DOI:
10.1039/C0AY00341G
(Paper)
Anal. Methods, 2010,
2, 1756-1763
Received
26th May 2010
, Accepted 30th July 2010
First published on
5th October 2010
Abstract
In this study IR and UV spectroscopic procedures are describe for the quantitative determination of Zidovudine (AZT) from solid dosage form. For IR spectroscopic method (KBr disc technique) has been used and Ursodeoxycholic acid (UDCA) was used as an internal standard. The specific absorption bands at 2105 and 2931 cm−1 were chosen for AZT and UDCA respectively. In this method, beer's law was obeyed in the concentration range 0.8–2.0% w/w in KBr disc. The regression equation was found to be y = 1.088x + 1.194 with correlation coefficient 0.9819, and the assay was found to be 99.8% with RSD of 2.157%. The UV spectroscopic method was also used in the quantitative determination of AZT in solid dosage form. 266 nm was chosen as λmax. The regression equation was found to be y = 0.0434x + 0.027, and the correlation coefficient 0.9989. The assay was found to be 99.81% with RSD of 0.273% by the UV spectroscopic method.
1. Introduction
Zidovudine (formerly called azidothymidine [AZT]) (Fig. 1), a pyrimidine nucleoside analogue chemically designated as 3′-azido-3′-deoxythymidine is active against HIV-1 infection. It is used in combination with other medications to control HIV-1 infection. It also lowers the risk of getting HIV disease complications (e.g., new infections, cancer). It belongs to a class of drugs known as nucleoside reverse transcriptase inhibitors (NRTI). It is not a cure for HIV infection and it does not prevent the spread of HIV to others through sexual contact or blood contamination (e.g., sharing needles). It is also used for the prevention of maternal-fetal transmission of HIV-1 infection.1,2
The previously published methods for AZT analysis described only using high performance liquid chromatography assay with either UV, tandem mass spectroscopy, LC-MS/MS or HPTLC. The earlier methods are expensive, time consuming and high technical skills are required. IR spectroscopic method for the determination of AZT in solid dosage form was suggested for the first time. The objectives of this study were firstly to develop and then validate two analytical methods for the quantification of AZT containing solid dosage form.3–6
2. Experimental
2.1. Apparatus
FT-IR spectrophotometer: Perkin Elmer, Spectrum Rx-1(Version 5.3.1, Operated on HP computer with Windows 2003). UV-Vis spectrophotometer: double-beam Perkin Elmer, Lambda-25 loaded, WinLab software (Version 5.2.0, operated on HP computer with Windows 2003). A 1 cm quartz cell over the range of 200–400 nm was employed.
2.2. Chemicals
AZT supplied by Matrix Pharma, Nashik, UDCA internal standard for IR by Sigma Aldrich USA, potassium bromide (IR spectroscopy grade), all the reagents and chemicals were of analytical grade. Retrovir® tablets containing 300 mg of AZT were purchased from local pharmacies in Dhulia-India.
2.3. Base line technique
The baseline technique was used for the quantitative analysis of drug substance. The following Fig. 2 showed the application of baseline technique. The values of PB and PO were calculated using the infra-red spectrum of the samples and, using the equation of regression, the quantitative analysis was carried out.
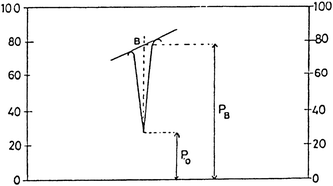 |
| | Fig. 2 Measurement of PB and PO points of the absorption peaks by base line technique (Application of base line technique). | |
2.4. IR spectroscopic method
2.4.1. Stock solutions.
The stock solution of AZT (2 mg ml−1) and UDCA (1 mg ml−1) were prepared in chloroform. These solutions were stable at 2–8 °C for 2 weeks.
2.4.2. Method validation.
2.4.2.1. Linearity.
1.0, 1.5, 2.0, 2.5, 3 ml of solution of AZT and 1.5, 1.0, 2.0, 1.5, 2.0 ml of solution UDCA were pipetted and poured into a 250 mg KBr powder in porcelain dishes separately. The dishes were kept in a hot air oven for 20 min at 60 °C to evaporate the moisture and solvent from the mixture. Then a series of synthetic standard mixtures of AZT-UDCA (2–1.5), (3–1), (4–2), (5–1.5), (5–2) mg were transferred into dishes separately. The powders were mixed through with an agate pestle and homogenous fine powder was obtained, after this 125 mg discs were prepared for each mixture and employed for quantitative measurement. Each disk of 125 mg contained 2 mg, 3 mg, 4 mg, 5 mg and 6 mg AZT respectively so a linear curve could be plotted. The calibration curve for AZT was prepared using the LogPB–LogPO values of AZT-UDCA in synthetic standard mixtures. The correlation of coefficient for AZT was found to be 0.9819 with equation line of y = 1.0883x + 1.1944.
2.4.2.2. Accuracy and precision.
Varying concentrations of AZT were prepared with internal standard in KBr disk range from 80%, 100% and 120%. PB and PO points of the absorption peaks were determined and LogPB–LogPO values of AZT-UDCA calculated by base line technique as depicted in Fig. 2. The intra-day and inter-day accuracy precision was studied. The percentage RSD of the method was calculated at these three levels.
2.4.2.3. Recovery study.
The recovery study was performed at 80%, 100% and 120%. 4 mg of AZT and concentration 3.2 mg, 4 mg, and 4.8 mg added into the disk containing AZT-UDCA standard mixture of 4 mg AZT. The recoveries of three levels were estimated and %RSD was calculated.
2.4.2.4. Ruggedness.
Ruggedness of the proposed methods was determined by analysis of aliquots from homogeneous slot in by different analysts and at different percentage relative humidity (%RH ± 5%) using similar operational and environmental conditions; the % R.S.D. was calculated.
2.4.3. Assay of AZT tablet by IR spectroscopy method.
Ten tablets were weighed powdered and a portion of powder equivalent to 40 mg AZT was accurately weighed and extracted with chloroform. The solution was filtered in to 20 ml volumetric flask and the volume was made up to the mark to get the solution of 2 mg ml−1. The solution of UDCA, 1 mg ml−1 was prepared by dissolving 20 mg of UDCA in 20 ml of chloroform.
Then 2 ml of solution of AZT and 2 ml of solution of UDCA were pipetted and poured into a 250 mg KBr powder in porcelain dishes separately. The dishes were kept in hot air oven for 20 min at 60 °C to evaporate the moisture and solvent from the mixture. The synthetic standard mixture of AZT-UDCA (4–2) mg was transferred into dishes separately. The powders were mixed through with an agate pestle and homogenous fine powder was obtained of which 125 mg of disc was prepared by using KBr. Likewise five homogeneous discs were prepared and employed for quantitative measurement.
2.5 UV spectroscopic method
2.5.1. Preparation of stock solution.
Accurately weighed 20 mg of AZT transferred to 20 ml volumetric flasks. It was dissolved in 10 ml methanol shaken manually for 5 min. The volume was made up to the mark with methanol to obtain final strength 1000 μg ml−1.
2.5.2. Determination of λ max.
From the stock solution, 0.1 ml and 0.3 ml of AZT solution was transferred to 10 ml volumetric flasks and the volume was adjusted to the mark with the same solvent to obtain concentrations of 10 and 30 μg ml−1 respectively. The solutions were scanned in the UV range 200–400 nm. The scanning spectrums of the drug are shown in Fig. 3 and Fig. 4 and the wavelength 266 nm was selected for further study.
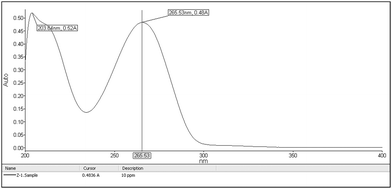 |
| | Fig. 3 Scanning spectrum of 10 μg ml−1 AZT solution in the UV range of 200–400 nm. | |
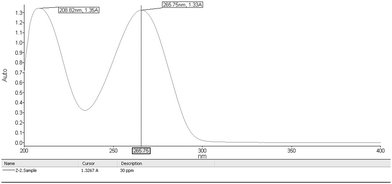 |
| | Fig. 4 Scanning spectrum of 30 μg ml−1 AZT solution in the UV range of 200–400 nm. | |
2.5.3 Determination of E (1%, 1 cm).
0.3 ml aliquot of AZT from stock solution was transferred to six 10 ml volumetric flaks and volume was adjusted to the mark with methanol to obtain six individual concentrations of 30 μg ml−1. The absorbance of each solution was measured at 266.0 nm. E (1%, 1 cm) values of drug were calculated using eqn (1) and results of E (1%, 1 cm) of the drug are shown in Table 1:| | | E (1%, 1 cm) = Absorbance/Concentration (g/100ml) | (1) |
Table 1 E (1%, 1cm) values of AZT at 266.0 nm
| Absorptivity at 266 nm |
|
Absorptivity values are the mean of six determinations.
S.D. is Standard Deviation.
RSD is Relative Standard Deviation.
|
|
Mean |
449.83 |
| ±SDb |
0.81 |
| %RSDc |
0.1798 |
2.5.4. Method validation.
2.5.4.1. Linearity.
Different concentration of AZT from 10–50 μg mL−1 were prepared using the stock solution to prepare the calibration curve of AZT. The slope and intercept was calculated from the regression line equation.
2.5.4.2. Precision (intra-day and inter-day).
Accuracy and precision study was carried out at three concentration levels from 80%, 100% and 120% of 30 μg mL−1. The study was performed for intra-day and inter-day to assess the variation in analysis.
2.5.4.3. Ruggedness.
Ruggedness of the proposed methods was determined by analysis of aliquots from homogeneous slot in different laboratories, by different analysts, using similar operational and environmental conditions; the % R.S.D. was calculated.
2.5.4.4. Recovery study.
To check the accuracy of the developed methods and to study the interference of formulation additives, analytical recovery experiments were carried out by standard addition methods, at 80, 100 and 120% level. From the total amount of drug found, the percentage recovery was calculated.
2.5.6. Assay of marketed formulation (Retrovir).
Contents of twenty ‘Retrovir’ tablets containing 300 mg of AZT were weighed and ground to fine powder. A quantity of sample equivalent to 20 mg of AZT was transferred into 20 ml volumetric flask containing approximately 15 ml methanol, sonicated for 10 min; the volume was made up to the mark and filtered through Whatmann filter paper (No. 41). An appropriate volume 0.3 ml of this solution was transferred to 10 ml volumetric flasks and dissolved, the volume was adjusted to mark with methanol. The absorbance's of the solutions were measured at 266.0 nm against blank.
3.0. Results
3.1.1. Linearity.
The calibration curve for AZT was prepared using the LogPB–LogPO values of AZT-UDCA in synthetic standard mixtures. The correlation of coefficient for AZT was found 0.9819 with equation line of y = 1.0883x + 1.1944 (Fig. 5). The linearity values of LogPB–LogPO are presented in Table 2.
Table 2 LogP–LogPO values found for AZT-UDCA in synthetic standard mixturesa
| Synthetic Mixture |
Disc Wt/mg |
AZT 2105 cm−1 |
UDCA 2931 cm−1 |
X = CAZT/CUDCA |
Y = TAZT/TUDCA |
| Conc./mg |
PB–PO |
Log PB − Log PO |
Conc./mg |
PB − PO |
Log PB − Log PO |
|
Where, CAZT = AZT conc. in KBr disc, CUDCA= UDCA conc. in KBr disc, TAZT= LogPB–LogPO values of AZT, TUDCA= LogPB–LogPO values of UDCA.
|
| St1 |
125.2 |
2 |
11.78 |
0.091 |
1.5 |
3.94 |
0.033 |
1.33 |
2.7576 |
| St2 |
126 |
3 |
13.52 |
0.123 |
1 |
3.73 |
0.028 |
3 |
4.3746 |
| St3 |
124 |
4 |
14.98 |
0.163 |
2 |
3.96 |
0.05 |
2 |
3.26 |
| St4 |
123.8 |
5 |
22.88 |
0.238 |
1.5 |
4.28 |
0.048 |
3.33 |
4.9633 |
| St5 |
123.5 |
6 |
23.53 |
0.312 |
2 |
3.95 |
0.081 |
2.5 |
3.8574 |
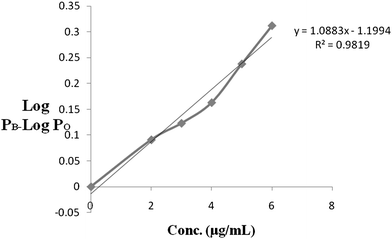 |
| | Fig. 5 Linearity of AZT. | |
3.1.2. Accuracy and precision.
The developed method of AZT shows high levels of accuracy and precision at 80%, 100% and 120% levels as given in Table 3 and 4. The intra-day and inter-day percentage %RSD for three levels is found below 1.72.
Table 3 Intra-day accuracy and precision
| Levels |
Disc Wt/mg |
AZT 2105 |
| Conc./mg |
PB–PO |
Log PB–Log PO |
| 80% |
124.500 |
3.200 |
14.340 |
0.132 |
| 124.300 |
3.200 |
14.310 |
0.131 |
| 124.600 |
3.200 |
14.370 |
0.133 |
|
Mean
|
124.467
|
3.200
|
14.340
|
0.132
|
|
SD
|
0.153
|
0.000
|
0.030
|
0.001
|
|
%RSD
|
0.123
|
0.000
|
0.209
|
0.758
|
| 100% |
125.900 |
4.000 |
15.020 |
0.166 |
| 125.300 |
4.000 |
14.990 |
0.165 |
| 125.700 |
4.000 |
15.030 |
0.168 |
|
Mean
|
125.633
|
4.000
|
15.013
|
0.166
|
|
SD
|
0.306
|
0.000
|
0.021
|
0.002
|
|
%RSD
|
0.243
|
0.000
|
0.139
|
0.918
|
| 120% |
1.26.7 |
4.800 |
17.810 |
0.192 |
| 126.200 |
4.800 |
17.830 |
0.198 |
| 126.800 |
4.800 |
17.780 |
0.196 |
|
Mean
|
126.500
|
4.800
|
17.807
|
0.195
|
|
SD
|
0.424
|
0.000
|
0.025
|
0.003
|
|
%RSD
|
0.335
|
0.000
|
0.141
|
1.564
|
Table 4 Inter-day accuracy and precision (3 days)
| Levels |
Disc Wt/mg |
AZT 2105 |
| Conc./mg |
PB–PO |
Log PB–Log PO |
| 80% |
124.500 |
3.200 |
14.354 |
0.136 |
| 124.300 |
3.200 |
14.324 |
0.134 |
| 124.600 |
3.200 |
14.325 |
0.138 |
|
Mean
|
124.467
|
3.200
|
14.334
|
0.136
|
|
SD
|
0.153
|
0.000
|
0.017
|
0.002
|
|
%RSD
|
0.123
|
0.000
|
0.119
|
1.471
|
| 100% |
125.900 |
4.000 |
15.420 |
0.165 |
| 125.300 |
4.000 |
14.980 |
0.169 |
| 125.700 |
4.000 |
15.450 |
0.164 |
|
Mean
|
125.633
|
4.000
|
15.283
|
0.166
|
|
SD
|
0.306
|
0.000
|
0.263
|
0.003
|
|
%RSD
|
0.243
|
0.000
|
1.722
|
1.594
|
| 120% |
1.26.7 |
4.800 |
17.860 |
0.195 |
| 126.200 |
4.800 |
17.810 |
0.192 |
| 126.800 |
4.800 |
17.700 |
0.199 |
|
Mean
|
126.500
|
4.800
|
17.790
|
0.195
|
|
SD
|
0.424
|
0.000
|
0.082
|
0.004
|
|
%RSD
|
0.335
|
0.000
|
0.460
|
1.798
|
3.1.3. Recovery study.
At the level of three concentrations of 3.2 mg, 4 mg, and 4.8 mg, the recovery was more than 78% and the %RSD was found to be less than .059. as data presented in Table 5 show.
Table 5 Recoveries of AZT at three level 80%, 100% and 120%
| Levels |
Disc Wt/mg |
AZT 2105 |
% found |
| Added Conc./mg |
PB-PO |
LogPB–LogPO |
| 80% |
122.500 |
3.200 |
14.295 |
0.139 |
99.81 |
| 128.700 |
3.200 |
14.289 |
0.136 |
99.79 |
| 127.600 |
3.200 |
14.332 |
0.136 |
99.79 |
|
Mean
|
126.267
|
3.200
|
14.305
|
0.137
|
99.797
|
|
SD
|
3.308
|
0.000
|
0.023
|
0.002
|
0.012
|
|
%RSD
|
2.620
|
0.000
|
0.163
|
1.264
|
0.012
|
| 100% |
123.980 |
4.000 |
15.457 |
0.169 |
99.87 |
| 127.300 |
4.000 |
14.893 |
0.170 |
99.89 |
| 128.750 |
4.000 |
15.278 |
0.164 |
99.78 |
|
Mean
|
126.677
|
4.000
|
15.209
|
0.168
|
99.847
|
|
SD
|
2.445
|
0.000
|
0.288
|
0.003
|
0.059
|
|
%RSD
|
1.930
|
0.000
|
1.895
|
1.917
|
0.059
|
| 120% |
125.780 |
4.800 |
17.895 |
0.189 |
99.97 |
| 129.250 |
4.800 |
17.938 |
0.199 |
99.99 |
| 125.880 |
4.800 |
17.849 |
0.192 |
99.92 |
|
Mean
|
126.970
|
4.800
|
17.894
|
0.193
|
99.960
|
|
SD
|
1.975
|
0.000
|
0.045
|
0.005
|
0.036
|
|
%RSD
|
1.556
|
0.000
|
0.249
|
2.566
|
0.036
|
3.1.4. Ruggedness.
Ruggedness of the proposed method shows that the different analysts and different percentage relative humidity (%RH ± 5%) does not affect the method and the accuracy of the method is more than 98% and %RSD was found below 3.728 as shown in Table 6–7.
Table 6 Ruggedness of AZT performed by two analysts
| Analyst-I |
Analyst-II |
| Disc Wt |
AZT 2105 |
Disc Wt |
AZT 2105 |
| Conc./mg |
PB-PO |
LogPB |
Conc./mg |
PB-PO |
LogPB |
| −LogPO |
−LogPO |
| AZT |
125.900 |
4.000 |
15.756 |
0.161 |
AZT |
125.900 |
4.000 |
15.980 |
0.175 |
| 125.300 |
4.000 |
14.834 |
0.167 |
125.300 |
4.000 |
14.950 |
0.170 |
| 125.700 |
4.000 |
15.678 |
0.169 |
125.700 |
4.000 |
15.890 |
0.176 |
|
Mean
|
125.633
|
4.000
|
15.423
|
0.166
|
Mean
|
125.633
|
4.000
|
15.607
|
0.174
|
|
SD
|
0.306
|
0.000
|
0.511
|
0.004
|
SD
|
0.306
|
0.000
|
0.570
|
0.003
|
|
%RSD
|
0.243
|
0.000
|
3.315
|
2.513
|
%RSD
|
0.243
|
0.000
|
3.655
|
1.968
|
Table 7 Ruggedness of AZT performed at different relative humidity
| 35% RH |
40% RH |
| Disc Wt |
AZT 2105 |
Disc Wt |
AZT 2105 |
| Conc./mg |
PB-PO |
LogPB |
Conc./mg |
PB-PO |
LogPB |
| −LogPO |
−LogPO |
| AZT |
128.400 |
4.000 |
14.348 |
0.179 |
AZT |
128.400 |
4.000 |
14.798 |
0.169 |
| 122.380 |
4.000 |
15.278 |
0.174 |
122.380 |
4.000 |
15.898 |
0.177 |
| 128.790 |
4.000 |
15.350 |
0.171 |
128.790 |
4.000 |
15.450 |
0.178 |
|
Mean
|
126.523
|
4.000
|
14.992
|
0.175
|
Mean
|
126.523
|
4.000
|
15.382
|
0.175
|
|
SD
|
3.594
|
0.000
|
.559
|
0.004
|
SD
|
3.594
|
0.000
|
0.553
|
0.005
|
|
%RSD
|
2.840
|
0.000
|
5.295
|
3.728
|
%RSD
|
2.840
|
0.000
|
3.596
|
2.824
|
| 45% RH |
| Disc Wt |
AZT 2105 |
| Conc./mg |
PB–PO |
LogPB |
| −LogPO |
| AZT |
128.400 |
4.000 |
15.120 |
0.165 |
| 122.380 |
4.000 |
14.560 |
0.167 |
| 128.790 |
4.000 |
15.140 |
0.174 |
|
Mean
|
126.523
|
4.000
|
14.940
|
0.169
|
|
SD
|
3.594
|
0.000
|
0.329
|
0.005
|
|
RSD
|
2.840
|
0.000
|
2.204
|
2.802
|
3.2. Assay of AZT tablet by IR spectroscopy method
The proposed method was used for the tablet formulation of AZT (Retrovir) and found the % assay is 99.8% with %RSD of 2.15 as given in Table 8.
Table 8 Assay results of commercial samples (Retrovir tablet 300 mg of AZT) by IR spectroscopy.b
| Parameters |
IR-Spectroscopy |
| AZT |
|
Value for Drug content (%) is the mean of five estimations.
S.D. is standard deviation and R.S.D. is relative standard deviation.
|
| Label Claim |
300 |
| %Drug Contenta |
99.8 |
| ±SD |
2.152
|
| %RSD |
2.157
|
4.1. Linearity
The method is linear in the range of 10–50 μg mL−1. The equation of regression lie was found to be at y = 0.0427 + 0.038 where the intercept is 0.038 and slope is 0.0427 for AZT as depicted in Fig. 6. The equation was used to determine the content of AZT in tablet formulation under the method.
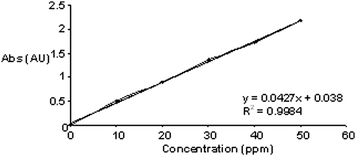 |
| | Fig. 6 Calibration curve of AZT over the concentration range of 10–50 ppm. | |
4.2. Recovery studies by UV-spectroscopy
To check the accuracy of the developed methods and to study the interference of formulation additives, analytical recovery experiments were carried out by standard addition method, at 80, 100 and 120% level. From the total amount of drug found, the percentage recovery was calculated. The results are reported in Table 9.
Table 9 Recovery studies of AZT
| Excess Drug |
%Recoverya |
%RSD |
|
%Recovery is mean of three estimations.
|
| 80 |
100.09 |
0.208 |
| 100 |
99.85 |
0.375 |
| 120 |
99.94 |
0.205 |
4.3. Precision and ruggedness parameters
Precision was determined as repeatability, intra-day and inter-day variations. Repeatability was determined by analyzing AZT solution (30 μg mL−1) for six times. Intra-day precision was determined by analyzing 30 μg ml−1 solution of AZT three times on the same day. Inter-day precision was determined by analyzing the same concentration of solutions for three different days over a period of one week. The results were reported in Table 10. Ruggedness of the proposed methods was determined by analysis of aliquots from homogeneous slot in different laboratories, by different analysts, using similar operational and environmental conditions; the %RSD reported in Table 11 was found to be less than 2%.
Table 10 Summary of repeatability, precision and ruggedness
| Parameters (UV Spectroscopy) AZT |
%RSD |
| Repeatability |
0.416 |
| Intra-day |
0.225 |
| Inter-day |
0.112 |
|
Ruggedness
|
| Analyst I |
0.555 |
| Analyst II |
0.337 |
Table 11 Determination of AZT in bulk
| Amount taken/μg ml−1 |
Absorbance |
Amount found/μg ml−1 |
% Amount found |
| 30 |
1.353 |
30.00 |
100 |
| 1.346 |
29.84 |
99.48 |
| 1.35 |
29.93 |
99.78 |
| 1.351 |
29.96 |
99.85 |
| 1.349 |
29.91 |
99.7 |
|
Mean
|
1.349
|
29.93
|
99.76
|
| ±SD |
0.19
|
|
%RSD
|
0.192
|
4.4 Determination of AZT in bulk and tablet using the developed method
The developed UV spectroscopy method was also used for the determination of AZT in bulk and tablet. The percentage amount found in bulk is 99.76% with %RSD of 0.192 and in tablet 99.81% with %RSD of 0.272 as given in Table 11 and 12.
Table 12 Determination of AZT in tablet formulation
| Amount taken/μg ml−1 |
Absorbance |
Amount found/μg ml−1 |
% Amount found |
| 30 |
1.355 |
30.04 |
100.14 |
| 1.349 |
29.91 |
99.7 |
| 1.345 |
29.82 |
99.41 |
| 1.351 |
29.96 |
99.85 |
| 1.352 |
29.98 |
99.93 |
|
Mean
|
1.35
|
29.94
|
99.81
|
| ±SD |
0.27
|
|
%RSD
|
0.273
|
5.0. Discussion
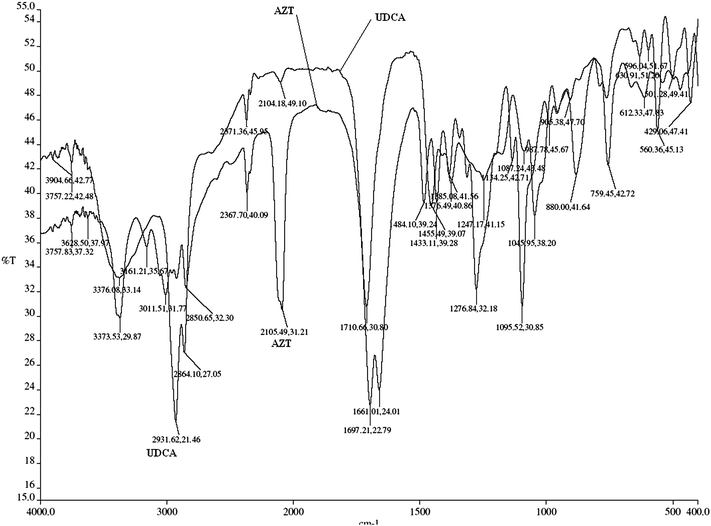
The IR spectra of discs were taken, for which 2105 cm−1 for AZT (Fig. 7) and 2931 cm−1 for UDCA (Fig. 7) absorption bands were used. Fig. 8 represents the IR Spectrum of commercial tablet (Retrovir) containing UDCA. The base line technique was used for the determination of PB and PO values of absorption bands. The logarithmic differences of these values as absorbance (y) were plotted against concentration (x) in order to calculate the regression equation. Internal standard was used in order to eliminate some unforeseen defaults which originated from the application of the method. For this purpose UDCA was chosen as the internal standard with the absorption band at 2931 cm−1 where no absorption band is available for AZT. On the other hand, the internal standard has no absorption band at 2105 cm−1 where AZT has absorption. In this method, the linear concentration range was obtained as 0.8–2.0% w/w. This range is so narrow because in this study more attention was paid to hold PB and PO points between 80–20% as the transmittance value which were used for AZT and UDCA. Especially when the PO point is under 20% transmittance, any small error for the determination of this point fairly affect the results.7–13 Quantitative determination is based on concentration-absorption relationship of Beer's law. PB and PO points of the absorption peaks were assigned with base line technique as shown in Fig. 2. The regression equation was formed by using the AZT/UDCA concentration ratio as (x) values and the ratio of Log PB–Log PO of AZT and Log PB–Log PO of UDCA values as (y). Absorbance values were measured by logarithmic subtraction of PB and PO points. The regression equation was calculated by using the ratio concentration/absorbance of AZT and UDCA (Table 2). At 2105 cm−1 the regression equation was found to be y = 1.088x + 1.194, R2 = 0.9819 and the assay was found to be 99.8% with RSD 2.157%.
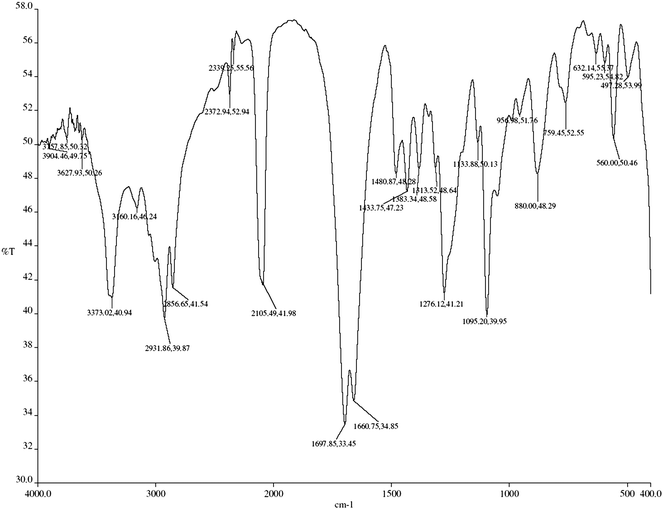 |
| | Fig. 8 IR Spectrum of commercial tablet (Retrovir) containing UDCA in KBr. | |
UV spectroscopic method was used as another comparison method for quantitative determination of AZT in commercial tablets. A linear concentration was obtained as 10–50 μg ml−1 in this method. The regression equation was found to be y = 0.0434x + 0.027 and the correlation coefficient 0.9984. E (1%, 1 cm) was calculated as 449.83 in methanol. The assay was found to be 99.81% with %RSD 0.273%.
Although various methods like HPLC-UV, LC-MS/MS, HPTLC are available, these methods are expensive due to use of expensive detectors, costly solvents, columns and also more time needed to develop and validate the method.14–16 The present IR spectroscopy method is a simple, rapid, accurate and cost effective analytical tool for AZT determination in tablet formulations.
6.0. Conclusion
This paper describes the application of IR spectroscopic method and UV spectroscopic method to determine AZT in formulation. The IR spectroscopic method can be use as an alternative for the determination of AZT in solid dosage forms. The proposed method is simple, precise, accurate and rapid for the determination of Zidovudine (AZT) in tablet dosage form. Analysis of authentic samples containing AZT showed no interference from the common additives and excipients, demonstrating that the recommended procedure is well suited for the assay and evaluation of drugs in pharmaceutical preparations. The developed method can be easily and conveniently adopted for routine quality control analysis.
Acknowledgements
We are thankful to Matrix Pharma, Nashik and Sigma Aldrich, USA for providing us the gift sample of Zidovudine (AZT) and Ursodeoxycholic acid (UDCA) respectively. We are also thankful to SPTM, SVKM's NMIMS (Shirpur campus) for providing the research facilities to carryout work.
References
-
H. P. Rang, M. M. Dale, J. M. Ritter and R. J. Flower, Pharmacology, Churchill Livingstone, Elsevier, 6th edn, pp 684 Search PubMed.
-
L. B. Laurence, S. L. John and L. P. Keith, Goodman and Gilman's: The Pharmacological Basis of Therapeutics, McGraw-Hill, Medical Publishing Division, 11th edn, pp 1283 Search PubMed.
- V. P. Nagulwar and K. P. Bhusari, A validated UV spectrophotometric method for the simultaneous estimation of Lamivudine, Nevirapine and zidovudine in combined tablet dosage form, J. Pharm. Res., 2009, 2(4), 666–669 Search PubMed.
- C. Sharada, K. Channabasavaraj and M. T. Tamizh, Development of two derivative spectrophotometric methods for quantitative estimation of Zidovudine in bulk and pharmaceutical dosage forms, I.J.P.R.D, 2010, 2(3), 001 Search PubMed.
- K. B. Kenney, S. A. Wring, R. M. Carr, G. N. Wells and J. A. Dunn, Simultaneous determination of Zidovudine And Lamivudine in human serum using HPLC with tandem Mass Spectrometry, J. Pharma. Bio. Ana., 2005, 22(6), 967–983 Search PubMed.
- S. Anbazhagan, N. Indumathy, P. Shanmugapandiyan and S. K. Sridhar, Simultaneous quantification of stavudine, lamivudine and nevirapine by UV spectroscopy, reverse phase HPLC and HPTLC in tablets, J. Pharma. Bio. Ana., 2005, 39(3), 801–804 Search PubMed.
- G. Mehtap and A. Okan, Quantitative determination of Amisulpride in pharmaceuticals by IR, UV Spectroscopy and HPLC, Turkish J. Pharm. Sci., 2004, 1(1), 17–29 Search PubMed.
- O. Berna and A. Okan, Quantitative determination of Disulfiram-containing pharma- ceuticals by IR spectroscopy and HPLC methods, FABAD J. Pharm. Sci., 2003, 28, 193–200.
- S. R. Matkovic, G. M. Valle and L. E. Briand, Quantitative analysis of Ibuprofen in pharmaceutical formulations through FT-IR spectroscopy, Latin Ame. Appli. Res., 2005, 35, 189–195 Search PubMed.
- O. Atay and F. Dincol, Quantitative determination of piroxicam by IR spectrometry, Sci. Pharm., 1997, 65, 131–142 CAS.
- O. Atay and F. Dincol, Quantitative determination of tenoxicam by IR spectrometry, Anal. Lett., 1997, 30(9), 1675–1684 CAS.
- O. Atay and F. Selcuk, Quantitative determination of fluconazole by IR spectrometry, Anal. Lett., 1996, 29(12), 2163–2176 CAS.
- D. Nebioglu and O. Atay, Quantitative analysis of cyclophosphamide containing drugs by IR spectrophotometry, J. Fac. Pharm. Gazi., 1987, 4(2), 75–84 Search PubMed.
- D. A. Kumar, M. V. N. Babu, J. V. L. N. S. Rao and V. J. Rao, Simultaneous estimation of lamivudine, zidovudine and nevirapine in tablet dosage forms by RP-HPLC method, Rasayan J. Chem., 2010, 3(1), 94–99 Search PubMed.
- B. Uslu and S. A. Ozkhan, Determination of lamivudine and zidovudine in binary mixtures using first derivative spectrophotometric, first derivative of the ratio-spectra and high-performance liquid chromatography-UV methods, Anal. Chim. Acta, 2002, 466(1), 175–185 CrossRef CAS.
- R. E. Estrela, M. C. Salvadori and G. S. Kurtz, A rapid and sensitive method for simultaneuous determination of lamivudine and zidovudine in human serum by on-line solid-phase extraction couplet to liquid chromatography/tandem mass spectrometry, Rapid Comm. Mass. Spectrometry., 2004, 18(10), 1147–1155 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here.