Synchronized detection and removal of thiols using a Gd(OH)3 nanosheet-sodium pentacyanonitrosylferrate(II) complex†
Vyom
Parashar
* and
Avinash C.
Pandey
Nanophosphor Application Centre, Faculty of Science, University of Allahabads, Allahabad, 211002, India. E-mail: vyomparashar@gmail.com; Fax: +91(532)2460675; Tel: +91(532)2460675
First published on 9th July 2010
Abstract
We described a novel procedure for visible detection and separation of thiols and disulfides. The method uses a Gd(OH)3 nanosheets–sodium pentacyanonitrosylferrate(II) paramagnetic complex which produce an intermediate chromophore when coupled with a sulfur containing analyte, which is detectable with the naked eye and simultaneously can be removed by applying an external magnetic field.
Instant detection of sulfur containing compounds especially thiols is of central importance in innumerable applications such as, chemical warfare agents, petroleum, coal, natural gas processing, sewage treatment plants, pharmaceutical, food, drinking water, clinical diagnostics and environmental laboratories.1 Depending upon the need some applications only require detection whereas a few demand separation of the thiol compounds (or sulfhydryl derivatives) apart from the detection. For example, in clinical diagnostics rapid detection of glutathione, cysteine and cystine is required whereas the removal of sulfur analyte from gasoline fractions is necessary due to new environmental regulations.2 The economics of fuels and diagnostics can be regulated if new simple procedures are developed to detect and remove sulfur compounds, because many of these compounds have commercial value. Several strategies encompassed for the detection of sulfur compounds include: high-performance liquid chromatography, commercial detectors, fluorescence probes, microchips, gold nanoparticles decorated carbon nanotubes, electrochemical detection, quantum dots, X-ray absorption spectroscopy, paper chromatography and commercial kits are being utilized;3 whereas hydrodesulfurization is effective for removing sulfur from certain petroleum feedstocks. The aforementioned techniques for the determination of thiols usually suffer from a few substantial difficulties in terms of time, equipment cost, complexity, sample processing and environment toxic materials.
Gd(OH)3 of various shapes (nanotubes, nanorods, nanowires and nanobundles) is predominantly used as a precursor of Gd2O34,5. However, in contrast to the many available reports on the applications of Gd2O3 we found only one report by Byeon et al. for the application of Gd(OH)3 nanosheets as a potential MRI contrast agent.6 Here we demonstrate a novel application for Gd(OH)3 nanosheets by fabricating a solid state complex with sodium pentacyanonitrosylferrate(II) (SPII) for visual detection and removal of thiols. To the best of our knowledge this work is the first to report Gd(OH)3 nanosheets application for analytical procedures. Out of so many available structures for Gd(OH)3, nanosheets was an obvious choice due to its large surface area which can sorb a high payload of thiols. This complex will be of fundamental importance not only for first-line screening of sulfur containing compounds but for easy removal from various industrial concoctions as a consequence of its low cost, synthetic convenience and benignancy to environment.
Our approach is directed towards the simultaneous determination and removal of thiols or disulfides in which a sulfur containing analyte is combined with the [Gd(OH)3/SPII] nanosheets complex to produce an intermediate. The intermediate comprises a chromophore and is detectable with the naked eye and simultaneously can be removed from the solution by applying an external magnetic field (Fig. 1a and c) (Supporting Information MPEG file).†Fig. 1b shows the colour appearance with the [Gd(OH)3/SPII] nanosheets complex on a microtiter plate with various thiols at a concentration of 200 μM. Water was used as a negative control. Interestingly, with this system different sulfur analytes develop different colors. The developed color is stable for more than 24 h at room temperature, and the concentration dependent color intensity is reproducible over a wide range of concentrations.
![a) Synchronized detection and removal of sulfur analyte using the [Gd(OH)3/SPII] nanosheets complex. b) Visual detection of various sulfur analytes (1 mM) with the [Gd(OH)3/SPII] nanosheets complex. c) Glutathione (1 mM) was detected by the nanosheets complex and simultaneously attracted towards the magnetic poles when a permanent magnet was applied externally.](/image/article/2010/AY/c0ay00366b/c0ay00366b-f1.gif) | ||
| Fig. 1 a) Synchronized detection and removal of sulfur analyte using the [Gd(OH)3/SPII] nanosheets complex. b) Visual detection of various sulfur analytes (1 mM) with the [Gd(OH)3/SPII] nanosheets complex. c) Glutathione (1 mM) was detected by the nanosheets complex and simultaneously attracted towards the magnetic poles when a permanent magnet was applied externally. | ||
Layered gadolinium hydroxides (LGdH) have been synthesized via various routes utilizing water, formamide and oleate ions.5,6 Further it has been noticed that the phase stability is more or less variable depending on the hydration state.4 Particles in the colloidal LGdH solution were investigated by transmission electron microscopy (TEM) as shown in Fig. 2c–d and indicate multiple overlap of faint sheet-like objects of lateral sizes 130 (±35) nm. Further, an X-ray diffraction pattern of the samples can be well indexed to the hexagonal phase of Gd(OH)3 (JCPDS No. 83-2037). Moreover, sharp diffraction peaks indicate good crystallinity of Gd(OH)3 at low temperatures (Fig. 2a). Electron diffraction (selected area electron diffraction, SAED) patterns are (Fig. 2b) in good agreement with previous literature.4 The exact growth process of Gd(OH)3 of different morphology is still unclear. Growth mechanisms for formation of nanotube and nanorods have been proposed to depend on temperature, pH and different alkaline source. Interestingly, though our method is similar to what Guang et al. have reported for fabricating Gd(OH)3 nanotube and nanorods, we successfully fabricated nanosheets by keeping the concentration of GdCl3·6H2O very low (0.5 mmol) and by the addition of ammonia solution at short tammonia (as short as 1 s by pouring the entire ammonia solution into the chloride solution) and low temperature (75 °C) (solution was stirred continuously for 16 h). It has been noticed that the diffusion rate of Gd(OH)3 on the edges of the seeds determine the morphology of the final products.7 Guang and co-workers were able to fabricate nanotubes at 75 °C but in our study we found that the role of temperature is significant only at higher concentrations of precursor salt. In this case, low concentration of precursor salt slows down the diffusion rate and could not provide enough atoms for the growth of solid rod or tube like crystal. This would lead to undersaturation in the central part of the growing regions of the surface whereas an instant addition of excess ammonia enhances the concentration of Gd(OH)3 on the diffusion layer. Hence, it is rational to speculate that combining the difference of low concentration of Gd ions versus the high diffusion rate of Gd(OH)3 on the nanoparticle seeds, crystalline sheet like nanostructures can be grown. The chemical composition of LGdH is [Gd2(OH)5(H2O)1.5]Cl. The thermogravimetric curve of LGdH is shown in Fig. 3. In view of adsorbed water on the large surface of the LGdH nanosheets, the observed weight loss below 200 °C is 6.23% which is close to the 5.85% calculated loss from the dehydration of [Gd2(OH)5(H2O)1.5]Cl. An observed total ignition loss of 20.2%, which is close to the calculated loss of 21.54%, supports this composition.
 | ||
| Fig. 2 a) XRD patterns of the Gd(OH)3 nanosheets. The standard data for Gd(OH)3 (JCPDS No. 83-2037) is also presented in the figure (red) for comparison. b) Selected area electron diffraction (SAED) pattern of Gd(OH)3 nanosheets. Low (c) and high (d) magnification of TEM images of Gd(OH)3 nanosheets. | ||
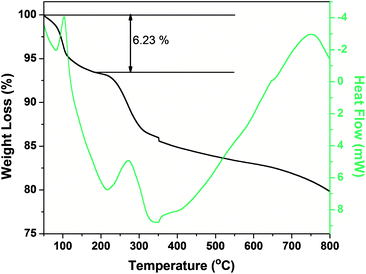 | ||
| Fig. 3 Thermogravimetric analysis curve of Gd(OH)3 nanosheets. | ||
Early monitoring of amino acids, proteins and polypeptides containing sulfhydryl derivatives (e.g., glutathione, methionine, homocysteine and cysteine) in various biological samples (e.g. human blood serum, urine, tissues and tumors) of patients is of foremost importance due to their contribution to several severe human diseases such as Alzheimer’s disease, cardiovascular disease, neural tube defect, inflammatory bowel disease, osteoporosis, retarded growth, hair depigmentation, lethargy, liver damage, muscle and fat loss, and skin lesions or metabolic disorders which manifest themselves in hypercystinuria, cystinlithiasis or hyperhomocystinuria.8,9 Sodium pentacyanonitrosylferrate(II) is widely used for qualitative and quantitative biochemical analysis.10 Sodium pentacyanonitrosylferrate(II) based tests although well characterized and established in clinical routine procedures, suffer from several disadvantages: first, the developed color lasts only a few minutes, second, a few of the co-reagents used (e.g. NaCN) are highly toxic and finally and most importantly, the method is insensitive to levels lower than 500 μM of the thiol compound.10 Where as, we found the [Gd(OH)3/SPII] nanosheets complex to be sensitive enough up to 50 μM for various sulfhydryl derivatives. Glutathione can be visually detected upto 50 μM in the presence of the [Gd(OH)3/SPII] nanosheets complex (see Fig. 4a, water is taken as a negative control). For detecting and quantifying disulfides such as cystine, a variety of reducing agents can be used which do not interfere with the final results such as TCEP or NaBH4. After thoroughly mixing these reducing agents the procedure is identical to the procedure adopted for glutathione (data not shown). To further demonstrate the advantages of the [Gd(OH)3/SPII] complex, we explore the possibility to remove sulfur analyte restrain on the nanosheets complex from the fluids. Recently, Stefanakis et al. have shown that Gd(OH)3 is paramagnetic at room temperature.11 The unique combination of paramagnetic Gd(OH)3 nanosheets and the SPII complex prompts us to embark on simultaneous removal of sulfur analyte apart from visual detection. Herein, the [Gd(OH)3/SPII] system did not only detect sulfur analytes within seconds, but also removed them from the solution when placed in an magnetic field. To a 2 ml vial containing 100 mM solution of glutathione, a constant concentration of the [Gd(OH)3/SPII] complex was added at room temperature with gentle shaking. After incubation, the samples were left on a permanent external magnet for different time intervals (5, 10, 20, 30, 40, 50 and 60 min). The supernatant was carefully removed by pipetting and was centrifuged to remove any remaining [Gd(OH)3/SPII] complex. Further, supernatant absorbance spectra was recorded (Fig. 4b). It is evident from Fig. 4b that maximum removal occurred in the first 5 min after which the absorbance peaks were found to be insignificantly changing. This is may be due to the saturation of the surface of the nanosheets exposed to the glutathione. This observation indicates that maximum removal of sulfur analyte will depend upon the concentration of the [Gd(OH)3/SPII] complex used.
![a) Concentration dependent color intensity of the [Gd(OH)3/SPII] nanosheets complex with various amounts of glutathione. b) Glutathione removal efficiency of the [Gd(OH)3/SPII] nanosheets complex monitored at various time intervals in the presence of an external electromagnetic field.](/image/article/2010/AY/c0ay00366b/c0ay00366b-f4.gif) | ||
| Fig. 4 a) Concentration dependent color intensity of the [Gd(OH)3/SPII] nanosheets complex with various amounts of glutathione. b) Glutathione removal efficiency of the [Gd(OH)3/SPII] nanosheets complex monitored at various time intervals in the presence of an external electromagnetic field. | ||
To summarize, a novel, single step, environment friendly, time and cost effective method has been developed for the detection and removal of sulfhydryl derivatives. The method disclosed herein can find application in various realms for example petroleum industries, clinical diagnostics, purifying water, pharmaceuticals and environment laboratories. This method can be developed as a portable analyzers for researchers working in fields where they need to collect and get the fundamental information regarding the presence of sulfur compounds instantly. Our further research efforts will be focused on developing methods for the recovery of the sulfur analytes from the [Gd(OH)3/SPII] complex.
This work was supported by Department of Science and Technology, Government of India.
Notes and references
- (a) R. A. Meyers in Encyclopedia of Analytical Chemistry, J. Wiley and Sons, Chichester, 2000, pp. 899 Search PubMed; (b) G. Douglas and B. J. Mair, Science, 1965, 147, 499 CrossRef; (c) V. P. Hanko, W. R. Lacourse, C. O. Dasenbrock and J. S. Rohrer, Drug Dev. Res., 2001, 53, 268 CrossRef CAS; (d) X. Fan, C. H. Sommers, D. W. Thayer and S. J. Lehotay, J. Agric. Food Chem., 2002, 50, 4257 CrossRef CAS; (e) World Health Organization, Guidelines for drinking-water quality, Vol. 2 (Eds: 2), Geneva, 1996 Search PubMed; (f) A. B. Rogers, K. S. Cormier and J. G. Fox, Lab. Invest., 2006, 86, 526 Search PubMed; (g) D. Gabriel and M. A. Deshusses, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6308 CrossRef CAS.
- Can be found under http://www.access.gpo.gov/, Document ID fr18ja01R.
- (a) H. P. Tuan, H. G. M. Janssen, C. A. Cramers, E. M. K. Loo and H. Vlap, J. High Resolut. Chromatogr., 1995, 18, 333 CrossRef; (b) L. Yi, H. Li, L. Sun, L. Liu, C. Zhang and X. Zhen, Angew. Chem., Int. Ed., 2009, 48, 4034 CrossRef CAS; (c) W. Jiang, Q. Fu, H. Fan, J. Ho and W. Wang, Angew. Chem., Int. Ed., 2007, 46, 8445 CrossRef CAS; (d) T. Revermann, S. Götz and U. Karst, Electrophoresis, 2007, 28, 1154 CrossRef CAS; (e) S. Mubeen, T. Zhang, N. Chartuprayoon, Y. Rheem, A. Mulchandani, N. V. Myung and M. A. Deshusses, Anal. Chem., 2010, 82, 250 CrossRef CAS; (f) W. Wang, L. Li, S. Liu, C. Ma and S. Zhang, J. Am. Chem. Soc., 2008, 130, 10846 CrossRef CAS; (g) Y. Zhang, Y. Li and X. P. Yan, Anal. Chem., 2009, 81, 5001 CrossRef CAS; (h) http://probes.invitrogen.com/ ; (i) C. L. Spiro, J. Wong, F. W. Lytle, R. B. Greegor, D. H. Maylote and S. H. Lamson, Science, 1984, 226, 48 CAS; (j) H. M. Winegard, G. Toennies and R. J. Block, Science, 1948, 108, 506 CrossRef CAS.
- G. Jia, K. Liu, Y. Zheng, Y. Song, M. Yang and H. You, J. Phys. Chem. C, 2009, 113, 6050 CrossRef CAS.
- K. H. Lee, B. Lee, J. H. You and S. H. Byeon, Chem. Commun., 2010, 46, 1461–1463, 10.1039/b922612e.
- B. I. Lee, K. S. Lee, J. H. Lee, I. S. Lee and S. H. Byeon, Dalton Trans., 2009,(14), 2490 RSC.
- Y. B. Mao, J. Y. Huang, R. Ostroumov, K. L. Wang and J. P. Chang, J. Phys. Chem. C, 2008, 112, 2278 CrossRef CAS.
- X. Chen, S. K. Ko, M. J. Kim, I. Shin and J. Yoon, Chem. Commun., 2010, 46, 2751 RSC.
- Y. Fu, H. Li, W. Hu and D. Zhu, Chem. Commun., 2005, 3189–3191 RSC.
- V. N. Bernshtein and V. G. Belikov, Russ. Chem. Rev., 1961, 30, 227 Search PubMed.
- D. Stefanakis and D. F. Ghanotakis, J. Nanopart. Res., 2010, 12, 1285 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: MPEG file. See DOI: 10.1039/c0ay00366b |
| This journal is © The Royal Society of Chemistry 2010 |
