Non-radioactive labelling of calcium oxalate crystals for investigations of crystal-cell interactions and internalization
Sakdithep
Chaiyarit
abc,
Siriwan
Mungdee
a and
Visith
Thongboonkerd
*ac
aMedical Proteomics Unit, Office for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University, 12th Floor Adulyadej Vikrom Building, 2 Prannok Road, Bangkoknoi, Bangkok, 1007, Thailand. E-mail: thongboonkerd@dr.com; vthongbo@yahoo.com; Fax: +66-2-4184793; Tel: +66-2-4184793
bDepartment of Immunology and Immunology Graduate Program, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
cCenter for Research in Complex Systems Sciences, Mahidol University, Bangkok, Thailand
First published on 8th September 2010
Abstract
The study of interactions between renal tubular cells and calcium oxalate (CaOx) crystals and their internalization was limited in the past due to lack of a simple method for visualization of CaOx crystals during such processes. We have developed non-radioactive techniques for efficiently labelling and imaging CaOx crystals in the study of crystal-cell interactions and internalization. A total of 12 ionic dyes, as well as AlexaFluor-488, FITC-conjugated IgG and Cy3-conjugated IgG were used to stain/label CaOx crystals. Thereafter, the crystals were incubated with MDCKcells for 48 h. The crystal images were obtained using light, phase-contrast, fluorescence, or laser-scanning confocal microscopy. The internalized CaOx crystals were finally quantified by flow cytometry. From 12 ionic dyes tested, CaOx monohydrate (COM) crystals were stainable only with CBB-R250 (blue) and Ponceau-S (red), whereas CaOx dihydrate (COD) crystals were stainable only with CBB-R250 (blue) and CBB-G250 (blue), which did not stain COM crystals but transformed them to COD. Additionally, only COM could be labelled and imaged with AlexaFluor-488 (green), FITC-conjugated IgG (green) and Cy3-conjugated IgG (red). Crystal-cell interactions (indicated by interrupted borders of crystals) and adhesion were successfully visualized under a light, phase-contrast, or fluorescence microscope. Moreover, laser-scanning confocal microscopic examination successfully identified internalized crystals, which could be quantified by flow cytometry. These non-radioactive techniques are very simple and effective for labelling and imaging COM and COD crystals for the study of crystal-cell interactions, adhesion and internalization, and will be very useful to investigate mechanisms of kidney stone formation.
Introduction
Calcium oxalate (CaOx) crystals are the most common crystalline compounds found in kidney stone matrices and are frequently observed in the urine of stone formers.1 In addition to kidney stone disease, CaOx crystals are also found in oxalate crystal deposition diseases; e.g., CaOx osteopathy, acute and chronic arthropathy with chondrocalcinosis, synovial calcification, and miliary skin CaOx deposits.2 Also, CaOx crystal formation and function have been widely studied in plants.3,4 Moreover, fetal bovine serum, which is generally used in cell culture, also contains CaOx crystals that may affect the study of molecular biology.5 Therefore, investigation of interactions between CaOx crystals and cells are crucial. CaOx crystals are able to interact with several eukaryotic cells including renal tubular cells, endothelial cells, fibroblasts, leukocytes, erythrocytes and pulmonary cells, leading to inflammation and cell death.6Generally, CaOx crystals are present as three different hydrate types; i.e., CaOx monohydrate (COM), dihydrate (COD) and trihydrate (COT).7 In kidney stone disease, COM crystals are the major components in stone nidus, whereas COD crystals are frequently found in normal human urine.8,9CaOxcrystallization, growth, aggregation and interaction with renal tubular epithelial cells are well known mechanisms of kidney stone formation.6,10 Therefore, many previous and recent studies have focused on the interaction between crystals and renal tubular cells, and have demonstrated that COM and COD crystals can adhere to and be engulfed by these cells.11–13 These processes then lead to cellular injury, alterations in cellular structure, compositions, physiology and gene expression, initiation of DNA synthesis, cell growth, and ultimately cell death.14–17 Moreover, in vitro studies have suggested that tubular cell injury, in turn, increases affinity of crystal-binding, which is a critical process of crystal retention within the kidney.18 Subsequent studies therefore have attempted to investigate crystal-cell interactions.
In the past, several methods had been applied to investigate CaOx crystal adhesion and internalization; e.g., microscopic examinations18,19 and radiolabelling.19 Although COM crystals could be occasionally (not usually) detected by light reflection using a laser-scanning confocal microscope, interference from the glass slide, cover slip and non-specific light reflection could affect such detection. For radiolabelled CaOx crystals,19,20 using a radioactive compound with a long half-life (14C) is an issue of concern and limits its use. Its long half-life (5730 years) is a major concern for accumulation and eradication. Moreover, its carbon compound, which can be easily transferred to any biological system, needs proper waste management to avoid potential effects on living organisms and the atmosphere.21 It is therefore crucial to develop a novel simple technique for imaging CaOx crystals for the study of crystal-cell interactions and internalization.
In the present study, we have developed non-radioactive techniques for labellingCOM and COD crystals using various dyes and labels, which are commonly used in life-science laboratories worldwide, particularly for staining of proteins, SDS-PAGE gels, 2-D PAGE gels, immunoblot membranes, cells, organelles, and microorganisms. Some of these dyes and/or labels could be effectively used for the study of crystal-cell interactions, adhesion and internalization. Moreover, the internalized crystals could be successfully quantified by flow cytometry.
Results and discussion
In our present study, we have developed non-radioactive techniques for labellingCOM and COD crystals for the study of crystal-cell interactions, adhesion and internalization of crystals into the cells. COM and COD were crystallized as described in our previous study.7 To stain or label these CaOx crystals, we incorporated various ionic dyes or labels into crystal matrices during crystallization. For staining, we initially examined many ionic dyes to find the ones that could stain crystals including anionic dyes; i.e., CBB-R250 (USB Corp.), CBB-G250 (USB Corp.) and Ponceau-S (Sigma-Aldrich), as well as cationic dyes; i.e., silver (Fluka), Colloidal Gold (Bio-Rad), Janus Green B (Merck), Neutral Red (Merck), Crystal Violet (Merck), Methylene Blue (Merck), Safranin-O (Merck), and Grams' Iodine (Merck). Among 12 ionic dyes tested, the data showed that only CBB-R250 (in blue) and Ponceau-S (in red) could stain COM crystals (Fig. 1A; left column), whereas COD crystals could be stained only by CBB-R250 (in blue) and CBB-G250 (in blue) (Fig. 1B; left column). All of these are negatively charged anionic dyes, which could simply form ion-pair complexes with positively charged molecules. Therefore, calcium ions in COM and COD crystals were most likely the target molecules to form the detectable complexes with these dyes. The incorporation of these dyes into COM and COD crystals had no effects on the crystal morphology (i.e. monoclinic prismatic shape and twin form of COM and bipyramidal shape of COD). However, while crystal morphology was not changed, the staining pattern of Ponceau-S in COM crystals showed a dumbbell-shape, consistent with a previous study demonstrating that synthetic polypeptides adsorbed preferentially to (121) faces of COM crystals formed in the presence of poly-asp (4–11 g mL−1) under high supersaturation conditions.22 Note that CBB-G250 could not stain COM crystals, but transformed them to COD. This might be due to a chemical effect of CBB-G250 that exhibited inhibitory activity on COM crystals; thus could transform them to COD,23,24 which has less adsorptive capability.25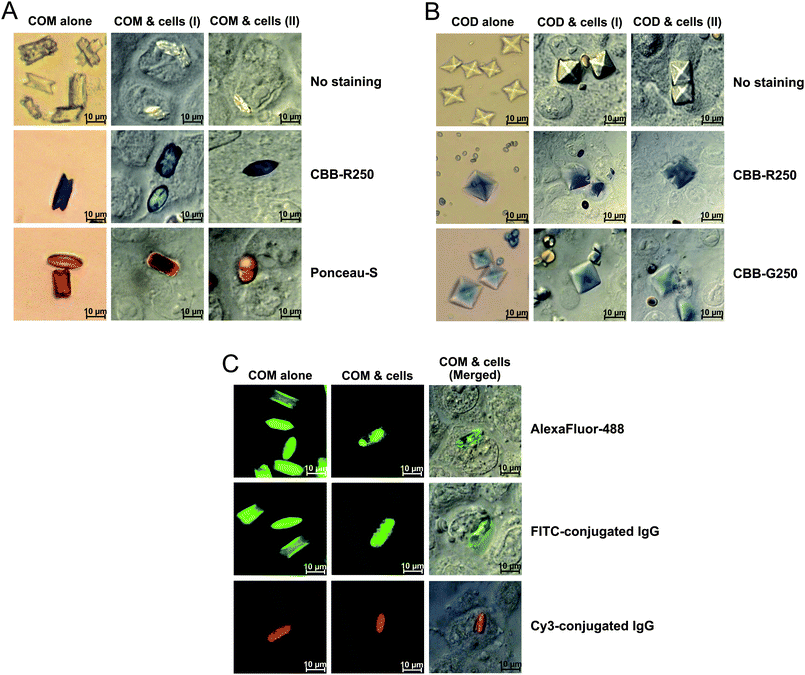 | ||
| Fig. 1 Morphologies and adhesion of stained/labelled CaOx crystals on the surface of renal tubular (MDCK) cells . (A): COM crystals stained with CBB-R250 or Ponceau-S with monoclinic prismatic shape or in twin form. (B): COD crystals stained with CBB-R250 or CBB-G250 with typical bipyramidal shape. (C): COM crystals stained with AlexaFluor-488, or labelled with FITC-conjugated IgG or Cy3-conjugated IgG. In (A) to (C), the left columns demonstrate crystals without cells, whereas the middle and right columns illustrate crystal-cell interactions (with interrupted borders on the interacted or adhered crystals) after 48 h incubation of crystals with MDCKcells (100 μg crystals per mL of culture medium) followed by vigorous washing with PBS three times to remove the non-adherent crystals. The images in the left columns of (A) and (B) were taken from Olympus CKX41 (Olympus Co. Ltd.), whereas those in the middle and right columns of (A) and (B) and all panels of (C) were taken from Nikon ECLIPSE 80i (Nikon Corp.; Tokyo, Japan) with differential interference contrast (DIC) mode. | ||
For fluorescent dye staining, we used the green fluorescent AlexaFluor-488 dye (Molecular Probes), which is spectrally similar to fluorescein. This fluorescent dye is brighter and more photostable than fluorescein. In the present study, the data showed that only COM crystals could be stained with AlexaFluor-488 dye (Fig. 1C; upper panel of left column), consistent with a previous study, which has demonstrated that fluorescein binds selectively to (101) faces of COM crystals.26
For labelling, we employed FITC-conjugated IgG (in green) and Cy3-conjugated IgG (in red). We used IgG because our preliminary study showed that mammalian IgG could bind to COM crystals (unpublished data), consistent with the findings in previous studies demonstrating that IgG could be identified in COM kidney stone matrices.27 In our present study, the data revealed that only COM could be labelled by these fluorescent labels (Fig. 1C; middle and lower panels of left column). This might be due to the more potent adsorptive capability of COM crystals as compared to COD crystals.25
To investigate crystal-cell interactions and adhesion, we incubated COM and COD crystals with MDCKcells. Fig. 1A–1C (middle and right columns) demonstrate that the crystals were retained on the cellular surface after 48 h incubation followed by vigorous washing with PBS three times, indicating crystal adhesion on the cell surface. The data also revealed interrupted borders of adherent crystals (normally COM and COD crystals have very sharp borders), indicating crystal-cell interactions (we used the term “interactions” because crystals have some biochemical effects to the cells, which in turn affect crystals).28,29 Indeed, several recent studies have simply applied a fluorescence microscope to visualize adherent plain COM crystals.18 Although some plain COM crystals could be occasionally visualized or detected based on their light reflection by the 633-nm Kr-laser, there are several factors that could easily limit such detection/visualization. For example, there are some interferences from the glass slide, cover slip, other crystals (e.g.uric acid crystals), and non-specific light reflection.18 Moreover, COM crystals must be in the right orientation for effective light reflection to be visualized; otherwise, they are invisible by fluorescence microscopy. These limitations had made analysis of crystal-cell interactions and adhesion difficult in the past. In our present study comparing plain (non-stained/non-labelled) crystals to stained/labelled crystals, the stained/labelled crystals offered clearer images of crystal-cell interactions and adhesion, thus will be beneficial for data interpretation in further functional studies. Moreover, the staining and labelling will be also useful for the investigation of CaOxcrystallization, degradation and transformation.
However, it should be noted that light, phase-contrast and fluorescence microscopes could not clearly discriminate extracellular crystals from internalized crystals. We therefore employed a laser-scanning confocal microscope to visualize and analyze the internalized crystals. The cells were incubated with fluorescence-stained COM crystals and fluorescence-labelled COM crystals for 48 h and the non-adherent crystals were removed by vigorous washing with PBS three times. Among the remaining crystals, the extracellular crystals were eliminated by detaching and dissolving with trypsin/EDTA solution. After these vigorous washing and dissolving steps, we successfully identified some internalized COM crystals as illustrated in Fig. 2. However, it was not simple to quantify the percentage of cells with internalized crystals compared to total number of cells using a laser-scanning confocal microscope.
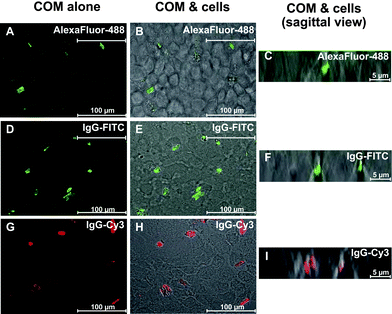 | ||
| Fig. 2 The internalization of fluorescent COM crystals into MDCK cells. After MDCKcells were incubated with fluorescent COM crystals (100 µg crystals per mL of culture medium) for 48 h, the non-adherent crystals were removed by vigorous washing with PBS three times, whereas the adherent crystals were finally detached and/or dissolved with trypsin/EDTA solution. (A, D and G): Fluorescent COM crystals without cells. (B, E and H): Internalized crystals (remaining crystals after removal of non-adherent and adherent (extracellular) crystals). (C, F and I): The internalized crystals are clearly illustrated with sagittal view of confocal sections. All panels were taken from a laser-scanning confocal microscope equipped with LSM5 Image Browser (LSM 510 META, Carl Zeiss; Oberkochen, Germany). | ||
In many previous studies, quantitative data of adherent and internalized cells were obtained by using COM crystals labelled with (14C)-oxalic acid.18,19,30 However, the use of a radioisotope with a long-half-life might be a drawback. In another study, a flow cytometric analysis was performed to measure endocytic activity of renal tubular cells after incubation with COM crystals, using the increasing side scatter of the cells to indirectly infer crystal internalization.31 However, this approach is not precise and is frequently erroneous. Therefore, we combined the high-throughput manner of flow cytometry with the specificity of fluorescence-stained/labelled COM crystals to quantify the internalized crystals and percentage of cells with internalized crystals. Fig. 3A shows a comparative analysis of fluorescence signal intensity obtained from plain COM crystals compared to that obtained from the fluorescence-labelled COM crystals (using COM crystals labelled with FITC-conjugated IgG as the representative). From the same gate “R1” shown in the left panels, the fluorescent COM crystals had markedly greater fluorescence signal intensity compared to the plain crystals (“M1” in the right panels). In addition, the percentage of the crystal particles with fluorescence signal intensity above the threshold level was much greater in the fluorescent COM crystals compared to the plain crystals (99.18 ± 0.02 vs. 1.85 ± 0.71%; p < 0.0001) (note that 1.85 ± 0.71% of the plain crystals represented the background of the flow cytometric analysis).
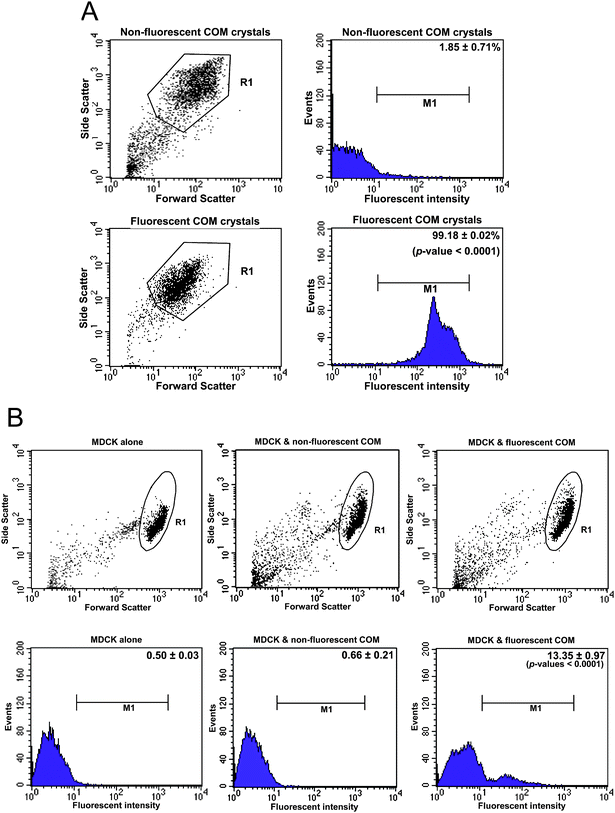 | ||
| Fig. 3 Quantitative analysis of the internalized fluorescent COMcrystals. COM crystals labelled with FITC-conjugated IgG were used as the representative for fluorescent crystals. After MDCKcells were incubated with fluorescent COM crystals (100 μg crystals per mL of culture medium) for 48 h, the non-adherent crystals were removed by vigorous washes with PBS three times, whereas the adherent crystals were finally detached and/or dissolved with trypsin/EDTA solution. (A): Dot plots and histograms of fluorescent and non-fluorescent plain COM crystals. Fluorescence intensities of COM crystals in “R1” gate in the dot plots were obtained and are present as histograms in the right panels. Percentages of fluorescent and plain crystals with fluorescence intensities above the threshold (indicated as “M1”) were then compared (99.18 ± 0.02 vs. 1.85 ± 0.71%, respectively; p < 0.0001). (B): Dot plots and histograms of MDCKcells with or without internalized fluorescent COM crystals. Fluorescence intensities of MDCKcells in “R1” gate in the dot plots were obtained and are present as histograms in the lower panels. Percentages of MDCKcells with fluorescence intensities above the threshold (indicated as “M1”) were then compared (13.35 ± 0.97 vs. 0.50 ± 0.03% and 0.66 ± 0.21% for cells with internalized fluorescent COM crystals vs. controlled cells and cells with plain crystals, respectively; p < 0.0001). Note that all the data in this figure were taken from a flow cytometer (FACScan, Becton Dickinson Immunocytometry System; San Jose, CA). | ||
After the MDCKcells were incubated with fluorescent COM crystals for 48 h, the non-adherent crystals were removed by vigorous washing with PBS three times, whereas the adherent crystals were finally detached and/or dissolved with trypsin/EDTA solution. The internalized crystals were then successfully quantified by flow cytometry using the untreated MDCKcells (without crystals) and cells with plain crystals as the controls (Fig. 3B). From the same gate “R1” in the left panels, the percentage of cells with the internalized crystals (“M1” in the right panels) could be efficiently obtained (13.35 ± 0.97 vs. 0.50 ± 0.03% and 0.66 ± 0.21% for cells with internalized fluorescent COM crystals vs. controlled cells and cells with plain crystals, respectively; p < 0.0001) (note that 0.50 ± 0.03% in the controlled cells represented the background of the flow cytometric analysis).
In conclusion, we have developed novel, simple and effective techniques for labelling and imaging COM and COD crystals for the study of crystal-cell interactions, adhesion and internalization. The crystal internalization into the cells was successfully demonstrated by a laser-scanning confocal microscope, and could be efficiently quantified by flow cytometric analysis. Our methods will therefore be very useful for further functional studies on crystal cell interactions, adhesion and internalization and may lead to a better understanding of molecular mechanisms of stone formation in kidney stone disease.
Materials and methods
COM crystal preparation and labelling
COM crystals were prepared as described previously.7 Briefly, 5 mM CaCl2·2H2O was mixed with 0.5 mM Na2C2O4 in a buffer containing 90 mM Tris-HCl and 10 mM NaCl (pH 7.4). The solutions were incubated at 25 °C overnight and COM crystals were harvested by centrifugation at 2000 × g for 5 min. The supernatant was discarded and COM crystals were resuspended in methanol. After another centrifugation at 2000 × g for 5 min, methanol was discarded and the crystals were air-dried overnight at room temperature. For staining/labelling, COM crystals were crystallized in the presence of 2.25 μg mL−1 CBB-R250 (USB Corp.; Cleveland, OH), 22.5 μg mL−1 Ponceau-S (Sigma-Aldrich; St. Louis, MO), 0.11 μg mL−1AlexaFluor-488 (Invitrogen/Molecular Probes; Burlington, Canada), 0.58 μg mL−1 rabbit anti-mouse IgG conjugated with FITC (DAKO; Glostrup, Denmark), or 0.01 μg mL−1 goat anti-mouse IgG conjugated with Cy3 (Jackson Immunoresearch Laboratories, Inc.; West Grove, PA). COM crystals were imaged by light microscopy, phase-contrast microscopy (Olympus CKX41, Olympus Co. Ltd.; Tokyo, Japan) or fluorescence microscopy (Nikon ECLIPSE 80i, Nikon Corp.; Tokyo, Japan). Note that all of the reagents and dyes/labels were sterilized before use for subsequent intervention with cells.COD crystal preparation and labelling
COD crystals were prepared as described previously.7 Briefly, 25.08 mM CaCl2·2H2O was added into a buffer containing 19.26 mM trisodium citrate dihydrate (C6H5Na3O7·2H2O), 23.1 mM magnesium sulfate heptahydrate (MgSO4·7H2O) and 127.4 mM potassium chloride (KCl). The pH of the mixture was then adjusted to 6.5 and the mixture was then further incubated at 25 °C for 15 min. Thereafter, 6.4 mM sodium oxalate (Na2C2O4) with or without dyes (2.4 mg mL−1 CBB-R250 or 8 mg mL−1 CBB-G250) (USB Corp., Cleveland, OH) was added into the mixture with gentle continuous stirring. After incubation overnight at 25 °C, COD crystals were harvested by centrifugation at 2000 × g for 5 min. The supernatant was discarded and the crystals were resuspended in methanol. After another centrifugation at 2000 × g for 5 min, methanol was discarded and the crystals were air-dried overnight at room temperature. COD crystals were imaged by light microscopy or phase-contrast microscopy (Olympus CKX41, Olympus Co. Ltd.). Note that all of the reagents and dyes/labels were sterilized before use for subsequent intervention with cells.MDCK cell culture
Madin-Darby Canine Kidney (MDCK) cells were grown with complete Eagle's minimum essential medium (MEM) (GIBCO, Invitrogen Corporation; Grand Island, NY) supplemented with 10% fetal bovine serum, 1.2% penicillin G/streptomycin and 2 mM glutamine in 75 cm2 tissue culture flasks. The cultured cells were maintained in a humidified incubator at 37 °C with 5% CO2.Crystal-cell interactions and adhesion
For the evaluation of COM and COD crystal-cell interactions and adhesion, MDCKcells (approximately 7.5 × 104cells) in MEM were subcultured on a coverslip (cleaved mica disk diameter: 9.5 mm, V-1 grade, SPI Supplies; Toronto, Canada). The cells were maintained at 37 °C with 5% CO2 for 24 h in a humidified incubator. Thereafter, the cells were washed with phosphate buffered saline (PBS) to eliminate detached cells and the culture medium was replaced by plain (non-stained/non-labelled) or stained/labelled crystals in MEM (100 μg of crystals/mL of medium). The cells were grown further for 48 h. The non-adherent crystals were then removed by three washes with PBS. The coverslip was then mounted with 50% glycerol, and crystal-cell interactions and adhesion were then examined under a light, phase-contrast, or fluorescence microscope with differential interference contrast (DIC) mode.COM crystal internalization
The cells were prepared as aforementioned for the investigation of crystal-cell interactions and adhesion. After washing with PBS three times, extracellular and/or intracellular localization of fluorescence-stained/labelled (with AlexaFluor-488, FITC-conjugated IgG, or Cy3-conjugated IgG) COM crystals were visualized by a laser-scanning confocal microscope equipped with LSM5 Image Browser (LSM 510 META, Carl Zeiss; Oberkochen, Germany).For quantitative analysis of internalized crystals by flow cytometry, approximately 3 × 106MDCKcells were cultured in 75 cm2 tissue culture flasks. The cells were maintained in a humidified incubator at 37 °C with 5% CO2 for 24 h and then treated with fluorescence-stained/labelled (with AlexaFluor-488, FITC-conjugated IgG, or Cy3-conjugated IgG) COM crystals (100 μg mL−1). After a subsequent 48 h incubation, the cells were washed with PBS and incubated with trypsin-EDTA solution to eliminate non-internalized (both adherent and non-adherent crystals) COM crystals. The internalized crystals were then quantified as compared to the blank control (MDCK alone without crystals) and cells with plain crystals using a flow cytometer (FACScan, Becton Dickinson Immunocytometry System; San Jose, CA).
Statistical analysis
Quantitative data are present as mean ± SEM. Comparisons of the two data sets (i.e. fluorescence intensity of plain vs. fluorescence-labelled crystals; % cells with internalized fluorescent crystals of controlled cellsvs. fluorescent crystal-treated cells) were performed by unpaired Student's t test (SPSS version 13.0). P-values less than 0.05 were considered statistically significant.Abbreviations
| CaOx | calcium oxalate |
| CBB | Coomassie Brilliant Blue |
| COD | calcium oxalate dihydrate |
| COM | calcium oxalate monohydrate |
| HSA | human serum albumin |
| MDCK | Madin-Darby Canine Kidney |
| MEM | minimum essential medium |
Acknowledgements
This study was supported by Mahidol University, The Thailand Research Fund, Commission on Higher Education, and National Research Council of Thailand (to VT). SC is supported by Siriraj Graduate Thesis Scholarship. VT is also supported by “Chalermphrakiat” Grant, Faculty of Medicine Siriraj Hospital.References
- F. L. Coe, A. Evan and E. Worcester, J. Clin. Invest., 2005, 115, 2598–2608 CrossRef CAS.
- I. Maldonado, V. Prasad and A. J. Reginato, Curr. Rheumatol. Rep., 2002, 4, 257–264 Search PubMed.
- M. A. Webb, Plant Cell, 1999, 11, 751–761 CAS.
- V. R. Franceschi and P. A. Nakata, Annu. Rev. Plant Biol., 2005, 56, 41–71 CrossRef CAS.
- C. E. Pedraza, Y. C. Chien and M. D. McKee, J. Cell. Biochem., 2008, 103, 1379–1393 CrossRef CAS.
- C. F. Verkoelen and A. Verhulst, Kidney Int., 2007, 72, 13–18 CrossRef CAS.
- V. Thongboonkerd, T. Semangoen and S. Chutipongtanate, Clin. Chim. Acta, 2006, 367, 120–131 CrossRef CAS.
- J. S. Elliot and I. N. Rabinowitz, J. Urol., 1980, 123, 324–327 Search PubMed.
- X. Sheng, M. D. Ward and J. A. Wesson, J. Am. Soc. Nephrol., 2005, 16, 1904–1908 CrossRef CAS.
- J. R. Asplin, J. H. Parks, M. S. Chen, J. C. Lieske, F. G. Toback, S. N. Pillay, Y. Nakagawa and F. L. Coe, Kidney Int., 1999, 56, 1505–1516 CrossRef CAS.
- J. C. Lieske, B. H. Spargo and F. G. Toback, J. Urol., 1992, 148, 1517–1519 Search PubMed.
- J. C. Lieske, M. M. Walsh-Reitz and F. G. Toback, Am. J. Physiol, 1992, 262, F622–F630 CAS.
- J. C. Lieske, F. G. Toback and S. Deganello, Calcif. Tissue Int., 1996, 58, 195–200 CAS.
- M. S. Schepers, E. S. van Ballegooijen, C. H. Bangma and C. F. Verkoelen, Kidney Int., 2005, 68, 1543–1553 CrossRef.
- S. R. Khan, K. J. Byer, S. Thamilselvan, R. L. Hackett, W. T. McCormack, N. A. Benson, K. L. Vaughn and G. W. Erdos, J. Am. Soc. Nephrol., 1999, 10(Suppl. 14), S457–S463 CAS.
- M. S. Hammes, J. C. Lieske, S. Pawar, B. H. Spargo and F. G. Toback, Kidney Int., 1995, 48, 501–509 CrossRef CAS.
- A. Bhandari, S. Koul, A. Sekhon, S. K. Pramanik, L. S. Chaturvedi, M. Huang, M. Menon and H. K. Koul, J. Urol., 2002, 168, 253–259 CrossRef CAS.
- C. F. Verkoelen, B. G. van der Boom, A. B. Houtsmuller, F. H. Schroder and J. C. Romijn, Am. J. Physiol, 1998, 274, F958–F965 CAS.
- M. S. Schepers, R. A. Duim, M. Asselman, J. C. Romijn, F. H. Schroder and C. F. Verkoelen, Kidney Int., 2003, 64, 493–500 CrossRef CAS.
- V. Kumar, S. Yu, G. Farell, F. G. Toback and J. C. Lieske, Am. J. Physiol.: Renal Physiol., 2004, 287, F373–F383 CrossRef CAS.
- T. L. Yankovich, J. Koarashi, S. B. Kim and P. A. Davis, Appl. Radiat. Isot., 2008, 66, 1726–1729 CrossRef CAS.
- A. Taller, B. Grohe, K. A. Rogers, H. A. Goldberg and G. K. Hunter, Biophys. J., 2007, 93, 1768–1777 CrossRef CAS.
- T. Jung, W. S. Kim and C. K. Choi, Mater. Sci. Eng., C, 2004, 24, 31–33 CrossRef.
- B. B. Tomazic and G. H. Nancollas, Invest. Urol., 1979, 16, 329–335 Search PubMed.
- B. B. Tomazic and G. H. Nancollas, J. Urol., 1982, 128, 205–208 Search PubMed.
- L. A. Touryan, R. H. Clark, R. W. Gurney, P. S. Stayton, B. Kahr and V. Vogel, J. Cryst. Growth, 2001, 233, 380–388 CrossRef CAS.
- B. Dussol, S. Geider, A. Lilova, F. Leonetti, P. Dupuy, M. Daudon, Y. Berland, J. C. Dagorn and J. M. Verdier, Urol. Res., 1995, 23, 45–51 CrossRef CAS.
- V. Thongboonkerd, T. Semangoen, S. Sinchaikul and S. T. Chen, J. Proteome Res., 2008, 7, 4689–4700 CrossRef CAS.
- T. Semangoen, S. Sinchaikul, S. T. Chen and V. Thongboonkerd, J. Proteome Res., 2008, 7, 2889–2896 CrossRef CAS.
- J. C. Lieske, R. Norris, H. Swift and F. G. Toback, Kidney Int., 1997, 52, 1291–1301 CrossRef CAS.
- F. T. Borges, Y. M. Michelacci, J. A. Aguiar, M. A. Dalboni, A. S. Garofalo and N. Schor, Kidney Int., 2005, 68, 1630–1642 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
