First detection of free D-amino acids in human nails by combination of derivatization and UPLC-ESI-TOF-MS
Jun Zhe
Min
*a,
Suguru
Hatanaka
a,
Hai-fu
Yu
b,
Tatsuya
Higashi
a,
Shinsuke
Inagaki
a and
Toshimasa
Toyo'oka
a
aDivision of Analytical and Bio-Analytical Chemistry, School of Pharmaceutical Sciences and Global COE Program, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526, Japan. E-mail: junzhe@u-shizuoka-ken.ac.jp; Fax: +81-54-264-5655; Tel: +81-54-264-5655
bFengxian Branch of Shanghai Sixth People's Hospital, Shanghai, 201400, China. E-mail: yuhaifu78@163.com; Tel: +86-21-5741-0145
First published on 3rd August 2010
Abstract
The resolution of free DL-amino acids in the human nail was carried out by combination of the R(−)-DBD-PyNCS derivatives and UPLC-ESI-TOF-MS. As we know, for the first time, these five kinds of D-amino acids, were determined from human nail samples. Fifteen kinds of L-amino acids were also recognized from human nails. Application to DL-amino acids in healthy person and diabetes patients nails is also described in this communication.
Introduction
Amino acids in mammalian tissues and body fluids have been considered to consist solely of L-enantiomers, whether free or as the components of peptides and proteins.1 However, with the recent progress in analytical technologies, several key reports have shown the presence, function and origin of some D-amino acids in mammals such as D-Asp in humans, neonatal rat brains, young rat pineal glands, AD (Alzheimer's disease) patients' brains and D-Ser in human and rat brains.2–7 Along with the elucidation of their distributions, origins and physiological functions, the D-amino acids have been recognized as candidates of novel physiologically active substances and the marker molecules of diseases. For instance, D-Asp is observed in various endocrine and neuroendocrine tissues and regulates hormonal synthesis and secretion in tissues. D-Ser and D-Ala are localized to the frontal brain areas and regulate N-methyl-D-aspartate (NMDA) receptor-mediated neurotransmission.8–10 Progress in the biochemical field has also been accelerated with the development of analytical methodologies for the effective separation and sensitive detection of amino acid enantiomers. There have been many reports about the resolution of amino acid enantiomers. There are mainly two methodologies for the enantiomeric determination of amino acids. One uses enzymes such as D-amino acid oxidases, and the other uses chromatography such as gas chromatography (GC),11 two-dimensional high-performance liquid chromatography (2D-HPLC)10,12–14 and high-performance capillary electrophoresis (HPCE).15 Among the HPLC separation and detection systems, it seems that fluorescence (FL) detection is one of the most reliable methods for the determination of DL-amino acids. Analysis of amino acid enantiomers has commonly been carried out by HPLC either directly with a chiral stationary phase column or indirectly after derivatization with a chiral reagent.16–18 Indirect resolution utilizing a fluorescent chiral tagging reagent is predominant in terms of sensitivity, versatility and separability. Although a number of fluorescent tagging reagents for amino acids have been developed, only a few chiral reagents are successfully used for highly sensitive resolution of amino acid enantiomers. We have developed a series of fluorescent chiral isothiocyanate reagents, i.e., 4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazole (DBD-PyNCS) and 4-(3-isothiocyanatopyrrolidin-1-yl)-7-nitro-2,1,3-benzoxadiazole (NBD-PyNCS) [R(−)- and S(+)-enantiomers],19,20 and have applied them to the separation of racemic amines.21,22 As described in a previous paper, the separations of each pair of DL-amino acids after labelling with the reagents are possible by reversed-phase liquid chromatography. These methods successfully determined the DL-amino acid in real samples, such as foodstuffs. However, the simultaneous determination of reported DL-amino acids with very low concentration in complex matrices is fairly difficult even with the highly sensitive FL detection. Therefore, a more sensitive and simultaneous determination method is required for the trace analysis of DL-amino acids in real samples. Tissues and blood samples have been extensively investigated for the DL-amino acid assay in biological specimens. The inherent problems of blood, such as the fluctuation in its composition during the day, and its analysis should be considered. Hygienic practice during its collection and handling is also another consideration. In contrast, the human nail is relatively clean and the sampling is easy. Nail analysis provides one important means for determining the individual past history of long-term chemical exposures, because many substances have been detected in the nail.23,24 Many studies concerning nail analysis have dealt with drugs of abuse, such as cocaine, itraconazole and amphetamines. Basic compounds are efficiently incorporated into the nail. Because of the stability of drugs in nails, it is very useful to analyze the drugs when doing post mortem investigations, especially when it is impossible to perform other tests because of the lack of common body fluids or when decomposition of the remains can produce false results. (e.g., negative tests due to the instability of analytes in body fluids or false positives from low specificity screening tests because of the presence of interferents).25,26 In the last decade, however, interest in nail analysis has gradually shifted to other drug species, e.g., doping agents and therapeutic drugs. Indeed, certain kinds of endogenous biogenic amino acids have been detected in the human nail.24 However; a method for the simultaneous determination of hydrophilic amino acid enantiomers including all of the human nails has not been reported. The UPLC system using an anti-pressurized column is possible for rapid separation. On the other hand, the selective detection of the target compound is carried out by ESI-TOF-MS due to its excellent accuracy and precision of the m/z values. However, the determination of trace amounts of amino acid enantiomers in the nail was very difficult because of the interference by endogenous substances in the samples. The reason seemed to be due to their high polarity, high hydrophilicity and low molecular weight. To increase the hydrophobicity, the primary amino functional groups in the structures were labeled with a fluorogenic reagent, R(−)-DBD-PyNCS.22 The resulting derivatives separated by UPLC were detected by ESI-TOF-MS.Results and discussion
In this communication, we describe the resolution of free DL-amino acids in human nails labeled with R(−)-DBD-PyNCS by UPLC-ESI-TOF-MS. The amino acid enantiomers in the nails of diabetes patients (age: 40–82; 3 men and age: 58–76; 3 women) and healthy persons (age: 69–83; 3 men and age: 47–78; 2 women) were also determined by the recommended method.Fig. 1 shows the labeling reaction of amino acids with the fluorescent chiral tagging reagent, DBD-PyNCS, which proceeds in a basic medium to form the corresponding diastereomers. The proposed derivatization conditions at 55 °C for 20 min in aqueous acetonitrile containing 1% TEA as base catalyst were also adopted in the present research.
 | ||
| Fig. 1 Reaction of DBD-PyNCS with D-amino acids. | ||
Initially, an anti-pressurized column packed with small porous resins, ACQUITY UPLC™ BEH C18 (100 mm × 2.1 mm i.d., 1.7 μm), was used for the rapid separation of the R(−)-DBD-PyNCS-labeled DL-amino acids by UPLC. For the detection of the derivatives, FL and ESI-TOF-MS instruments were directly connected to the outlet of the column in this order. Separation of each pair of DL-amino acids was studied by isocratic elutions with water–acetonitrile containing 0.1% formic acid (FA). However, it seems difficult to resolve all 17 DL-amino acids with a single isocratic run. Therefore, a gradient elution method was tried for the total resolution of the DL-amino acids. Fig. 2-I shows the separation of the racemic mixtures of His, Lys, Arg, Glu, Asp, Thr, Ala, Pro, Tyr, Met, Val, Trp, Phe, Gly and 6-aminohexanoic acid (I.S.) using water–acetonitrile containing 0.1% FA, by gradient elution, which provided a good separation of the derivatives. However, some peaks, i.e., D-Asn and L-Asn; L-Ser and D-Ser; DL-Ile and DL-Leu, overlapped each other. Therefore, the addition of methanol to the mobile phase and trifluoroacetic acid (TFA) instead of FA was tried in order to improve the separation. However, the separation of some amino acids was still difficult. Because the separation seems to depend on the pH of the eluent, the separation using 5 mM ammonium acetate buffer (pH 6.67) was tried finally. Fig. 2-II shows the separation of the racemic mixtures of Asn, Ser, Ile and Leu using 5 mM ammonium acetate buffer (pH 6.67) 0.1% FA acetonitrile by gradient elution. As shown in Fig. 2-II, all four DL-amino acids were completely separated with the buffer solution by linear gradient elution. Thus, the derivatives of 17 DL-amino acids were well separated using both elution systems: the gradient elution with water–acetonitrile containing 0.1% FA, and the gradient elution with 5 mM ammonium acetate buffer (pH 6.67) 0.1% FA acetonitrile. Based on these observations, the two gradient elution systems were recommended for the resolution of 17 DL-amino acids in real samples. Because the highly sensitive detection of the derivatives was carried out by the FL detection, the detection method seemed to be adequate for the determination of DL-amino acids. However, the determination in complex matrices such as plasma and nails seems to be fairly hard by FL detection. Indeed, the determination of several DL-amino acids in human nails by FL detection was interfered with by peaks from endogenous substances. Although the interference seemed to be evitable by optimization of the elution conditions, the determination with a short run time failed. Furthermore, no structural information could be obtained from the FL detection. On the other hand, the TOF-MS analysis is an efficient means to obtain structural information, because an exact m/z-value corresponding to the molecular mass is easily obtained. Thus, the simultaneous determination of DL-amino acids by UPLC-ESI-TOF-MS was next attempted. In the mass spectra, the protonated-molecular ions [M + H]+ were identified in all the DL-amino acids derivatives. Fig. 2 shows the selected-ion chromatogram (SIC) spectra obtained from the derivatives of 17 authentic DL-amino acids. The sensitivities were relatively higher than those with the FL detection. Furthermore, the information from the m/z values seems to be one of the important features for the structural elucidation of the DL-amino acids. The detection limits of the proposed method for the gradient elution of water–acetonitrile containing 0.1% FA, instead of water, are 1.0 fmol (L-Ala)–150 fmol (L-Glu), and the gradient elution with 5 mM ammonium acetate buffer (pH 6.67) 0.1% FA acetonitrile are 0.3 pmol (D-Leu)–1.0 pmol (D-Ser).The CV of the intra-day and inter-day determinations was 0.960–5.45% and 1.08–8.93%, respectively. Acceptable accuracy and precision were obtained from the three different concentrations for all of the DL-amino acids. Based upon these observations, the UPLC–ESI-TOF-MS system was adopted for the simultaneous determination of the DL-amino acids in human nails after FL labeling with R(−)-DBD-PyNCS.
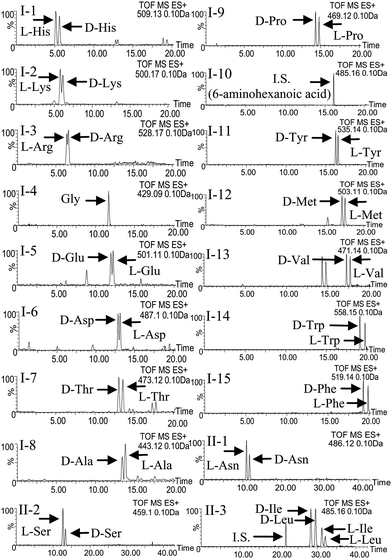 | ||
| Fig. 2 Mass chromatogram separation of DL-amino acid derivatives by UPLC-ESI-TOF-MS. UPLC conditions: instrument, ACQUITY™ UPLC (Waters, Milford, USA); column, ACQUITY UPLC™ BEH C18 (1.7 μm, 100 mm × 2.1 mm i.d., Waters); column temperature, 40 °C; flow rate, 0.4 mL min−1; eluent: I-1–15, A = 0.1% HCOOH in H2O, B = 0.1% HCOOH in CH3CN a linear gradient from B 20–20–23–45% (0–2–10–20 min); II-1–3, A = 5 mM CH3COONH4, B = 0.1% HCOOH in CH3CN a linear gradient B 14–14–21–21% (0–17–17–35 min). TOF-MS conditions: instrument, Micromass LCT Premier™ XE Mass Spectrometer (high sensitivity orthogonal time-of-flight instrument; Waters, Milford, USA); ion mode, ESI+; capillary voltage, 3000 V; cone voltage, 10 V; desolation temperature, 300 °C; source temperature, 120 °C; cone gas flow, 50 L h−1; desolation gas flow, 700 L h−1; MS range, 100–1000 m/z. | ||
The concentrations of DL-amino acids in the nails of diabetes patients and healthy persons were determined by the proposed procedures. The extraction solvent of the amino acids was first studied. Because the amino acids are hydrophilic and are strong basic compounds, water-soluble solvents MeOH, CH3CN and (MeOH and CH3CN) containing acids (HCl and TFA) and were tried as the extraction solution. Among the tested solutions, the amino acids were efficiently extracted with the MeOH solution. The extracted DL-amino acids from human nails were then labeled with R(−)- and S(+)-DBD-PyNCS and determined by the UPLC-ESI-TOF-MS method. The peaks corresponding to the DL-amino acid derivatives were completely separated without any interference from the endogenous substances in the samples. Furthermore, a rapid separation within 20 min and 35 min was performed by the combination of the anti-pressurized column and the UPLC instrument. Of course, the structures of the derivatives were identified from a comparison of the positive and negative ion mode MS of the authentic DL-amino acids. Fig. 3 shows the concentration of the representative DL-amino acids in the healthy person nails, as the mean amounts of Leu, Ala, Ile, Pro and Val in 1 mg of nails (n = 5). Almost all the amino acids were of the L-enantiomer, and high concentrations of Leu, Ser, Ile, Val, Ala, Phe, Pro, D-Leu, D-Ile, D-Ala, D-Pro and D-Val appeared in the methanol extracts of the nails. Of course, the opposite elution order was observed with the resulting derivatives with S(+)-DBD-PyNCS as tagging reagent.
 | ||
| Fig. 3 Amounts of DL-amino acids in healthy persons (3 men and 2 women). UPLC-ESI-TOF-MS conditions are the same as those in Fig. 2. | ||
Conclusion
In the present research, we have described a rapid method for the resolution of DL-amino acids in human nail derivatives by UPLC-ESI-TOF-MS. The resolution of free DL-amino acids in the human nail was carried out by combination of the R(−)-DBD-PyNCS derivatives and UPLC-ESI-TOF-MS. As we know, for the first time, these five kinds of D-amino acids, which were D-Leu, D-Ile, D-Ala, D-Pro and D-Val, were determined from human nail samples. Fifteen kinds of L-amino acids were also recognized from human nails. Thus, only the TOF-MS detection after separation by UPLC is described in this paper. In addition, when comparing the index from diabetes patients to those from healthy persons, there is no significant difference in the content of L-amino acids in the nail. However, the quantity of D-amino acids from diabetes patients decreased significantly from those in healthy persons. We will examine the correlation of D-amino acids with diabetes in detail now, and the results will be reported elsewhere in the near future.The present research was supported in part by a Grant-in-Aid for Young Scientists (B) (KAKENHI, No. 21790039) and the Global COE program from the Ministry of Education, Science, Sports and Culture of Japan.
Notes and references
- J. J. Corrigan, Science, 1969, 164, 142 CAS.
- K. Hamase, A. Morikawa and K. Zaitsu, J. Chromatogr. B., 2002, 781, 73 CrossRef CAS.
- N. Fujii, Origins Life Evol. Biosphere, 2002, 32, 103 CrossRef CAS.
- A. Hashimoto, T. Nishikawa, T. Oka and K. Takahashi, J. Neurochem., 1993, 60, 783 CrossRef CAS.
- K. Imai, T. Fukushima, K. Hagiwara and T. Santa, Biomed. Chromatogr., 1995, 9, 106 CAS.
- A. Neidle and D. S. Dunlop, Life Sci., 1990, 46, 1517 CrossRef CAS.
- R. Shapira, G. E. Austin and S. S. Mirra, J. Neurochem., 1988, 50, 69 CAS.
- S. H. Snyder and P. M. Kim, Neurochem. Res., 2000, 25, 553 CrossRef CAS.
- N. W. Kleckner and R. Dingledine, Science, 1988, 241, 835 CrossRef CAS.
- K. Hamase, H. Homma, Y. Takigawa, T. Fukushima, T. Santa and K. Imai, Biochim. Biophys. Acta, 1997, 1334, 214 CrossRef CAS.
- H. Bruckner and A. Schieber, Biomed. Chromatogr., 2001, 15, 166 CrossRef.
- Y. Miyoshi, K. Hamase, Y. Tojo, M. Mita, R. Konno and K. Zaitsu, J. Chromatogr. B, 2009, 877, 2506 CrossRef CAS.
- H. Bruckner, S. Haasmann, M. Langer, T. Westhauser, R. Wittner and H. Godel, J. Chromatogr., A, 1994, 666, 259 CrossRef CAS.
- T. Fukushima, J. Kawai, K. Imai and T. Toyo'oka, Biomed. Chromatogr., 2004, 18, 813 CrossRef CAS.
- S. Terabe, K. Otsuka and H. Nishi, J. Chromatogr., A, 1994, 666, 295 CrossRef CAS.
- D. W. Armstrong, M. P. Gasper, S. H. Lee, N. Ercal and J. Zukowski, Amino Acids, 1993, 5, 299 CrossRef CAS.
- M. Zief, L. J. Crane (ed.), Chromatographic Chiral Separation, Marcel Dekker, New York, 1988 Search PubMed.
- N. R. Srinivas and L. N. Igwemezie, Biomed. Chromatogr., 1992, 6, 163 CAS.
- T. Toyo'oka and Y.-M. Liu, Analyst, 1995, 120, 385 RSC.
- T. Toyo'oka and Y.-M. Liu, J. Chromatogr., A, 1995, 689, 23 CrossRef CAS.
- D. Jin, K. Nagakura, S. Murofushi, T. Miyahara and T. Toyo'oka, J. Chromatogr., A, 1998, 822, 215 CrossRef CAS.
- T. Toyo'oka, M. Toriumi and Y. Ishii, J. Pharm. Biomed. Anal., 1997, 15, 1467 CrossRef CAS.
- C. Ralph Daniel III MD., Bianca Maria Piraccini MD. and Antonella Tosti, J. Am. Acad. Dermatol., 2004, 50, 258 CrossRef.
- M. S. Greaves and J. M. H. Moll, Clin. Chem., 1976, 22, 1608 CAS.
- A. Palmeri, S. Pichini, R. Pacifici, P. Zuccaro and A. Lopez, Clin. Pharmacokinet., 2000, 38, 95 CrossRef CAS.
- R. C. Irving and S. J. Dickson, Forensic Sci. Int., 2007, 166, 58 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
