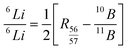Determination of lithium by potentiometry using fluoride ion selective electrode
R.
Govindan
a,
D.
Alamelu
b,
Raju V.
Shah
b,
T. V.
Vittal Rao
b,
Y. R.
Bamankar
b,
A. R.
Parab
b,
K.
Sasi Bhushan
b,
S. K.
Mukerjee
b and
S. K.
Aggarwal
*b
aProduct Development Division, Bhabha Atomic Research Centre, Trombay, Mumbai, 400 085, India
bFuel Chemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai, 400 085, India
First published on 28th September 2010
Abstract
A potentiometric method was developed for the determination of lithium in ethanol medium using ammonium fluoride as titrant and fluoride ion selective electrode for end point detection. The precision of the measurements for samples containing 1 to 3 mg of Li was found to be better than 0.5%. The developed methodology was then employed for the determination of concentration of lithium (Li) in a complex chemical mixture containing hexamethylenetetramine (HMTA), formaldehyde and ammonium nitrate, which are the wash streams generated in the sol–gel process employed for preparation of Li2TiO3 microspheres. The concentration data profile agreed with the isotopic profile obtained by thermal ionization mass spectrometry.
1. Introduction
Determination of elemental concentrations is of great importance in various fields of science and technology. These data are required for chemical quality assurance of various ingredients used in the processing of industrial materials. Elemental determination is also of interest in nuclear industry as a part of the chemical quality assurance of nuclear fuels, for obtaining their fissile content.Sol–Gel process is promising for obtaining ceramic nuclear fuel materials such as UO21,2 as well as non-nuclear materials in the form of microspheres (pebbles). In recent years, Li based ceramics3 have received considerable importance as tritium breeders in Test Blanket Modules (TBMs) for fusion reactors.4,5 Li2O is promising for obtaining suitable tritium breeding ratio (TBR)6 but not used because of its high sensitivity towards moisture. Recently, Li-meta-titanate (Li2TiO3) was proposed as an important material for TBM because of its good chemical stability and possibility of tritium recovery at relatively low temperature.7 Sol–gel process was hence developed for the preparation of Li containing ceramic materials in the form of pebbles of required size and characteristics as well as with the required Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti stoichiometry.8
Ti stoichiometry.8
A simple and fast technique would be desirable for the determination of Li with a view to standardizing the various steps in the sol–gel process. Conventional analytical methods such as ICP-AES, AAS etc. cannot be employed directly in an organic matrix containing HMTA, urea, etc. Methodologies used generally for destruction of organic components cannot be employed in the sol–gel process, since it has been reported that the mixture containing HMTA and NH4NO3 while heating may be explosive in nature.9 An acid–base titration method after separation of Li using cation exchange separation is also possible, but is not suitable as a fast approach. It was, therefore, considered worthwhile to investigate the possibility of employing potentiometric titration for the determination of Li.
Recently, a simple methodology was reported for the stoichiometric analysis of lithium carbonate, a raw material for the solid-state synthesis of lithium metazirconate (Li2ZrO3).10 The reported methodology consisted of decomposition of Li2CO3 sample in hydrochloric acid followed by drying and redissolving in ethanol. Li was determined by titrating with ammonium fluoride in water–ethanol (1 + 1). The results were compared with those obtained from titration with 0.1 M NaOH after cation exchange separation of Li and a precision of 0.3–0.4% was reported.
In the present work, a modified methodology was developed for the determination of lithium based on the precipitation of Li by adding a known excess of NH4F solution, followed by determining excess fluoride using ISE. The method was then adopted for the determination of lithium in the various process streams generated during Li2TiO3 preparation where significant amount of organic constituents are present along with lithium. The Li concentration data in the wash streams was then compared with isotope profile obtained by thermal ionization mass spectrometry (TIMS). The method is simple and rapid and an accuracy of about 0.5% can be achieved for the determination of Li in the range of 1 to 3 mg in the organic matrix.
2. Experimental
2.1. Determination of lithium by potentiometry
A combination fluoride ion selective electrode (M/s. Orion) and an Orion 5-Star ion analyzer were employed to measure the potential developed during the titration. A magnetic stirrer was employed throughout the experiment for continuous stirring of the electrolytic solution during analysis.Synthetic mixtures were prepared employing LiNO3, with and without the organic constituents, to study the effect of the presence of the organic constituents. Furthermore, synthetic mixtures containing varying amounts of Li were prepared in an aqueous solution of 0.5 M NH4OH containing the different organic constituents, namely HMTA (0.75 M) and urea (0.75 M). Concentrations of the different components were higher than those actually used (0.25 M) for gel preparation, to find out interferences, if any, during the determination of Li by this method. All the chemicals used were of A.R. grade.
0.1 mL of each of these standard solutions was added to 15 mL of absolute ethanol which was used as a titration medium. Lithium was precipitated using 0.5M NH4F solution and the end point was detected from the variation in emf measured vs. volume of 0.5 M NH4F. F− ion selective electrode was used to detect the free F−. Titrations were also performed for the sol–gel wash solutions for the determination of lithium in the same manner as above. The titrations were possible only in alcohol medium owing to high solubility of LiF in aqueous medium.11 Though it is reported that the NH4F titrant is to be mixed in ethanol + water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),10 we found that it is sufficient to mix the titrant in pure water.
1),10 we found that it is sufficient to mix the titrant in pure water.
2.2. Preparation of lithium titanate microspheres
The lithium titanate microspheres were obtained by the internal gelation process.8 Natural lithium nitrate and titanium chloride solutions were mixed with 3 M solution containing a mixture of HMTA/urea at 0 °C to obtain feed solution. This feed solution was then dispersed as droplets through a stainless steel capillary of 0.8 to 1.0 mm internal diameter into a glass column in which hot silicone oil pre-heated to 90 °C was circulated. These droplets get hardened (gelled microspheres) as they travel down the column. The gelled microspheres were separated from oil, washed initially with CCl4 (4 to 5 times), to remove the adhering silicone oil, dried and then digested in 1.55 M LiOH at 60 °C in an air oven for 16 to 18 h. Washing with 1.55 M LiOH was done 6 to 7 times to remove the reaction products and unused chemicals from the microspheres to prevent their cracking during sintering. The microspheres were then dried at 100 °C. In order to study the pickup/leaching of Li from the microspheres during the washing, LiOH used for washing was prepared from depleted 6Li.2.3. Determination of 6Li/7Li atom ratio by thermal ionization mass spectrometry (TIMS)
The atom ratios of Li in different wash samples were determined using a Finnigan MAT 261 (Bremen, Germany) thermal ionization mass spectrometer with multiple Faraday cup detectors employing Li2BO2+ ions for analysis12 since simultaneous multicollection using Li+ ions was not possible due to the large relative mass difference in the two isotopes of Li (16%). The sample solution was first mixed with high purity boric acid (NIST-SRM-951) so as to obtain a mole ratio around 2 for B/Li. The solution was then loaded on to the single filament assembly of high purity rhenium filament. The solution was slowly dried at a heating current of 1.5 A for 5 min. The filament current was then increased to 2 A and maintained for 10 min to convert the loaded mixture into Li compound which gives high intensity of Li2BO2+ ions during TIMS analysis. The intensity of the ions corresponding to m/z 56 and 57 were measured by employing static mode of multicollection.3. Results and discussion
In order to ascertain that there is no interference in the determination of lithium due to the presence of organic constituents, potentiometric titrations were carried out using the two solutions of known Li concentration (as LiNO3), one aqueous and the other containing the organics (0.75 M HMTA and 0.75 M urea). It was found that the concentration of Li in the two samples matched within 0.5% of the expected values which proved the absence of any significant interference.Table 1 shows the data on the determination of lithium in synthetic mixtures containing organic components (HMTA, urea). It can be seen that the results agree well with the expected values in the synthetic samples. The precision of the method was also ascertained by repetitive determination of Li in one of the samples and was found to be better than 0.5%. These data are shown in Table 2.
| Sample | Li concentration (M) | A/Ba | |
|---|---|---|---|
| Determined using F− ISE (A) | Expected (B) | ||
| a Average A/B = 1.003 ± 0.006. b Denotes 1σ calculated from three measurements. | |||
| Li-MIX-1 | 0.852 ± 0.004b | 0.84 | 1.014 |
| Li-MIX-2 | 1.001 ± 0.003 | 1.00 | 1.001 |
| Li-MIX-3 | 0.780 ± 0.003 | 0.78 | 1.001 |
| Li-MIX-4 | 0.892 ± 0.004 | 0.89 | 1.002 |
| Li-MIX-5 | 1.795 ± 0.003 | 1. 80 | 0.997 |
The above method was then employed for determining the lithium concentrations in the wash solutions of the sol–gel process of Li2TiO3. As explained earlier, the washing of the microspheres was done using LiOH, the pH of the titration medium varied significantly during the analysis of these samples. We also observed that the Li hydroxide wash solutions containing HMTA were in the pH working range of the fluoride ISE (namely 5 to 7), while those samples without HMTA, the pH was above 11. Since the pH range of the F− ISE's is limited (pH 5 to 7), it was not possible to employ the same approach for all the wash solutions. In order to analyze all samples within the pH range of the fluoride electrode, two methodologies were investigated, (i) adjusting the pH using known volume of 0.5 M HNO3 and (ii) employing a titrant solution containing the buffer solution (TISAB III supplied by M/s. Orion for the F− ISE's). The response of the electrode was sluggish when the ionic strength adjuster was used, which resulted in identification of the end point of titration difficult. So for the wash solutions with pH higher than 11, the pH adjustment was carried out using known amounts of 0.5 M HNO3.
The developed potentiometric method was employed for studying the leaching/pickup of lithium from/by the Li2TiO3 microspheres. Any leaching or pickup that occurs during the washing would change the 6Li/7Li isotope ratio of the wash solution, due to the difference in the isotopic signatures of lithium in the two systems. Fig. 1 shows the data obtained for the successive wash solutions by using F− ISE. It can be seen that washing the microspheres with 1.55 M LiOH, leads to significant leaching of Li+ from the pebbles to the wash liquid at the first stage of washing. Subsequently, small amount of leaching of Li+ from the microspheres (pebbles) was observed, finally reaching to a saturation level.
 | ||
| Fig. 1 Data on Li concentration (M) by ISE and 6Li/7Li atom ratio by TIMS in different wash solutions. | ||
The data on the TIMS analyses of the wash solutions are given in Table 3. The Table also contains the details on the ion current data of the major peak at m/z 57. The isotopic ratio of Li in the sample was then obtained using the formula12
| Sample | Ion intensity (in mV) at m/z 57 | Ion intensity ratio (56/57) | 6Li/7Li atom ratio | Remarks |
|---|---|---|---|---|
| a Note: The isotope ratio of 10B/11B in the samples was taken from the certified value in NIST-SRM-951 (0.2473 ± 0.1%) and was used to calculate 6Li/7Li atom ratio from the observed ion intensity ratio at m/z 56/57. | ||||
| Li-I | 295 | 0.40242 (0.02%) | 0.0776 | Natural Li used for preparation of pebbles |
| Li-II | 355 | 0.33504 (0.1%) | 0.0438 | Depleted 6Li used for washing |
| OD-1 | 33.45 | 0.35902 (2.36%) | 0.0558 | Wash solutions after overnight digestion |
| OD-2 | 298.6 | 0.33830 (0.24%) | 0.0455 | |
| OD-3 | 323.3 | 0.33546 (0.10%) | 0.0440 | |
| OD-4 | 924.4 | 0.33754 (0.06%) | 0.0451 | |
| OD-5 | 122.0 | 0.33517 (0.04%) | 0.0439 | |
| OD-6 | 670.2 | 0.33642 (3.6%) | 0.0446 | |
| OD-7 | 1186.0 | 0.33541 (0.15%) | 0.0440 |
It can be seen from the TIMS data that there is a significant change in the isotopic ratio of lithium in the first two stages of washings, in comparison to that of the solution (Li-II) (depleted lithium) which was used for washing. After two washings, no significant change in the isotopic ratio of Li was observed. Thus the leaching of lithium from pebbles during first two stages of washings was confirmed by employing mass spectrometry data as well. Also, it was confirmed that there is no significant isotopic exchange during the later stages of washings, since no significant change in the isotopic ratio of Li in the wash solutions was observed.
The data on the change in the isotopic ratios in the different wash solutions with respect to that of the stock are also shown in Fig. 1. It can be clearly seen that there is significant leaching of lithium during the first two washings, and it becomes insignificant from the third washing onwards. These studies indicate that further increase in the amount of lithium in wash liquid might be helpful in minimizing the loss of Li from the microspheres as well as leaching of Li from the microspheres. This is essential to maintain the required Li to Ti stoichiometry in the final product.
The results from these experiments demonstrate the usefulness of the present method for lithium determination in complex chemical matrix containing various organic constituents. These studies would be highly useful in the optimization of the washing parameters in sol–gel process for obtaining phase pure lithium titanate pebbles. In addition to maintaining the stoichiometry, the investigations would also be helpful to reduce the loss of enriched 6Li. This is desirable due to the high cost of enriched 6Li as well as the strategic importance of this isotope. The ISE methodology developed in this work is fast and precise and can be employed on a routine basis for lithium determination in solutions containing organic constituents. The same ISE has been in use for nearly three years and there is no significant change in its performance.
Acknowledgements
The authors express their sincere thanks to Dr V.Venugopal, Director, Radiochemistry and Isotope Group at BARC for his interest and support for this work.References
- V. N. Vaidya, S. K. Mukerjee, J. K. Joshi, R. V. Kamat and D. D. Sood, J. Nucl. Mater., 1987, 148, 324–331 CrossRef CAS.
- S. Suryanarayana, N. Kumar, Y. R. Bamankar, V. N. Vaidya and D. D. Sood, J. Nucl. Mater., 1996, 230, 140–147 CrossRef CAS.
- H. Hamilton, J. Nucl. Mater., 1996, 219, 274–283.
- C. E. Johnson, K. R. Kummerrer and E. Roth, J. Nucl. Mater., 1988, 155–157, 188–201 CrossRef.
- C. E. Johnson, J. Nucl. Mater., 1991, 179–181, 42–46 CrossRef CAS.
- D. L. Smith, J. Nucl. Mater., 1984, 122–123, 51–65 CrossRef.
- N. Roux, J. Avon, A. Florencig, J. Mougin, B. R. Asneur and S. Rabel, in Proc. 4th International Workshop on Ceramic Breeder Blanket Interactions, 1995, p. 452 Search PubMed.
- T. V. Vittal Rao, Y. R. Bamankar and S. K. Mukerjee, Proceedings of the XIV International Sol–Gel Conference (ISGC), Montpellier, France, September 2–7, 2007, p. 210.
- Ashok Kumar, T. V. Vittal Rao, S. K. Mukerjee and V. N. Vaidya, J. Nucl. Mater., 2006, 350, 254–263 CrossRef CAS.
- M. Taddia and M. Mattioli, Inorg. Chim. Acta, 2007, 360, 1226–1229 CrossRef CAS.
- C. B. Stubblefield and R. O. Ricardo, J. Chem. Eng. Data, 1972, 17, 491–492 CrossRef CAS.
- B. P. Datta, P. S. Khodade, A. R. Parab, A. H. Goyal, S. A. Chitambar and H. C. Jain, Int. J. Mass Spectrom. Ion Processes, 1992, 116, 87–114 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |