Rapid Raman mapping for chocolate analysis†
I. A.
Larmour
,
K.
Faulds
and
D.
Graham
*
Centre for Molecular Nanometrology, WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, UK. E-mail: duncan.graham@strath.ac.uk; Tel: +44 (0)141 548 4701
First published on 10th August 2010
Abstract
Raman microspectroscopy mapping capabilities have advanced significantly and have been applied to cell and pharmaceutical tablet formulation analysis. Bulk Raman investigations of food and their constituents have been carried out but little work exists on the application of Raman mapping capabilities to food. Here, we assess the applicability of Raman microspectroscopy mapping to the analysis of chocolate and examine both white and milk chocolate samples. It was found that the sucrose, lactose and fat constituents of white chocolate could be extracted and spatially resolved, indicating that the sucrose and lactose formed particles within a matrix of ‘fats’. Fluorescence from cocoa solids present in milk chocolate prevented chemical mapping with the instrumentation used. Raman mapping should provide a powerful analytical technique for the analysis and development of food products.
Introduction
Raman mapping can give a wealth of information about the spatial distribution and sizes of constituents within a complex sample. It has been used for the spatial analysis of active pharmaceutical in the excipient matrix within a final tablet1–4 and has also been applied to in vivo studies where either a cell or a tissue section is analysed.5,6 The quality of data obtained has improved significantly as technological advances have been incorporated into the latest Raman microscope systems. Raman mapping can now be routinely carried out at a resolution set by the optical diffraction limit. However, one area where the possibilities of the information rich technique of Raman mapping have not yet been fully exploited is within the food industry.7,8 Raman investigations of constituents and final food products have been carried out by obtaining spectra at individual points.9–13 However, this approach provides low resolution data and gives no indication of particulate sizes or distribution within final products.Since Cadbury introduced the first chocolate bar in 1842, the global chocolate market has grown to an estimated value of $145 bn per annum.14 Several analytical techniques have been applied to the analysis of chocolate or its constituents. Solid phase micro-extraction (SPME) followed by gas chromatograph toroidal ion trap mass spectrometry has been used to analyse the volatile and non-volatile compounds in cocoa beans, cocoa butter and final chocolate products.15 The free fatty acids in chocolate were probed with liquid chromatograph–mass spectrometry (LC-MS)16 while the majority of reported work has been conducted with infrared spectroscopy.17–20
Diffuse reflectance near-infrared Fourier transform spectroscopy (DRIFTS) was used to monitor the nutritional parameters of chocolate.17 Sucrose, lactose, fat and moisture levels within bulk chocolate or cocoa powder have all been reported.18–20 However, to the best of our knowledge, there have been no reports in the primary literature of chemical mapping of chocolate.
Raman spectroscopy has been used to investigate the individual ingredients present within chocolate,9,21,22 as well as their phase changes;10 however, the final product has not been the focus of analysis. We report the application of rapid Raman microspectroscopy mapping to final chocolate products. This technique provides a powerful development tool, not just for chocolate, but for the food industry as a whole.
Experimental
Materials
The individual raw constituents were obtained from Mars Chocolate Ltd. These included: sucrose, lactose, whey, skimmed milk powder, anhydrous milk fat, vegetable fat, lecithin, cocoa butter and cocoa mass. Whey consists of ∼80% lactose, skimmed milk powder contains whey, lactose and anhydrous milk fat. Cocoa mass is approximately 50% cocoa solids and 50% cocoa butter. Final preparation white and milk chocolate samples were also provided. All materials were used without further purification.Instrumentation
A WITec Alpha 300R confocal Raman microscope was used for all spectral acquisitions with independent spectrographs for the two laser wavelengths investigated: 532 and 785 nm. The gratings were 600 g mm−1 (BLZ = 500 nm) and 300 g mm−1 (BLZ = 750 nm) and were coupled to thermoelectrically cooled charge-coupled devices (CCDs) which gave spectral resolutions of 4 cm−1 and 3.7 cm−1, respectively, across a spectral range of 3936 cm−1 and 3324 cm−1. Unfocussed laser powers at the sample were measured at ∼37 mW and ∼80 mW respectively.Reference and map spectra were recorded using an Olympus LMPlanFI 50×/0.50 long working distance objective. The cocoa mass reference had to be recorded using a Nikon EPlan 10×/0.25 LWD objective and accumulated for 5 seconds to prevent sample degradation and detector saturation. All reference spectra of the remaining pure constituents were accumulated for 60 seconds.
High-resolution mapping was carried out on an area measuring 80 × 80 µm. 160 points per line and 160 lines per image were recorded to give a resolution of 0.5 µm. Spectra were recorded for 50 ms per point. Laser powers were reduced for milk chocolate samples by means of attenuators on the fibre optic coupling on the laser heads. Data analysis was carried out using WITec Project software, version 2.02.
Results and discussion
Using 532 nm laser excitation it was found that skimmed milk powder, cocoa mass, whey and lecithin (an emulsifier) all fluoresced, Fig. S1†, and contained no vibrational spectral features suitable for component identification. The other raw ingredients produced spectra with good signal-to-noise levels. These constituents contained vibrational bands that could be used for component extraction, Fig. 1. | ||
| Fig. 1 Reference spectra of the non-fluorescent constituents of chocolate at 532 nm and accumulation time of 60 seconds. (a) Lactose, (b) sucrose, (c) cocoa butter, (d) anhydrous milk fat, and (e) vegetable fat. The bands used for identification of the lactose, sucrose and ‘fats’ are identified. | ||
The band at 636 cm−1 was used for identification of sucrose, while the band at 468 cm−1 identified lactose. Vegetable fat, anhydrous milk fat and cocoa butter all showed very similar spectral features and were therefore classified generally as ‘fats’ with the band at 1295 cm−1, assigned to –CH2 deformation,9 used for identification.
Initial mapping experiments were performed on white chocolate samples due to the reduced risk of sample degradation. It was found that spectra could be recorded at the maximum laser power without observable sample damage occurring. Fig. 2 shows the individual Raman maps extracted for each of the three main components: sucrose, lactose and ‘fats’, using the bands identified in Fig. 1. The combined map is also shown, which clearly shows that the sucrose and lactose particles were found within a matrix of ‘fats’. These maps contain 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 individual full range spectra and were recorded in approximately 40 minutes. The time discrepancy is largely due to stage movements.
600 individual full range spectra and were recorded in approximately 40 minutes. The time discrepancy is largely due to stage movements.
 | ||
| Fig. 2 Raman mapping of a white chocolate sample measured at 532 nm, constituent maps and the combined Raman map which shows the lactose and sucrose particles within a matrix of ‘fats’. Scale bar in each image is 10 µm. | ||
Raman mapping did not only give spatial distribution information about the constituents, it also provided sizing information. In the example shown the lactose and sucrose particles were measured to be 4.9 ± 2.1 µm and 4.4 ± 1.5 µm respectively. This was in agreement with the particle sizes of the raw materials measured via light scattering and the final crystal sizes observed under electron microscopy (data not shown), although significantly it gave non-destructive, unambiguous chemical information of what the crystals were made from within the final product.
Milk chocolate samples proved significantly more difficult to map due to sample damage from the laser excitation. The unfocussed laser power at the sample had to be reduced to <0.1 mW to prevent degradation of the chocolate surface. Once sample degradation was minimised sample fluorescence became the overriding problem. It has already been mentioned that skimmed milk powder, cocoa mass, whey and lecithin all fluoresce at 532 nm. However, cocoa mass was responsible for the observed fluorescence as this was the only fluorescent constituent missing from white chocolate, in which no problems with sample fluorescence were observed. Cocoa mass contains cocoa butter and cocoa solids. Since, the spectra from cocoa butter has already been recorded and showed no fluorescence, this means that the detrimental fluorescence was obtained from the cocoa solids component. It should be noted that although skimmed milk powder, whey and lecithin are used within white chocolate their fluorescent signals did not overwhelm the signals from the other constituents.
In an attempt to extend Raman mapping to milk chocolate samples the wavelength was increased to 785 nm. Fig. 3 shows the spectra recorded from the non-fluorescent constituents. Lactose, sucrose and the ‘fats’ could be discriminated with the same bands as those used for the 532 nm results, this resulted in similar maps being produced for white chocolate, indicating no wavelength dependence of the results, Fig. S2†. Skimmed milk powder and whey did not give fluorescent signals, although they did not contain individual vibrational bands which could be used for their discrimination without further data analysis such as modified alternating least squares regression.23 Unfortunately, cocoa mass and lecithin continued to be fluorescent at this wavelength.
 | ||
| Fig. 3 Reference spectra of the non-fluorescent chocolate constituents measured at 785 nm. (a) Lactose, (b) sucrose, (c) skimmed milk powder, (d) cocoa butter, (e) anhydrous milk fat, (f) vegetable fat, and (g) whey. All spectra were accumulated for 60 seconds. The bands used for identification of the lactose, sucrose and ‘fats’ are identified. | ||
Milk chocolate mapping at 785 nm suffered from laser induced sample damage and the power had to be significantly reduced from 80 mW to ∼2 mW to ensure no observable sample degradation occurred. Fig. 4 shows the map extracted using the lactose peak, it shows some structural features, however, these turned out to be fluctuations within the fluorescence signal rather than any ‘real’ features. Cocoa solids are again responsible for the poor Raman mapping of milk chocolate. We have observed that cocoa mass, containing cocoa solids, continues to show a fluorescent response, even when 830 nm laser excitation is used (data not shown). Therefore, either Raman mapping using longer wavelengths, such as 1064 nm, is required or more sophisticated fluorescence rejection techniques employed. Although it has not been possible to map milk chocolate samples, we have successfully demonstrated Raman mapping of white chocolate samples.
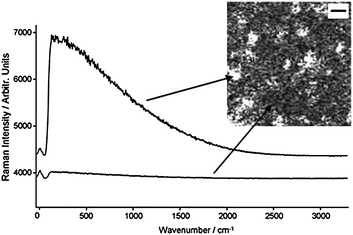 | ||
| Fig. 4 An extracted ‘lactose’ map from a milk chocolate sample showing features. These turned out to be variations within the fluorescence signal intensity as shown. Scale bar in the map is 10 µm. | ||
Conclusions
We have successfully applied rapid, high-resolution, large-area, Raman mapping to the analysis of chocolate samples. White chocolate was mapped with no special sample or instrumental alterations and showed that lactose and sucrose particles were embedded in a matrix of ‘fats’. Laser powers had to be significantly reduced to prevent sample damage with respect to milk chocolate samples. Milk chocolate mapping also suffered due to inherent fluorescence from the cocoa solid constituent. Importantly, Raman mapping did not only provide spatial distribution of constituents, it also allowed the particles to be sized and their chemical nature to be assigned unambiguously. Raman microspectroscopy mapping should become a significant addition to the food analysts' toolkit.Notes and references
- C. Gendrin, Y. Roggo and C. Collet, J. Pharm. Biomed. Anal., 2008, 48, 533 CrossRef CAS.
- M. J. Henson and L. Zhang, Appl. Spectrosc., 2006, 60, 1247 CrossRef CAS.
- S. Sasic, Appl. Spectrosc., 2007, 61, 239 CrossRef CAS.
- S. Wartewig and R. H. H. Neubert, Adv. Drug Delivery Rev., 2005, 57, 1144 CrossRef CAS.
- J. R. Beattie, S. Brockbank, J. J. McGarvey and W. J. Curry, Mol. Vision, 2007, 13, 1106 Search PubMed.
- C. Krafft, T. Knetschke, A. Siegner, R. H. W. Funk and R. Salzer, Vib. Spectrosc., 2003, 32, 75 CrossRef CAS.
- P. D. A. Pudney, T. M. Hancewicz and D. G. Cunningham, Int. J. Spectrosc., 2002, 16, 217 Search PubMed.
- P. D. A. Pudney, T. M. Hancewicz, D. G. Cunningham and M. C. Brown, Vib. Spectrosc., 2004, 34, 123 CrossRef CAS.
- V. Baeten, P. Hourant, M. T. Morales and R. Aparicio, J. Agric. Food Chem., 1998, 46, 2638 CrossRef.
- A. Celedon and J. M. Aguilera, Food Sci. Technol. Int. (Poznan), 2002, 8, 101 Search PubMed.
- L. F. C. de Oliveira, R. Colombara and H. G. M. Edwards, Appl. Spectrosc., 2002, 56, 306 CrossRef CAS.
- J. R. Beattie, S. E. J. Bell, C. Borgaard, A. M. Fearon and B. W. Moss, Lipids, 2004, 39, 897 CrossRef CAS.
- S. Okazaki, M. Hiramatsu, K. Gonmori, O. Suzuki and A. T. Tu, Forensic Toxicol., 2009, 27, 94 Search PubMed.
- Leatherhead Food Research, The Global Confectionery Market—Trends and Innovations, 2006, p. 212 Search PubMed.
- C. R. Bowerbank, A. McShea, S. B. Munro, E. D. Lee and D. W. Later, LCGC North Am., 2009, 27, 31.
- D. Perret, A. Gentili, S. Marchese, M. Sergi and L. Caporossi, Rapid Commun. Mass Spectrom., 2004, 18, 1989 CrossRef CAS.
- J. Moros, F. A. Inon, S. Garrigues and M. de la Guardia, Anal. Chim. Acta, 2007, 584, 215 CrossRef CAS.
- P. A. da Costa Filho, Anal. Chim. Acta, 2009, 631, 206 CrossRef.
- A. Vesela, A. S. Barros, A. Synytsya, I. Delgadillo, J. Copikova and M. A. Coimbra, Anal. Chim. Acta, 2007, 601, 77 CrossRef CAS.
- J. Tarkosova and J. Copikova, J. Near Infrared Spectrosc., 2000, 8, 251 CrossRef CAS.
- M. Liang, V. T. Chen, H. L. Chen and W. L. Chen, Talanta, 2006, 69, 1269 CrossRef CAS.
- H. G. M. Edwards, S. E. J. Villar, L. F. C. de Oliveira and M. L. Hyaric, Anal. Chim. Acta, 2005, 538, 175 CrossRef CAS.
- J. H. Wang, P. K. Hopke, T. M. Hancewicz and S. L. L. Zhang, Anal. Chim. Acta, 2003, 476, 93 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescent constituents at 532 nm and 785 nm maps. See DOI: 10.1039/c0ay00320d |
| This journal is © The Royal Society of Chemistry 2010 |
