DOI:
10.1039/C0AY00305K
(Paper)
Anal. Methods, 2010,
2, 1472-1478
A new fluorescein isothiocyanate-based screening method for the rapid discovery of electrophilic compounds
Received
10th May 2010
, Accepted 27th July 2010
First published on
27th August 2010
Abstract
In the present study, a simple fluorescein isothiocyanate (FITC)-based screening method was established for the rapid discovery of electrophilic compounds from natural products. The test sample is firstly allowed to alkylate a certain proportion of glutathione (GSH) under basic conditions (pH 8.0), then FITC is added to conjugate with the remaining GSH from the first step. By analyzing the fluorescence intensity of the reaction solution under acidic conditions (pH 3.0), it could be determined whether the test sample contained electrophilic compounds. A botanical sample, the ethyl acetate extract of Radix Salvia miltiorrhiza, was tested using this screening assay and we successfully discovered four electrophilic compounds from it, which were miltirone and its three derivatives (1-oxomiltirone, 4-methylenemiltirone, and 1,2-didehydromiltirone). The findings indicate that the screening method is effective and suitable for high-throughput screening (HTS) assays in the future.
1. Introduction
Electrophilic compounds are considered as redox-active compounds because of their electron-deficient centers.1 In cells, electrophilic compounds can activate or inhibit specific signal transduction pathways as a consequence of reacting with nucleophiles, including protein thiols, glutathione (GSH) or guanine bases in DNA.2,3 Thus they often manifest two disparate actions in the life processes: a toxic or a preventive effect. Some electrophilic compounds can attack macromolecules such as proteins and DNA3 or deplete the cellular level of GSH (e.g. menadione4 and doxorubicin5), resulting in inhibition of the transcription and replication of DNA or abrogation of the reducing capability of the cell. In contrast, numerous electrophilic compounds can be used as antitumor6–8 and neuroprotective agents.9–11 For example, miltirone and tert-butyl hydroquinone (tBHQ) have been proven to possess preventive effects because of their activities of inducing NAD(P)H: quinone oxidoreductase 1 (NQO1).12,13
Fluorescein isothiocyanate (FITC) is a common fluorophore with high fluorescence. It plays a particularly important role in biosciences because of its reactive properties towards amine and sulfhydryl groups. Previous studies have indicated that FITC could be used in the labeling and imaging of proteins14,15 and DNA primers.16 It could also be used as a labeling reagent in separation of chiral amino acids through laser-induced fluorescence detection techniques.17,18
In this study, a simple FITC-based screening method was established for the rapid discovery of electrophilic compounds from natural products based on the initial finding that the fluorescence intensities of FITC and FITC-GSH conjugate were different under acidic conditions (pH 3.0). As shown in Fig. 1, the screening method includes three main steps. First, the test sample alkylates a certain proportion of GSH under basic conditions (pH 8.0). Then FITC is added to conjugate with the remaining GSH that did not react with the test sample in the first step. Finally, the pH value of the solution is adjusted to 3.0 and the fluorescence intensity is measured with excitation at 485 nm and emission at 535 nm. Compared with the control group, electrophilic compounds can reduce the formation of FITC-GSH conjugates in the last step, resulting in a lower fluorescence intensity of the reaction solution.
 |
| | Fig. 1 Schematic of the FITC-based screening method for the discovery of electrophilic compounds. | |
2. Experimental
2.1 Materials and chemicals
FITC, menadione and GSH were purchased from Sigma-Aldrich (St. Louis, MO, USA). The roots of Salvia miltiorrhiza were collected at Shanxi Province, People's Republic of China, in December 2007. The plant material was identified by the authors and a voucher specimen (No. SM071211) has been deposited in the herbarium of the School of Pharmaceutical Sciences, Zhejiang University. Ten tanshinones including Miltirone, 1-oxomiltirone, 4-methylenemiltirone, 1,2-didehydromiltirone, 15,16-dihydrotanshinone I, 1,2-dihydrotanshinquinone, 17-hydroxycryptotanshinone, Tanshinone IIB, Tanshinone I and Methyl dihydronortanshinonate were isolated from the ethyl acetate extract of root of S. miltiorrhiza, the procedures of extraction and isolation were described previously.12 The structures of ten tanshinones were elucidated by NMR spectroscopic analysis and their purities were all greater than 95% according to HPLC analysis. HPLC-grade acetonitrile (Merck, Darmstadt, Germany), methanol (Merck, Darmstadt, Germany) and formic acid (Tedia, Fairfield, OH, USA) were utilized for the HPLC analysis. Deionized water was prepared using a Milli-Q system (Millipore, Bedford, MA, USA). All other chemicals and solvents were of analytical-reagent grade.
The stock solution of FITC (2 mM) was prepared in HPLC-grade acetonitrile. Briefly, FITC was incubated with 1 mM GSH in a total volume of 100 μL of 25 mM Tris-HCl buffer solution (pH 8.0) for 30 min at room temperature (the final concentration of FITC was 100 μM). The reaction solution was analyzed using a Thermo Finnigan LCQ Deca XPplus ESI ion trap mass spectrometer (San Jose, CA, USA) in positive ion mode equipped with an Agilent 1100 HPLC system (Waldbronn, Germany) and a Zorbax SB–C18 column (4.6 mm × 250 mm, 5 μm, Agilent Technologies, USA). The mobile phase consisted of A (HCOOH: H2O = 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and B (HCOOH: CH3CN = 0.2
100) and B (HCOOH: CH3CN = 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). A 30 min gradient was used from 90% A to 100% B and maintained at 100% B during the next 6 min. The flow rate was 0.5 mL min−1 and the column temperature was set at 30 °C. The ultraviolet (UV) spectra were recorded at 280 nm. The MS operating parameters were as follows: collision gas, ultra-high-purity helium (He); nebulizing gas, high-purity nitrogen (N2); ion spray voltage, −4.5 kV; sheath gas (N2), 5 arbitrary units; capillary temperature, 275 °C; capillary voltage, −15 V; tube lens offset voltage, −30 V. The collision energy for collision-induced dissociation (CID) was between 30% and 45%, and the isolation width of precursor ions was 3.0 Th.
100). A 30 min gradient was used from 90% A to 100% B and maintained at 100% B during the next 6 min. The flow rate was 0.5 mL min−1 and the column temperature was set at 30 °C. The ultraviolet (UV) spectra were recorded at 280 nm. The MS operating parameters were as follows: collision gas, ultra-high-purity helium (He); nebulizing gas, high-purity nitrogen (N2); ion spray voltage, −4.5 kV; sheath gas (N2), 5 arbitrary units; capillary temperature, 275 °C; capillary voltage, −15 V; tube lens offset voltage, −30 V. The collision energy for collision-induced dissociation (CID) was between 30% and 45%, and the isolation width of precursor ions was 3.0 Th.
2.3 LC-MS analysis of FITC-CH3OH conjugate
FITC was dissolved in methanol (the final concentration of FITC was 2 mM) for 1 h at room temperature. The reaction solution was analyzed using a Thermo Finnigan LCQ Deca XPplus ESI ion trap mass spectrometer in positive ion mode equipped with an Agilent 1100 HPLC system and a Zorbax SB–C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase, gradient program and MS operating parameters were the same as described above.
2.4 Fluorescence assay of FITC and FITC-GSH conjugate in different pH environments
The assay was performed in 96-well plates (Corning Costar). Briefly, 95 μL/well of a series of concentrations ranging from 0 to 1 mM GSH in 25 mM Tris-HCl buffer solution (pH 8.0) was added, followed by 5 μL/well FITC stock solution (the final concentration of FITC was 0.1 mM). For the basic condition, after 30 min incubation at room temperature, the fluorescence was measured using infinite F200 scanning fluorescence plate reader (Tecan, Mannedorf, Switzerland) with excitation at 485 nm and emission at 535 nm. For the acidic condition, after 30 min incubation at room temperature, 15 μL 1% HCl was added to each well and the fluorescence was measured using infinite F200 scanning fluorescence plate reader with excitation at 485 nm and emission at 535 nm.
2.5 Fluorescence assay for test sample
The assay was performed in 96-well plates. Typically, 90 μL/well 1 mM GSH in 25 mM Tris-HCl buffer solution (pH 8.0) was added, followed by 5 μL/well test sample (dissolved in acetonitrile). After 30 min incubation at room temperature, 5 μL/well FITC solution (dissolved in acetonitrile) was added and incubated for another 30 min, the final concentration of FITC was 0.1 mM. Then 15 μL/well 1% HCl was added into each well. The fluorescence was measured using infinite F200 scanning fluorescence plate reader with excitation at 485 nm and emission at 535 nm.
2.6 LC-MS analysis of GSH conjugation with the ethyl acetate extract of S. miltiorrhiza
The ethyl acetate extract of S. miltiorrhiza was incubated with 5 mM GSH for 1 h at 37 °C in a total volume of 200 μL of 25 mM Tris-HCl buffer solution (pH 8.0) (The final concentration of the ethyl acetate extract of S. miltiorrhiza was 2 mg mL−1). A control experiment was carried out in which the ethyl acetate extract of S. miltiorrhiza was incubated with 25 mM Tris–HCl buffer solution (pH 8.0) only. The reaction solution was analyzed using a Thermo Finnigan LCQ Deca XPplus ESI ion trap mass spectrometer in positive ion mode equipped with an Agilent 1100 HPLC system and a Zorbax SB–C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase consisted of A (HCOOH: H2O = 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and B (CH3CN). A gradient program was used according to the following profile: 0–90 min, 20–100% B and maintained at 100% B during the next 10 min. The MS operating parameters were the same as described above.
100) and B (CH3CN). A gradient program was used according to the following profile: 0–90 min, 20–100% B and maintained at 100% B during the next 10 min. The MS operating parameters were the same as described above.
3. Results and discussion
3.1 The fluorescence intensities of FITC and FITC-GSH conjugate in different pH environments
We first investigated the reaction between GSH and FITC. In the HPLC chromatogram, for which the wavelength of detection was 280 nm, the FITC peak eluting at 26.97 min diminished after incubation with GSH, and one extra peak formed, eluting at 15.93 min (Fig. 2A). In the corresponding tandem mass spectrum, the [M + H]+ ion at m/z 697 was identified as FITC-GSH conjugate (Fig. 2B). Considering the chemical structure of FITC, the carbon of the isothiocyanate group is electron-deficient because its electron density is drawn to the sulfur beside it, thus making FITC more reactive for the alkylation of the thiol group of GSH (Fig. 2C).
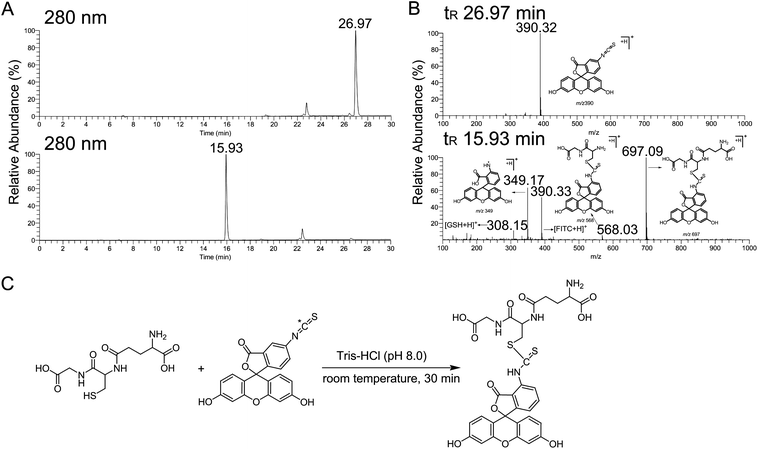 |
| | Fig. 2 The reaction between FITC and GSH. (A) The HPLC chromatograms before (upper graph) and after (lower graph) FITC incubation with GSH, UVλ = 280 nm. (B) Positive ion ESI mass spectrum of FITC (upper graph) and FITC-GSH conjugate (lower graph). (C) Proposed reaction of GSH with FITC under basic conditions (pH 8.0). | |
It is known that the fluorescence intensity of FITC is dependent on the pH value of the assay buffer.19 Thus we investigated the fluorescence spectra of FITC and FITC-GSH conjugate in different pH environments. FITC (0.1 mM) was allowed to incubate with GSH (1 mM) and then the pH value of the reaction solution was adjusted to 8.0, 5.0 and 3.0, respectively. We might consider the fluorescence intensity of the reaction solution in which 0.1 mM FITC was incubated with 1 mM GSH represented the fluorescence intensity of FITC-GSH conjugate because FITC formed FITC-GSH completely (as shown in Fig. 2A). When the pH values were 8.0 and 5.0, the fluorescence excitation and fluorescence emission spectra of FITC and FITC-GSH conjugate were almost the same (Fig. 3A). However, when the pH value was adjusted to 3.0, the fluorescence excitation and fluorescence emission spectra of FITC and FITC-GSH conjugate were different (Fig. 3A). We noticed that in the fluorescence excitation spectra of FITC and FITC-GSH conjugate at pH 3.0, there were two excitation peaks at 441 nm and 485 nm. It was known that there were often some fluorescent products in the botanical sample (e.g., coumarins) that could interfere with the results if 441 nm was chosen as the excitation wavelength, thus excitation at 485 nm was selected for further experiments. Subsequently, we chose a series of concentrations of GSH ranging from 0 to 1 mM for incubation with 0.1 mM FITC and measured the fluorescence intensity of the reaction solution under basic (pH 8.0) and acidic (pH 3.0) conditions, respectively. Compared with the control group, after 30 min incubation of GSH with FITC, the fluorescence intensity of the reaction solution increased in a dose-dependent manner under acidic conditions (pH 3.0) but was almost the same under basic conditions (pH 8.0) (Fig. 3B).
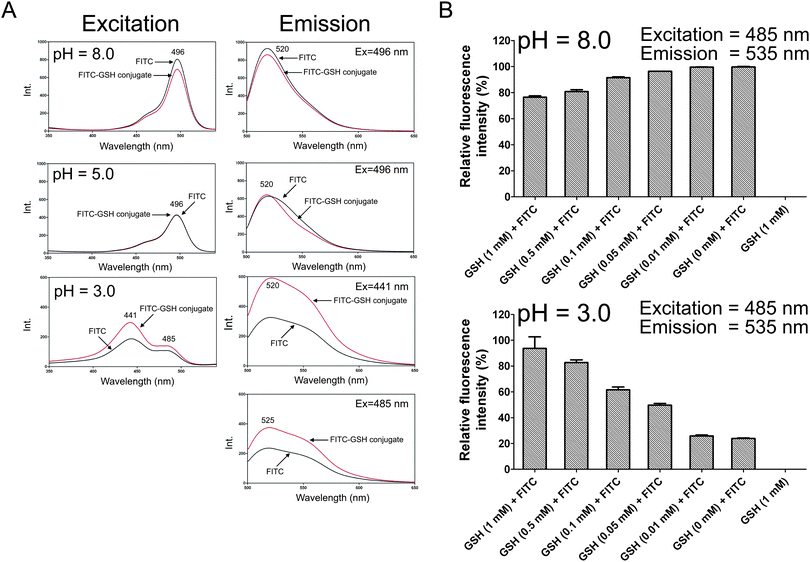 |
| | Fig. 3 The fluorescence spectra and intensities of FITC and FITC-GSH conjugate in different pH environments. (A) Fluorescence excitation (Ex) and fluorescence emission (Em) spectra of FITC and FITC-GSH conjugate at pH 8.0 (concentration of FITC = 0.1 μM), 5.0 (concentration of FITC = 0.1 μM) and 3.0 (concentration of FITC = 1 μM). (B) 0.1 mM FITC was incubated with a series of concentrations of GSH ranging from 0 to 1 mM for 30 min and the fluorescence intensities were measured with excitation at 485 nm and emission at 535 nm under basic (pH 8.0) and acidic (pH 3.0) conditions, respectively. | |
3.2 Assay development and optimization
A time course study showed that the reaction was complete within 10 min at pH 8.0 and the fluorescence intensity of FITC-GSH conjugate was almost unchanged during the next 50 min (Fig. 4A). So an incubation time of 30 min was selected for further experiments.
 |
| | Fig. 4 Assay development and optimization. (A) Time course of the reaction at room temperature. (B) The HPLC chromatogram and corresponding tandem mass spectrum of FITC in methanol for 1 h. | |
As methanol is a commonly used solvent for compounds in screening assays, the methanol tolerance of this assay was examined. FITC was dissolved in methanol and analyzed by LC-MS after 1 h. It was found that after 1 h incubation, FITC and methanol formed an FITC-CH3OH conjugate (Fig. 4B), which could be explained by the hydroxyl of CH3OH making a nucleophilic attack on the isothiocyanate group of FITC. When using acetonitrile as solvent, FITC was more stable (data not shown), thus acetonitrile was selected as the suitable solvent for FITC.
3.3 Screening assay for a known electrophilic compound menadione
Based on the above findings that the fluorescence intensities of FITC and FITC-GSH conjugate were different under acidic conditions (pH = 3.0), we established a simple screening method for the rapid discovery of electrophilic compounds. The schematic of the screening assay was shown in Fig. 1. A known electrophilic compound, menadione, was tested in this screening assay. Previous studies had shown that menadione could easily alkylate GSH.20 A series of concentrations of menadione (0–2.5 mM) were incubated with 1 mM GSH for 30 min, then 0.1 mM FITC was added and incubated for another 30 min. As shown in Fig. 5A, the fluorescence intensity of the reaction solution decreased as the concentration of menadione increased. The results of LC-MS analysis of the reaction solution indicated that when 2.5 mM menadione was added, FITC-GSH conjugate could not be detected in the HPLC chromatogram (Fig. 5B). When lower concentrations of menadione were added, the FITC-GSH conjugate signal gradually increased in a dose-dependent manner (Fig. 5B).
 |
| | Fig. 5 Screening assay for a known electrophilic compound, menadione. (A) Concentration course of the reaction. The fluorescence intensity of the reaction solution decreased as the concentration of menadione increased. (B) The HPLC chromatograms of the reaction solution. A series of concentrations of menadione (0–2.5 mM) were incubated with GSH (1 mM) for 30 min, and then FITC (0.1 mM) was added and incubated for another 30 min. The reaction solution was analyzed by LC-MS. (C) Tandem mass spectra of menadione-GSH conjugate in negative ion mode (upper graph) and FITC-GSH conjugate in positive ion mode (lower graph). | |
3.4 Screening assay for the ethyl acetate extract of S. miltiorrhiza and ten tanshinones isolated from it
A botanical sample, the ethyl acetate extract of S. miltiorrhiza, was tested using this screening method. As shown in Fig. 6A, the fluorescence intensity of the reaction solution decreased as the concentration of the ethyl acetate extract of S. miltiorrhiza increased, indicating there might be some electrophilic compounds contained in it, which was verified by the subsequent LC-MS analysis of the incubation solution of the ethyl acetate extract of S. miltiorrhiza and GSH. As shown in Fig. 6B, after incubation of the ethyl acetate extract of S. miltiorrhiza with GSH, plenty of extra peaks formed in the HPLC chromatogram, which represented the GSH conjugates. Then ten tanshinones were isolated from the ethyl acetate extract of S. miltiorrhiza (Fig. 6C) and tested for their alkylating capabilities. The results indicated that miltirone and its three derivatives (1-oxomiltirone, 4-methylenemiltirone and 1,2-didehydromiltirone) were electrophilic compounds while the other six were not (Fig. 6D and 6E), which was in accordance with the previous report,12 in which miltirone, 1-oxomiltirone, 4-methylenemiltirone and 1,2-didehydromiltirone could react with GSH completely (Table 1). The results were easily understood because the four electrophilic compounds all contained electron-deficient centers (as the asterisks indicated in Fig. 6C) and thus could be easily attacked by the thiol group of GSH.
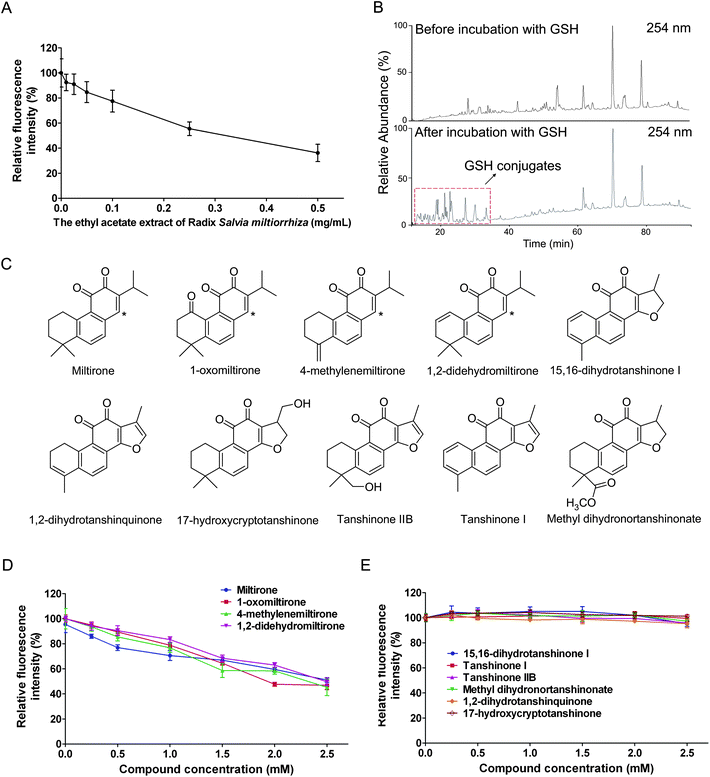 |
| | Fig. 6 Screening assay for the ethyl acetate extract of S. miltiorrhiza and ten tanshinones isolated from it. (A) A series of concentrations of the ethyl acetate extract of S. miltiorrhiza (0.5 mg mL−1, 0.25 mg mL−1, 0.1 mg mL−1, 0.05 mg mL−1, 0.025 mg mL−1 and 0.01 mg mL−1) were selected to test their alkylating capabilities using the screening assay. (B) The HPLC chromatograms before and after incubation of the ethyl acetate extract of S. miltiorrhiza with GSH, UVλ = 254 nm. (C) Chemical structures of ten tanshinones isolated from the ethyl acetate extract of S. miltiorrhiza. Asterisks represent electron-deficient centers. (D and E) Screening assay for ten isolated tanshinones. | |
Table 1 The fluorescence intensities of test samples and their conjugating capabilities with GSH
| Compound |
Fluorescence intensity of control (%) at the concentration 2.5 mM |
GSH conjugating rate (%) as literature reports12 |
| Miltirone |
51.0 ± 1.9 |
100 |
| 1-oxomiltirone |
46.8 ± 3.5 |
100 |
| 4-methylenemiltirone |
45.2 ± 6.6 |
100 |
| 1,2-didehydromiltirone |
49.6 ± 3.1 |
100 |
| 15,16-dihydrotanshinone I |
95.1 ± 2.2 |
0 |
| 1,2-dihydrotanshinquinone |
99.5 ± 2.9 |
0 |
| 17-hydroxycryptotanshinone |
95.9 ± 0.9 |
0 |
| Tanshinone IIB |
97.2 ± 1.8 |
0 |
| Tanshinone I |
95.2 ± 3.4 |
0 |
| Methyl dihydronortanshinonate |
101.2 ± 1.2 |
0 |
4. Conclusion
In this paper, a simple FITC-based screening method was established for the rapid discovery of electrophilic compounds. It was found that the fluorescence intensities of FITC and FITC-GSH conjugate were different under acidic conditions (pH 3.0). Based on these findings, we tested the ethyl acetate extract of S. miltiorrhiza and successfully identified four electrophilic compounds from it. Therefore, it is a fast and effective screening method and is suitable for high-throughput screening (HTS) assays in the future.
Acknowledgements
The work was financially supported by Zhejiang Key Science & Technological Program (2009C13028), Administration of Traditional Chinese Medicine of Zhejiang Province (2010ZA049) and Zhejiang Innovation Program for Graduates (YK2009015).
References
- T. Satoh, K. Kosaka, K. Itoh, A. Kobayashi, M. Yamamoto, Y. Shimojo, C. Kitajima, J. Cui, J. Kamins, S. Okamoto, M. Izumi, T. Shirasawa and S. A. Lipton, J. Neurochem., 2008, 104, 1116–1131 CrossRef CAS.
- J. Gayarre, K. Stamatakis, M. Renedo and D. Perez-Sala, FEBS Lett., 2005, 579, 5803–5806 CAS.
- J. P. Spencer, A. Jenner, O. I. Aruoma, P. J. Evans, H. Kaur, D. T. Dexter, P. Jenner, A. J. Lees, D. C. Marsden and B. Halliwell, FEBS Lett., 1994, 353, 246–250 CrossRef CAS.
- S. M. Nguyen, C. N. Alexejun and L. A. Levin, Antioxid. Redox Signaling, 2003, 5, 629–634 Search PubMed.
- M. Wetzel, G. A. Rosenberg and L. A. Cunningham, Eur. J. Neurosci., 2003, 18, 1050–1060 CrossRef CAS.
- A. T. Dinkova-Kostova, W. D. Holtzclaw and T. W. Kensler, Chem. Res. Toxicol., 2005, 18, 1779–1791 CrossRef CAS.
- F. Hong, M. L. Freeman and D. C. Liebler, Chem. Res. Toxicol., 2005, 18, 1917–1926 CrossRef CAS.
- B. Padmanabhan, K. I. Tong, T. Ohta, Y. Nakamura, M. Scharlock, M. Ohtsuji, M. I. Kang, A. Kobayashi, S. Yokoyama and M. Yamamoto, Mol. Cell, 2006, 21, 689–700 CrossRef CAS.
- T. Satoh, S. I. Okamoto, J. Cui, Y. Watanabe, K. Furuta, M. Suzuki, K. Tohyama and S. A. Lipton, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 768–773 CrossRef CAS.
- A. Y. Shih, P. Li and T. H. Murphy, J. Neurosci., 2005, 25, 10321–10335 CrossRef CAS.
- A. D. Kraft, D. A. Johnson and J. A. Johnson, J. Neurosci., 2004, 24, 1101–1112 CrossRef CAS.
- Z. Ma, M. Zhang and Z. Song, Rapid Commun. Mass Spectrom., 2009, 23, 2857–2866 CrossRef CAS.
- J. M. Lee, J. D. Moehlenkamp, J. M. Hanson and J. A. Johnson, Biochem. Biophys. Res. Commun., 2001, 280, 286–292 CrossRef CAS.
- P. J. Weber, J. E. Bader, G. Folkers and A. G. Beck-Sickinger, Bioorg. Med. Chem. Lett., 1998, 8, 597–600 CrossRef CAS.
- W. J. Wollack, N. A. Zeliadt, D. G. Mullen, G. Amundson, S. Geier, S. Falkum, E. V. Wattenberg, G. Barany and M. D. Distefano, J. Am. Chem. Soc., 2009, 131, 7293–7303 CrossRef.
- C. Gnoula, L. Guissou, J. Dubuis and P. Duez, Talanta, 2007, 71, 1886–1892 CrossRef CAS.
- N. Dias, J. F. Goossens, B. Baldeyrou, A. Lansiaux, P. Colson, A. Di Salvo, J. Bernal, A. Turnbull, D. Mincher and C. Baily, Bioconjugate Chem., 2005, 16, 949–958 CrossRef CAS.
- K. Pachmann, K. Reinecke, B. Emmerich and E. Thiel, Bioconjugate Chem., 1991, 2, 19–25 CrossRef CAS.
- J. Casanovas, D. Jacquemin, E. A. Perpete and C. Aleman, Chem. Phys., 2008, 354, 155–161 CrossRef CAS.
- Z. Ma and X. Zhang, Phytochem. Lett., 2009, 2, 152–158 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and B (HCOOH: CH3CN = 0.2
100) and B (HCOOH: CH3CN = 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). A 30 min gradient was used from 90% A to 100% B and maintained at 100% B during the next 6 min. The flow rate was 0.5 mL min−1 and the column temperature was set at 30 °C. The ultraviolet (UV) spectra were recorded at 280 nm. The MS operating parameters were as follows: collision gas, ultra-high-purity helium (He); nebulizing gas, high-purity nitrogen (N2); ion spray voltage, −4.5 kV; sheath gas (N2), 5 arbitrary units; capillary temperature, 275 °C; capillary voltage, −15 V; tube lens offset voltage, −30 V. The collision energy for collision-induced dissociation (CID) was between 30% and 45%, and the isolation width of precursor ions was 3.0 Th.
100). A 30 min gradient was used from 90% A to 100% B and maintained at 100% B during the next 6 min. The flow rate was 0.5 mL min−1 and the column temperature was set at 30 °C. The ultraviolet (UV) spectra were recorded at 280 nm. The MS operating parameters were as follows: collision gas, ultra-high-purity helium (He); nebulizing gas, high-purity nitrogen (N2); ion spray voltage, −4.5 kV; sheath gas (N2), 5 arbitrary units; capillary temperature, 275 °C; capillary voltage, −15 V; tube lens offset voltage, −30 V. The collision energy for collision-induced dissociation (CID) was between 30% and 45%, and the isolation width of precursor ions was 3.0 Th.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and B (CH3CN). A gradient program was used according to the following profile: 0–90 min, 20–100% B and maintained at 100% B during the next 10 min. The MS operating parameters were the same as described above.
100) and B (CH3CN). A gradient program was used according to the following profile: 0–90 min, 20–100% B and maintained at 100% B during the next 10 min. The MS operating parameters were the same as described above.





