, Anal. Lett., 2006, 39(9), 2007 CrossRef.
- G. Wei, C. Wei, G. Dang, H. Yao and H. Li, Determination of Puerarin in Pharmaceutical Injection by Flow Injection Analysis with Acidic Potassium Permanganate–Glyoxal Chemiluminescence Detection, Anal. Lett., 2007, 40(11), 2179 CrossRef CAS.
- S. Han, Determination of puerarin by capillary electrophoresis with chemiluminescence detection, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2009, 877(14–15), 1591 CrossRef CAS.
- Q. Zhang, A. Myint, L. Liu, X. Ge and H. Cui, Flow injection-chemiluminescence determination of puerarin in pharmaceutical preparations, J. Pharm. Biomed. Anal., 2004, 36(3), 587 CrossRef CAS.
- C. Wang and Z. Song, In vitro monitoring of nanogram levels of puerarin in human urine using flow injection chemiluminescence, Bioorg. Med. Chem. Lett., 2004, 14(16), 4127 CrossRef CAS.
- J. P. Lai, M. L. Yang, R. Niessner and D. Knopp, Molecularly imprinted microspheres and nanospheres for di(2-ethylhexyl)phthalate prepared by precipitation polymerization, Anal. Bioanal. Chem., 2007, 389(2), 405 CrossRef CAS.
- N. Maier and W. Lindner, Chiral recognition applications of molecularly imprinted polymers: a critical review, Anal. Bioanal. Chem., 2007, 389(2), 377 CrossRef CAS.
- C. D. Ki, C. Oh, S. G. Oh and J. Y. Chang, The Use of a Thermally Reversible Bond for Molecular Imprinting of Silica Spheres, J. Am. Chem. Soc., 2002, 124(50), 14838 CrossRef CAS.
- M. A. Markowitz, P. R. Kust, G. Deng, P. E. Schoen, J. S. Dordick, D. S. Clark and B. P. Gaber, Catalytic Silica Particles via Template-Directed Molecular Imprinting, Langmuir, 2000, 16(4), 1759 CrossRef CAS.
- M. S. Rao and B. C. Dave, Selective Intake and Release of Proteins by Organically-Modified Silica Sol-Gels, J. Am. Chem. Soc., 1998, 120(50), 13270 CrossRef CAS.
- S. R. Carter and S. Rimmer, Molecular Recognition of Caffeine by Shell Molecular Imprinted Core-Shell Polymer Particles in Aqueous Media, Adv. Mater., 2002, 14(9), 667 CrossRef CAS.
- S. R. Carter and S. Rimmer, Surface Molecularly Imprinted Polymer Core-Shell Particles, Adv. Funct. Mater., 2004, 14(6), 553 CrossRef CAS.
- S. B. Savvin and R. F. Gur'eva, 5-azo derivatives of rhodanine and its analogues in the analytical chemistry of the noble metals, Talanta, 1987, 34(1), 87 CrossRef CAS.
- J. Yu, Q. Ou, Y. Lu, L. Wang and T. Li, Study on the Synthesis of a New Reagent 3-(4′-Fluorophenyl)-5-(2′-Arsenoxylphenylazo)-Rhodanine and the Fluoresence Determination of Bismuth(III), Spectrosc. Spectr. Anal., 2004, 24(9), 1093 CAS.
- H. Shi, Z. Wang and H. Shi, Synthesis of a Fluorescent Reagent 3-Phenyl-5-(2 -carboxylphenylazo)rhodanine and Its Character, Chinese J. Anal. Chem., 1995, 23(2), 172 Search PubMed.
- J. Yu, P. Dai, S. Ge, Y. Zhu, L. Zhang and X. Cheng, Catalytic dynamic spectrofluorimetry determination of trace antimony using new type arsenoxylphenylazo rhodanine, Spectrochim. Acta, Part A, 2009, 72(1), 17 CrossRef.
- T. Hishiya, M. Shibata, M. Kakazu, H. Asanuma and M. Komiyama, Molecularly Imprinted Cyclodextrins as Selective Receptors for Steroids1, Macromolecules, 1999, 32(7), 2265 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |