In situ Raman and IR spectroscopic analysis of indigo dye
Anna
Baran
a,
Andrea
Fiedler
b,
Hartwig
Schulz
b and
Malgorzata
Baranska
*a
aFaculty of Chemistry, Jagiellonian University, Ingardena 3, 30-060, Cracow, Poland. E-mail: baranska@chemia.uj.edu.pl
bJulius Kühn-Institut, Institute for Ecological Chemistry, Plant Analysis and Stored Product Protection Pflanzenanalytik und Vorratsschutz, Erwin-Baur-Strasse 27, 06484, Quedlinburg, Germany
First published on 1st July 2010
Abstract
Non-destructive analysis of indigo dye in biological and textile samples is presented. Using different but complementary techniques of vibrational spectroscopy (FT-Raman and ATR-IR) detection of indigo in various samples is performed. This work shows the possibility of both vibrational analytical methods to identify indigo directly in plant tissue, commercially available pigments and cotton and silk textiles. The experimental work is supported by quantum-chemical calculations at the B3LYP/6-311++g(d,p) level of theory. Additionally, in order to follow the process of indigo formation upon leaf drying Raman mapping of Polygonum tinctorium is demonstrated.
Introduction
Raman scattering as a complementary method to IR absorption spectroscopy has become an important and popular analytical way to study pigments and dyes. The main advantage of Raman techniques is the requirement for only a small amount of analyte and the possibility of in situ analysis to be performed on various type of samples. Thus, Raman spectroscopy is very useful in the area of an art conservation1,2 as well as plant research.3 Excitation in the NIR range using a Nd:YAG laser emitting at 1064 nm provides the best option for the analysis of art pieces and biomaterials because in most cases the spectra are fluorescence free and the laser excitation does not cause any sample destruction.3Additionally, Raman mapping allows the consecutive collection of spectra over a distinctively defined sample area. With the help of chemometric processing the distribution of individual compounds is visualized according to their vibrational properties. Furthermore, a comparison of the corresponding visual image of the investigated sample provides additional chemical information. The important advantage of Raman compared to IR measurement is the weak absorption of water, which offers, especially for biological tissues, a fast analysis without further sample preparation. On the other hand, FT-IR spectroscopy is often more informative when polar functional groups are studied. In the last years, ATR-IR technique was widely used to investigate diverse materials because of its manifold applicability for solids or liquids.4
This work is focused on the spectroscopic analysis based on Raman and ATR-IR spectroscopy of the commonly known and widely used indigo dye occurring in plant, textiles and art pieces. The comparison between applied vibrational methods is presented and discussed.
Indigo was used in the textile industry in the traditional way of obtaining this dye from plants of the genus Indigofera, which are native to the tropics. In temperate climates indigo can also be obtained from woad (Isatis tinctoria) (Europe) and dyer's knotweed (Polygonum tinctorium) (Asia).5P. tinctorium is characterized by the presence of large amount of indoxyl-β-D-glucoside (indican), which acts as a precursor of indigo in this plant but its function in the secondary metabolism is still discussed. Indican can only be hydrolyzed at relatively low pH or in the presence of a native β-glucosidase normally occurring in the chloroplasts.6,7 When the leaves are crushed, indican is immediately hydrolyzed by β-glucosidase to β-D-glucose and indoxyl. This reaction is followed by the spontaneous conversion of free indoxyl by air oxidation into natural indigo dye6 (see Fig. 1).
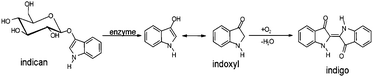 | ||
| Fig. 1 The process of indigo formation in biological samples. | ||
In order to develop an analytical method for the reliable determination of indigo in plant tissue, investigation of the drying process of P. tinctorium leaf leading to the blue dye of indigo was performed. The Raman mapping was obtained for fresh and consequently dried Polygonum tinctorium leaves to follow the process of indigo formation in biological samples.
This work presents also the application of vibrational spectroscopy in the area of art conservation. Various textiles such as cotton threads dyed with indigo have been investigated by using Raman spectroscopy by Coupry8 and Thomas.9 They analysed indigo textiles (jeans) with the laser excitation in the visible range. Textile analysis (i.e. wool and cotton) with NIR laser excitation and FT-Raman spectroscopy was reported by Schrader et al.10 In our research, modern cotton threads (jeans) and silk Tibetan tapestry (early 20th century), both dyed with indigo, are measured with FT-Raman spectroscopy.
Additionally, the possibilities of Raman and ATR-IR spectroscopy to distinguish between various artistic pigments called indigo with regard to their origin and impurities is demonstrated. This topic was discussed by Vandenabeele et al.,11 who showed the usefulness of Raman spectroscopy combined with chemometrics (PCA, Cluster Analysis, LDA) for the analysis of indigo samples. In this paper we present a similar approach using ATR-IR spectroscopy.
Most papers reporting vibrational analysis of indigo refer to calculations by Tatsch and Schrader12 performed at HF/3-21G level of theory. Later on the calculations were repeated at a higher level of theory or better basis sets, e.g. HF/6-31G(d,p),13 B3LYP/6-31G(d,p),13,14 B3LYP/6-31G+(d,p).15 The experimental work presented here is supported by quantum-chemical calculations at B3LYP/6-311++g(d,p) level and the assignment of vibration modes is based on the potential energy distribution.
Experimental
Standards, pigments, plant material and textiles
Polygonum tinctorium plants were cultivated in the experimental garden of the Julius Kühn Institute (JKI) in Quedlinburg, Germany.Natural indigo dyes were supplied by Kremer Pigmente company (Aichstetten, Germany). The samples named as ‘36000 – Genuine, Indian’ and ‘36003 – Indigo made from woad’, came from Indigofera tinctoria and Isatis tinctoria (woad), respectively.
Various silk and cotton textiles dyed with indigo were measured: samples of silk threads of two Mongolian thangkas from a private collection (thangka of Maitreia painting on canvas and White Tara, a woodcut painting on silk), a cotton threads dyed with indigo (jeans) commercially available and supplied by Winchester jeans (Poland). Raw silk threads and cotton textiles were taken as reference samples for the thangkas and jeans, respectively.
Spectroscopic measurements
Raman mapping of freshly crushed Polygonum tinctorium leaf was performed on a FT-Raman Spectrometer RFS 100 (Bruker, Germany) equipped with a diode-pumped Nd:YAG laser (1064 nm), using an xy motorized stage, a mirror objective and a prism slide for redirection of the laser beam. Compared with the standard vertical sampling arrangement, the samples of P. tinctorium leaf were mounted horizontally between two glass slides to avoid their movement and deformation during measurement. Laser power of 150 mW, 32 scans per spectrum and 4 cm−1 spectral resolution was applied. The experiment was repeated from the same leaf after one and then two days of drying. Raman mappings were collected over the same sample area of 3.5 mm × 2.5 mm with a spatial resolution of 40 μm. Additionally, single point measurements of P. tinctorium leaf and indoxyl-β-D-glucoside (Sigma Aldrich, Germany) were performed using 100 and 32 scans and 400 and 600 mW of laser power, respectively.Raman analysis of textiles, pigments, indigo and cotton standards were done using a FT-Raman Spectrometer (Nicolet, Model NXR 9650) equipped with cw. Nd:YAG laser 1064 nm and a germanium detector cooled with liquid nitrogen. The spectra were collected with laser power of 0.7 W and 1000–2000 scans for silk samples and about 0.8 W and 100–200 scans for pigments, indigo and cellulose samples. FT-IR spectra of these samples were obtained on a Varian 620-IR spectrometer equipped with a diamond ATR using 16 scans/sample.
Spectroscopic measurements of indigo were discussed based on quantum-chemical calculations. For the optimized molecule the frequency of vibrational modes were calculated with the Gaussian 03 program16 applying the B3LYP/6-311++g(d,p) level of theory. The potential energy distribution of the vibrations was calculated with Gar2PED program.17
Results and discussion
Synthetic and natural pigments of indigo
The result of FT-Raman and ATR-IR measurements of indigo samples are presented in Fig. 2 and 3, respectively. Besides a synthetic standard of indigo two pigments obtained from different plants, Indigofera tinctoria (36000) and Isatis tinctoria (36003), are investigated. Table 1 provides band assignment of the most characteristic vibrations based on theoretical calculations.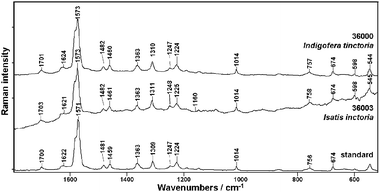 | ||
| Fig. 2 FT-Raman spectra of indigo standard and natural indigo dyes from Indigofera tinctoria (36000) and Isatis inctoria (36003). | ||
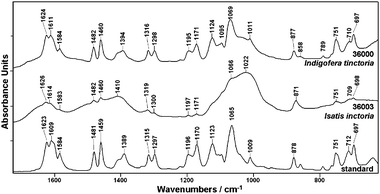 | ||
| Fig. 3 ATR-IR spectra of indigo standard and natural indigo dyes from Indigofera tinctoria (36000) and Isatis inctoria (36003). | ||
| Raman | IR | Band assignment |
|---|---|---|
a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C – vibrations of C atoms between two indoxyl parts of a molecule; ring5 – a 5-membered ring of the indigo molecule; ring6 – a 6-membered ring of the indigo molecule; ip – in plane; op – out of plane; iph – in phase; oph – out of phase; sym – symmetric; asym – asymmetric; breath – breathing vibrations; β – deformation modes; ν – stretching; ρ – rocking; ω – wagging; w – weak, m – medium, s – strong, vs. – very strong. C – vibrations of C atoms between two indoxyl parts of a molecule; ring5 – a 5-membered ring of the indigo molecule; ring6 – a 6-membered ring of the indigo molecule; ip – in plane; op – out of plane; iph – in phase; oph – out of phase; sym – symmetric; asym – asymmetric; breath – breathing vibrations; β – deformation modes; ν – stretching; ρ – rocking; ω – wagging; w – weak, m – medium, s – strong, vs. – very strong.
|
||
| 1700m |
ν
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C + νC C + νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + βring5ip O + βring5ip |
|
| 1623s |
ν
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O
|
|
| 1622m |
ν
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C + νC C + νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + βring5ip + βring6ip O + βring5ip + βring6ip |
|
| 1609s | ν CCring6ip | |
| 1584w | ν CCring6ip | |
| 1581vs |
ν
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C + νC C + νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
|
| 1571s | ν CCring6ip | |
| 1481m | ρ CH iph, asym + νCCring6 | |
| 1481m | ρ CHiph, sym + νCCring6 | |
| 1459m | ρ CHoph, sym + νCCring6 | |
| 1459s | ρ CHoph, asym + νCCring6 | |
| 1389m | ρ NH + νCN | |
| 1363s | ρ NH + νCN + ρCHiph, asym | |
| 1315m | ν CCring6 + νCCring5 + ρCHoph, asym | |
| 1309s | ν CCring6 + ρCHiph, asym | |
| 1297m | ρ CHiph, sym + βR5ip | |
| 1247w | ρ CHiph, asym + νCN + νCCring6 + νCCring5 | |
| 1224m |
ρ
NH + νCN + νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
|
| 1196w | ν CCring5 + ρCHoph, asym + βring6ip | |
| 1170s | ν CN + ρCHoph, asym + ρNH | |
| 1123m | ρ NH + νCN | |
| 1065s | ν CCring5 + ρCO | |
| 1014m | ν CCring6 breath,,sym | |
| 1009m | ν CCring6 breath,asym | |
| 878m | βring6ip + βring5ip | |
| 756w | βring5 + νCCring5 + ρC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C + νCN C + νCN |
|
| 751m | ωCH + βring6op | |
| 712m | βring6ip + βring5ip | |
| 697m | βring5op + βring6op + ωCO | |
| 674m | βring6ip + ρCO + ρC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
It can be seen (Fig. 2), that the FT-Raman spectra of indigo of various origin are very similar. The profile of indigo bands recognized in the spectrum of pure standard can be observed for both naturally obtained samples. The most intense signal of the ring stretching mode is observed near 1571 cm−1 with a pronounced shoulder at 1581 cm−1 due to the stretching vibrations of the conjugated system of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups.18 The vibration of this conjugated system gives rise also to bands near 1700, 1622 and 1363 cm−1. The band attributed to rocking vibrations of N–H groups is observed at about 1224 cm−1. Vibrations involving C–H rocking are recognized at 1481, 1459 and 1247 cm−1 whereas the vibrations of five- and six-membered rings are identified at 1309, 1014, 756 and 674 cm −1.
O and N–H groups.18 The vibration of this conjugated system gives rise also to bands near 1700, 1622 and 1363 cm−1. The band attributed to rocking vibrations of N–H groups is observed at about 1224 cm−1. Vibrations involving C–H rocking are recognized at 1481, 1459 and 1247 cm−1 whereas the vibrations of five- and six-membered rings are identified at 1309, 1014, 756 and 674 cm −1.
ATR-IR spectra of indigo (Fig. 3) in contrast to previously discussed data release significant differences between the investigated samples. Indigo made from Indigofera tinctoria (sample no. 36000) shows spectral profile similar to the standard, but for indigo obtained from Isatis tinctoria (sample no. 36003) new bands are observed in the measured ATR-IR spectrum. Only a few spectral features identified as indigo marker bands are observed in ATR-IR spectrum of 36003 sample and most of signals are overlapped by three broad bands (near 1614, 1410, 1002 cm−1) due to impurities in the pigment. It should be stressed that indigo vibrations due to its conjugated bond system do not demonstrate intensive bands in ATR-IR spectrum as previously observed by FT-Raman spectroscopy. The bands attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations are detected near 1623 cm−1 (stretching), 1065 cm−1 (rocking) and 697 cm−1 (wagging). The rocking vibration of N–H together with stretching vibration of C–N contributes to bands near 1389 cm−1 and 1123 cm−1. The signal at about 1170 cm−1 can be assigned to stretching vibration of C–N group composed with rocking of the C–H and N–H groups. The most intense band at 1065 cm−1 is attributed to the vibration of a five-membered ring, which gives rise also to the signals at 1297, 1196, 1065, 878, 712 and 697 cm−1. The stretching and bending vibrations of six-membered rings occur at 1609, 1584, 1481, 1459, 1315, 1196, 1009, 878, 751, 712, 697 cm−1 whereas bands attributed to vibrations of C–H arise at 1481, 1459, 1297 1196, 1170 cm−1 (rocking) and 751 cm−1 (wagging). It should be mentioned that some assignments are in discrepancy to previous papers, especially when calculations were performed at the HF level.
O vibrations are detected near 1623 cm−1 (stretching), 1065 cm−1 (rocking) and 697 cm−1 (wagging). The rocking vibration of N–H together with stretching vibration of C–N contributes to bands near 1389 cm−1 and 1123 cm−1. The signal at about 1170 cm−1 can be assigned to stretching vibration of C–N group composed with rocking of the C–H and N–H groups. The most intense band at 1065 cm−1 is attributed to the vibration of a five-membered ring, which gives rise also to the signals at 1297, 1196, 1065, 878, 712 and 697 cm−1. The stretching and bending vibrations of six-membered rings occur at 1609, 1584, 1481, 1459, 1315, 1196, 1009, 878, 751, 712, 697 cm−1 whereas bands attributed to vibrations of C–H arise at 1481, 1459, 1297 1196, 1170 cm−1 (rocking) and 751 cm−1 (wagging). It should be mentioned that some assignments are in discrepancy to previous papers, especially when calculations were performed at the HF level.
In summary, Raman spectroscopy has been shown to be a sensitive method to detect indigo, also in the presence of sample impurities. While, ATR-IR can provide information about the source and complexity of indigo pigments.
Detection of indigo in Polygonum tinctorium plant
Fig. 4 presents a Raman spectrum taken of dried leaves of Polygonum tinctorium and related to reference compounds of indigo and indican (indoxyl-β-D glucoside). Several marker bands of indigo can be clearly seen in the spectrum obtained from the dried leaf, whereas the characteristic signals of indican could not be detected due to the low concentration and low Raman sensitivity of that compound. Moreover, indigo due to its high molecular symmetry and conjugated bond system provides strong Raman signals.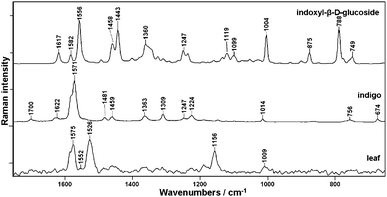 | ||
| Fig. 4 FT-Raman spectra of dried leaves of Polygonum tinctorium together with indigo and indican (indoxyl-β-D-glucoside). | ||
A comparison of the spectra shown in Fig. 4 indicates that the best marker of indigo occurring in the plant tissue is the band at 1571 cm−1, which can be used not only to identify but also to determine the individual concentration of this plant pigment based on a calibration model (the paper to be submitted soon).19 Neither of two major plant constituents, cellulose with the characteristic doublet at 1095 and 1120 cm−1 associated to νasym (C–O–C) and νsym (C–O–C) vibrations, respectively, nor lignin recognized by marker bands above 1650/1600 cm−1 could be detected in Polygonum tinctorium leaf.20
Besides indigo, carotenoids are clearly visible in the spectrum obtained from the plant. Carotenoids are present in the mg kg−1 range but the pre-resonance effect in FT-Raman spectroscopy gives strong enhancement of their vibration. Strong bands of carotenoids are observed in the Raman spectrum of the leaf at 1526 and 1156 cm−1 due to in-phase C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (ν1) and C–C stretching (ν2) vibrations of the polyene chain. Additionally, the in-plane rocking mode of CH3 groups attached to the polyene chain and coupled with C–C bonds can be seen as a peak of weak intensity at 1010 cm−1.21
C (ν1) and C–C stretching (ν2) vibrations of the polyene chain. Additionally, the in-plane rocking mode of CH3 groups attached to the polyene chain and coupled with C–C bonds can be seen as a peak of weak intensity at 1010 cm−1.21
Fig. 5 shows the corresponding ATR-IR spectra for dried leaf material obtained from Polygonum tinctorium and the reference compounds of indigo and indican. The presence of indigo in the leaf is recognized by several characteristic signals (e.g. at 1623 cm−1, 1584 cm−1, 1481 cm−1), but most of the signals show high coincidence with spectral data from other plant components. Indican due to its low concentration could not be detected in the spectrum obtained from P. tinctorium leaf.
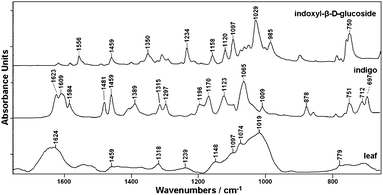 | ||
| Fig. 5 ATR-IR spectra of dried leaves of Polygonum tinctorium together with indigo and indican (indoxyl-β-D-glucoside). | ||
Spatiotemporal development of indigo in Polygonum tinctorium leaves
Raman mapping of Polygonum tinctorium leaf was performed in order to detect and follow the enzymatically catalyzed process of indigo formation upon leaf drying. Fig. 6A presents a visual image of a crushed P. tinctorium leaf whereas in Fig. 6B Raman maps are presented obtained from fresh leaf and after one and two days of drying at room temperature. The blue area on the leaf surface indicates a damaged plant tissue where indican was enzymatically transformed to indigo according to the reaction presented in Fig. 1.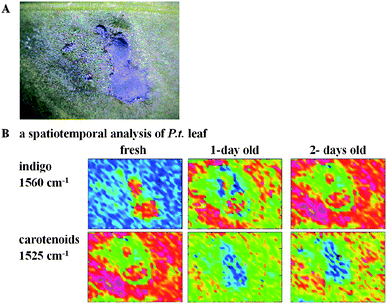 | ||
| Fig. 6 Visual image of Polygonum tinctorium leaf (A) and FT-Raman maps of indigo and carotenoids distribution (B). (Blue color = low concentration, red = high concentration of the analyte). | ||
The maps showing the distribution of indigo were calculated by the integration of the marker band near 1570 cm−1 (red = high, blue = low concentration of indigo). As can be seen, at first indigo is present only in the damaged area of the fresh leaf, however due to drying a dyeing process is observed. It has been found that the amount of indigo gradually increased to nearly homogenous concentration except in the area closely surrounding the damaged spots. In the neighborhood of primary indigo spots carotenoids are detected, which, here, most likely act as a plant protector following abiotic stress. This is not surprising since carotenoids in chloroplasts act as accessory pigments in light-harvesting complexes and transfer energy to chlorophylls.22 They are known to be effective protectants against photo-oxidative damage, working by quenching the triplet state of chlorophyll23 and acting as scavengers of singlet oxygen.24
Application of Raman spectroscopy in textile analyses
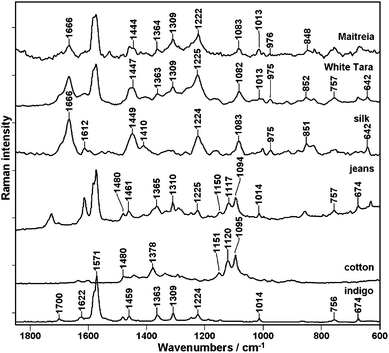
In order to investigate the possibility of Raman spectroscopy to detect indigo in dyed textiles several samples were analysed. Fig. 7 presents the Raman spectra of cotton and silk threads dyed with indigo together with ordinary silk and cotton. To make the discussion easier the indigo spectrum is shown.As an example of dyed silk textiles two Mongolian thangkas called Maitreia and White Tara were used. The most characteristic Raman spectral features of indigo are observed in both samples at 1571, 1363, 1309, 1014 and 757 cm−1. Additionally, broad Raman bands typical for silk can be seen.
The intense signals near 1666 cm−1 and 1224 cm−1 are attributed to the amide vibrations of silk β-sheet structure, accordingly amide I and III. The bending vibrations of CH2 and CH3 groups give rise to band near 1449 cm−1 and stretching vibrations are assigned to band near 1083 cm−1. The band which could be observed near 851 cm−1 is attributed to vibrations of tyrosine, an aromatic amino acid that occurs in silk.25 To conclude, the spectra of thangkas illustrate the sum of silk and indigo spectral features.
A similar observation can be made when the analysis of cotton textile dyed with industrial indigo, commonly called jeans, is performed (Fig. 7). The indigo bands are clearly present in the ’jeans’ pectrum together with other signals attributed to cotton. These bands can be assigned to vibrations of cellulose in the cotton fibers. The most characteristic bands of cellulose are seen near 1150 cm−1, 1120 cm−1 and 1094 cm−1 due to the C–C ring asymmetric stretching, C–O–C glycoside link symmetric stretching and C–O–C glycoside link asymmetric stretching, respectively. Other noticeable bands are attributed to different types of CH2 group vibration, e.g. at 1480 and 1378 cm−1 (scissoring) and at 1292 cm−1 (twisting modes), while at 1337 cm−1 a wagging vibration is additionally composed with scissoring of the C–OH groups, followed by rocking modes at 996 cm−1.26
Conclusions
In order to analyze indigo which occurs in natural and synthetic samples, and is also used as a dyeing agent for ordinary and art textiles, FT-Raman spectroscopy combined with ATR-IR technique is recommended. Such a complex study supported by theoretical calculations allows detailed interpretation of the obtained results.Detection of indigo is based on the Raman marker band at 1571 cm−1, attributed to the stretching vibrations of the cross-conjugated system of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups, whereas in the ATR-IR spectrum the band attributed to C
O and N–H groups, whereas in the ATR-IR spectrum the band attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations near 1623 cm−1 can be used. Raman spectroscopy has been shown to be a sensitive method to identify indigo even in the presence of pigment impurities. At the same time, the ATR-IR technique can provide information about the source and complexity of indigo samples.
O vibrations near 1623 cm−1 can be used. Raman spectroscopy has been shown to be a sensitive method to identify indigo even in the presence of pigment impurities. At the same time, the ATR-IR technique can provide information about the source and complexity of indigo samples.
In situ Raman mapping measurements offer the possibility of nondestructive analysis of plant or textile without any sample preparation.
Acknowledgements
The authors thank the Academic Computer Centre “Cyfronet”, Kraków, Poland for computing time. The research was supported by the Ministry of Science and Higher Education (MNiSW), grant no. N204121638, 2010–2011, and partly by grant no. N204013635, 2008–2011.References
- I. M. Bell, R. J. H. Clark and P. J. Gibbs, Spectrochim. Acta, Part A, 1997, 53, 2159 CrossRef.
- P. Vandenabeele, H. G. M. Edwards and L. Moens, Chem. Rev., 2007, 107, 675 CrossRef CAS.
- G. N. Andreev, B. Schrader, H. Shulz, R. Fuchs, S. Popov and N. Handijeva, Fresenius J. Anal. Chem., 2001, 371, 1009 CrossRef CAS.
- H. Schulz and M. Baranska, Vib. Spectrosc., 2007, 43, 13 CrossRef CAS.
- H. Schweppe, in Handbuch der Naturfarbstoffe, 1993, Nikol Verlagsgesellschaft, Hamburg Search PubMed.
- Y. Minami, H. Takao, T. Kanafuji, K. Miura, M. Kondo, I. Hara-Nishimura, M. Nishimura and H. Matsubara, Plant Cell Physiol., 1997, 38, 1069 CAS.
- E. Campeol, L. G. Angelini, S. Tozzi and M. Bertolacci, Environ. Exp. Bot., 2006, 58, 223 CrossRef.
- C. Coupry, G. Sagon and P. Gorguet-Ballesteros, J. Raman Spectrosc., 1997, 28, 85 CrossRef CAS.
- J. Thomas, P. Buzzini, G. Massonnet, B. Reedy and C. Roux, Forensic Sci. Int., 2005, 152, 189 CrossRef CAS.
- B. Schrader, H. Schulz, G. N. Andreev, H. H. Klump and J. Sawatzki, Talanta, 2000, 53, 35 CrossRef CAS.
- P. Vandenabeele and L. Moens, Analyst, 2003, 128, 187 RSC.
- E. Tatsch and B. Schrader, J. Raman Spectrosc., 1995, 26, 467 CAS.
- M. S. del Rio, M. Picquart, E. Haro-Poniatowski, E. van Elslande and V. H. Uc, J. Raman Spectrosc., 2006, 37, 1046 CrossRef.
- J. Tomkinson, M. Bacci, M. Piccolo and D. Colognesi, Vib. Spectrosc., 2009, 50, 268 CrossRef CAS.
- R. Giustetto, F. X. Llbarés i Xamena, G. Ricchiardi, S. Bordiga, A. Damin, R. Gobetto and M. R. Chierotti, J. Phys. Chem. B, 2005, 109, 19360 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision D.01, Gaussian, Inc., Wallingford CT, 2004 Search PubMed.
- J. M. L. Martin and C. Van Alesenoy, 1995, Gar2ped, University of Antverp Search PubMed.
- H. Bauer, K. Kowski, H. Kuhn, W. Luttke and P. Rademacher, J. Mol. Struct., 1998, 445, 277 CrossRef CAS.
- A. Fiedler, M. Baranska and H. Schulz, FT-Raman spectroscopy - a rapid and reliable quantification protocol for the determination of natural indigo dye in Polygonum tinctorium, J. Raman Spectrosc. DOI:10.1002/jrs.2726.
- R. Baranski, M. Baranska, H. Schulz, P. W. Simon and T. Nothnagel, Biopolymers, 2006, 81, 497–505 CrossRef CAS.
- H. Schulz, M. Baranska and R. Baranski, Biopolymers, 2005, 77, 212 CrossRef CAS.
- D. Siefermann-Harms, Physiol. Plant., 1987, 69, 561 CrossRef CAS.
- A. J. Young, Physiol. Plant., 1991, 83, 702 CrossRef CAS.
- N. I. Krinsky, Pure Appl. Chem., 1979, 51, 649 CrossRef CAS.
- J. Shao, J. Zheng, J. Liu and C. M. Carr, J. Appl. Polym. Sci., 2005, 96, 1999 CrossRef CAS.
- Y. Liu, S. Kokot and T. J. Sambi, Analyst, 1998, 123, 633 RSC.
| This journal is © The Royal Society of Chemistry 2010 |