Studying copper(II) ion induced interactions of β-amyloid peptides within living cells by gold nanoparticle probes†
Chengke
Wang
ab,
Jine
Wang
ab,
Dianjun
Liu
a and
Zhenxin
Wang
*a
aState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, China 130022. E-mail: wangzx@ciac.jl.cn; Fax: (+86) 431-85262243; Tel: (+86) 431-85262243
bGraduate School of the Chinese Academy of Sciences, Beijing, China
First published on 9th September 2010
Abstract
In this paper, a kind of gold nanoparticle (GNP)-based colorimetric assay was developed for studying copper(II) ion (Cu2+) induced interaction of β-amyloid (Aβ) peptide with living cells. In the presence of Cu2+, biotin modified β-amyloid peptides (Aβ1–16(biotin)) enable to attach on the SHG-44 (human glioma cell) cellular surface which results in significant color change of the cells when the indicators, streptavidin conjugated GNPs were introduced into the reaction mixture. We also demonstrate the assay ability to screen Aβ aggregation inhibitors because the aggregating/binding process can be easily reversed by adding chelators (e.g., EDTA, EGTA, histidine and clioquinol) of Cu2+ to the system. In addition, a time-dependent interaction of SHG-44 cells with Cu2+ and Aβ1–16(biotin) has been observed that may suggest different expression levels of Aβ related proteins in various cell cycles.
Introduction
Alzheimer's disease (AD) is a brain disorder disease that destroys brain cells with no known cause and no cure.1 AD is characterized by deposition of extracellular amyloid plaques, the major component being the β-amyloid peptide (Aβ).2–4 Metal-induced (e.g., Cu2+ and Zn2+) Aβ oligomerization is believed to play a key role in plaque formation and the course of the disease.5–8 In particular, a detailed knowledge of the Cu2+–Aβ interaction is the fundamental point of AD because the Cu2+ is able to influence conformational transformation of Aβ, and makes the Aβ monomers assemble to oligomers and eventually form fibrils or impact aggregates. In this aggregation process, the histidine residues in the position of 6, 13, 14 of the peptide are able to coordinate with Cu2+, which leads to plaque formation.9–12 The Aβ1–16 (i.e., the 16 amino acids residue at the N-terminal domain of Aβ) is the minimal fragment that is suitable for studying the role of Cu2+ in the aggregation of Aβ since the metallic ion–peptide interaction is the sole mechanism for forming the Aβ1–16 aggregate.13–15 In addition, Cu2+ chelators, such as clioquinol, 1,10-phenanthroline and EDTA, have been chosen to explore their effects on the removal of copper from Cu2+–Aβ species because metallic ion-chelation therapy has been used to prevent Aβ deposition and shown great promise for the cure of AD.16–19 Therefore, there is significant motive to develop a novel method requiring simple or no instrumentation yet still providing accurate study/analysis of Cu2+–Aβ interactions both in vitro and in vivo and screen amyloid aggregation inhibitors. A number of assays have already been developed for studying interactions of metallic ions with Aβ in solution.17,18 However, very little attention has been paid to metallic ion induced interactions of living cells with Aβ. In this regard, colorimetric methods based on functionalized gold nanoparticles (GNPs) are convenient and attractive, and can satisfy to meet these requirements. As versatile optical probes, GNPs have been extensively applied in analytical and biomedical research since the GNPs are bioinert and have strong surface plasmon resonance (SPR) absorption which is not only dependent on their shape and size, but also very sensitive to their local environments.20–27 In the past decade or so, numerous GNP-based assays have been developed for the detection of various targets, including: metal ions, small organic compounds, nucleic acids, proteins, cells etc.20–27Here we reported a simple, selective, and versatile GNP-based colorimetric assay, which can be employed for both studying the Cu2+ induced interaction of Aβ with living cells and assaying inhibition efficiencies of chelators. The assay is based on labeling Aβ bound living neurocytes by GNPs through streptavidin–biotin chemistry. In this proof-of-principle experiment, we demonstrate that the GNP-based approach is possible to (i) identify Cu2+ induced Aβ binding with living cells, and (ii) develop a simple assay for screening amyloid plaque inhibitors. In addition, a time-dependent interaction curve was observed, which may imply a time check point for Cu2+ in promoting Aβ pathology in AD.
Experimental
Materials and reagents
Peptides (CALNN, CALNNGK(biotin)G, (GK(biotin)GDAEFRHDSGYEVHHQK (Aβ1–16(biotin)) were purchased from Scilight Biotechnology Ltd. (Beijing, China). Hydrogen tetrachloroaurate trihydrate (HAuCl4·3H2O) was purchased from Sigma-Aldrich Co. (USA). Streptavidin was purchased from Promega Co. (USA). HeLa (human cervical cancer cell line) and SHG-44 (human glioma cell line) were obtained from Chinese Academy of Sciences Cell Bank. Other chemicals were analytical grade and used as received. Milli-Q water (18.2 MΩ.cm) was used in all experiments.Preparation of streptavidin-conjugated nanoparticles
The citrate stabilized 13 nm GNPs were synthesized by traditional Turkevich–Frens method.28,29 Peptide-stabilized nanoparticles were prepared by previously reported peptide capping procedure.30 Generally, an aqueous solution of peptide mixture (CALNN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CALNNGK(biotin)G = 9
CALNNGK(biotin)G = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the solution of 1 mL 13 nm GNPs (5 nM) to give a final concentration of total peptides of 1.5 mM. After 1 h incubation, excess peptides were removed by repeated centrifugation at 8800 rpm (∼7200 g, 3 times) using an Eppendorf centrifuge (Eppendorf, Germany). The purified GNPs were resuspended in 1 mL water. Then a 30 μL streptavidin (2 mg mL−1) aqueous solution was added to the solution. After 1 h incubation, excess streptavidin was removed by repeated centrifugation at 8800 rpm (∼7200 g, 3 times). The purified streptavidin-conjugated GNPs were resuspended in 1 mL HEPES buffer (pH 7.4, 10 mM HEPES, 0.8% NaCl (w/w)), which were named as streptavidin–GNPs. The streptavidin–GNPs were characterized by UV-visible spectroscopy and transmission electron microscopy (TEM, as shown in the ESI†). All experiments were carried out at room temperature unless mentioned otherwise.
1) was added to the solution of 1 mL 13 nm GNPs (5 nM) to give a final concentration of total peptides of 1.5 mM. After 1 h incubation, excess peptides were removed by repeated centrifugation at 8800 rpm (∼7200 g, 3 times) using an Eppendorf centrifuge (Eppendorf, Germany). The purified GNPs were resuspended in 1 mL water. Then a 30 μL streptavidin (2 mg mL−1) aqueous solution was added to the solution. After 1 h incubation, excess streptavidin was removed by repeated centrifugation at 8800 rpm (∼7200 g, 3 times). The purified streptavidin-conjugated GNPs were resuspended in 1 mL HEPES buffer (pH 7.4, 10 mM HEPES, 0.8% NaCl (w/w)), which were named as streptavidin–GNPs. The streptavidin–GNPs were characterized by UV-visible spectroscopy and transmission electron microscopy (TEM, as shown in the ESI†). All experiments were carried out at room temperature unless mentioned otherwise.
Cell experiment
HeLa cell line and SHG-44 cell line were grown with fresh Dulbecco's Modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum in a humidified 5% CO2 incubator at 37 °C.For the dose-dependent study, cells were seeded in 48 well plates (6000 cells in 200 μL DMEM supplemented with 10% fetal bovine serum) for 48 h. The medium was then replaced by 200 μL fresh DMEM supplemented with 4.1 μM Aβ1–16(biotin). Next, the desired amount of CuSO4 was added immediately and incubated for 12 h. Subsequently, the cells were washed with DMEM (200 μL, 3 times) and HEPES buffer (200 μL, 3 times), and incubated with streptavidin–GNPs (2.4 nM) in HEPES buffer (150 μL) for another 2 h, respectively. Then, the cells were washed with HEPES buffer (200 μL, 3 times), and imaged by a Soif 37XB inverted microscope (Shanghai Optical Instrument Factory, China) with an A470 IS digital camera (Canon, Japan).
For the chelator inhibition study, the cells was treated as described in a dose-dependent study, following the desired amount of chelators (EDTA, EGTA, histidine and clioquinol) were introduced into the reaction mixtures, respectively.
For the time-dependent study, Cu2+ and Aβ1–16(biotin) were introduced into the cell culture medium as previously described at different cell culture times (12, 24, 36, 48, 60, or 72 h), respectively. The cells were then incubated for another 12 h and treated as described in the dose-dependent study.
UV-visible absorption of GNP stained cells
Absorption spectra of GNP stained cells were measured by the microplate reader at room temperature. Generally, after being incubated with streptavidin–GNPs as previously described, GNP stained cells were washed with HEPES buffer carefully (200 μL, 3 times) to remove the unbounded nanoparticles, scraped from the culture plates, and re-dispersed in HEPES buffer with 2.25 × 105 cells mL−1. Then, 100 μL cell solution was transferred to 96 well plates, and recorded by the microplate reader (BioTek Powerwave XS2, BioTek Instruments Inc., USA), respectively. The corresponding streptavidin-GNP stained positive control sample (SHG-44 cells co-cultured with 4.1 μM Aβ1–16(biotin) alone) was used as background.Cyto-TEM measurement
The streptavidin-GNP stained cells were scraped from the culture plates and washed with HEPES buffer, concentrated by centrifugation (1500 rpm for 5 min). Then the cell pellet was incubated in 2.5% glutaraldehyde in PBS for 1 day at 4 °C. After post-fixed in 1% OsO4 for 2 h at 4 °C, the cells were washed again with PBS, dehydrated in an alcohol series, embedded in epoxy resin. Ultrathin sections of 70 nm were cut by a diamond knife and were taken up on 300 mesh copper grids. Finally, the samples were examined with a JEOL 2000FX (JEOL, Japan) transmission electron microscopy at an accelerating voltage of 120 kV.Results and discussion
The Cu2+ induced interaction of Aβ1–16(biotin) with cells
The schematic representation of the assay is shown in Fig. 1. In this case, Aβ1–16(biotin) was selected as a model molecule to study the Cu2+ induced interaction of Aβ with living cells. In the presence of Cu2+, the Aβ1–16(biotin) enables to bind with neurocytes. Through this reaction, together with the peptide, a biotin site is transferred to the cellular surface. In a subsequent step, the streptavidin-GNPs can be attached to the cell surfaces since the streptavidin has very high affinity with biotin.31 More efficient interaction of Aβ1–16(biotin) with cells can produce more biotin sites on the cellular surfaces, which leads to higher level of GNP staining. Furthermore, the GNPs can be dissociated when the chelators of Cu2+ were introduced into the reaction mixture. This process is manifested in a pronounced color change of the cells due to the strong plasmon resonance absorption of GNPs.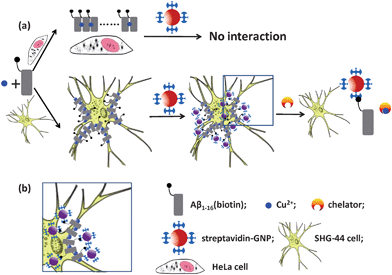 | ||
| Fig. 1 Schematic representation of Cu2+ induced interaction of Aβ1–16(biotin) with living cells (a); and details of attaching streptavidin–GNPs with Aβ1–16(biotin) binding cell (b). The illustration is not drawn to scale. | ||
To demonstrate the specificity and utility of this assay, Cu2+ induced interaction of Aβ1–16(biotin) with two living cell lines, SHG-44 and HeLa, were studied. SHG-44 cell line is a kind of neurocyte which derived from human frontal lobe astrocytoma which relates to several kinds of severe brain diseases. In this experiment, relatively high concentration (>0.5 μM) of Cu2+ was used because some components of culture medium (e.g., glutamine, tyrosine, and histidine residues) make it possible to coordinate with Cu2+ and decrease the concentration of free Cu2+ in the reaction mixture. After co-incubation with Cu2+ and Aβ1–16(biotin), SHG-44 cells exhibit a high level of streptavidin–GNP binding, the phenomena were able to be clearly observed by a conventional microscope and the amount of cellular binding particles was increased by increasing the concentration of Cu2+ while keeping Aβ1–16(biotin) concentration as a constant (as shown in Fig. 2a–f). In order to evaluate the selectivity of the assay, three kinds of control experiments (i.e., SHG-44 cells co-cultured with 50 μM Cu2+ alone (negative control), and SHG-44 cells co-cultured with 4.1 μM Aβ1–16(biotin) alone (positive control), and HeLa cells (non-neuronal cells) co-cultured with 50 μM Cu2+ and 4.1 μM Aβ1–16(biotin) under same experimental condition) have been designed. In the control experiments, there are only a few streptavidin–GNPs binding on the cellular surfaces (as shown in Fig. 2g–i). This experimental result demonstrates that our assay has great potential for studying the Cu2+ induced Aβ interaction with living neurocyte, which is one of mainly pathogenesis of AD. To further investigate the binding morphology of the GNPs with SHG-44 cell, cyto-TEM observations were performed. In this case, we clearly observed that the GNPs were attached on the microvilli of the SHG-44 cellular membranes (as shown in Fig. 3) and formed GNP clusters. The TEM experimental result indicates that Cu2+ may play as a mediator/bridge which links the Aβ1–16(biotin) to certain compositions of SHG-44 cellular membrane by coordinating with the histidine residues from both Aβ1–16(biotin) and relevant membrane proteins (e.g. amyloid precursor protein (APP)) which can generate Aβ by enzymatic cleavage in physiological conditions.5–7,32,33
 | ||
| Fig. 2 Microscopic images of streptavidin–GNP stained cells. The figures represent SHG-44 cells co-cultured with 4.1 μM Aβ1–16(biotin) in the presence of 0.5 (a), 1.0 (b), 2.0 (c), 5.0 (d), 20.0 (e), and 50.0 (f) μM Cu2+, respectively. SHG-44 cells co-cultured with 50 μM Cu2+ alone (negative control) (g), 4.1 μM Aβ1–16 (biotin) alone (positive control) (h), and HeLa cells co-cultured with 4.1 μM Aβ1–16(biotin) and 50 μM Cu2+ (i), respectively. The scale bars indicate 10 μm. | ||
 | ||
| Fig. 3 Cyto-TEM micrograph of streptavidin–GNP stained SHG-44 cell. The cell was co-cultured with 4.1 μM Aβ1–16(biotin) and 5 μM Cu2+. Red circles indicate nanoparticles adhered to the microvilli of the cell. The scale bar indicates 200 nm. | ||
The stained cells were analyzed by UV-visible spectroscopy to estimate the amount of Aβ1–16(biotin) binding with cells since GNPs have strong SPR absorption. As shown in Fig. 4, the SPR absorption intensity of stained SHG-44 cells is increased by increasing the concentration of Cu2+. This UV-visible spectroscopic result is consistent with the microscopic study and suggests that our assay could be applied to semi-quantitatively monitor metallic ion induced interaction of Aβ1–16(biotin) with cells.
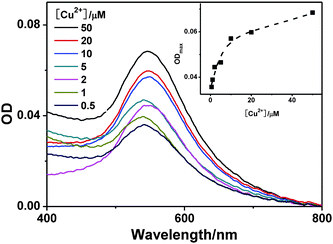 | ||
| Fig. 4 The UV-visible spectra of streptavidin–GNP stained SHG-44 cells (2.25 × 105 cells mL−1). The cells were co-cultured with 4.1 μM Aβ1–16(biotin) and various concentrations of Cu2+; then stained by streptavidin–GNPs (2.4 nM). Inset shows the corresponding maximum absorbance changes of the streptavidin–GNP stained SHG-44 cells. | ||
Time-dependent study
Most interesting, a cell culture time-dependent behavior was observed while SHG-44 cells co-incubated with Aβ1–16(biotin) and Cu2+. As shown in Fig. 5, the amount of cellular binding of streptavidin–GNPs is increased by increasing SHG-44 cells culture time from 0 h to 48 h and reaches peak value at 48 h. Then, the binding amount of particles on cell surfaces was gradually decreased by increasing the SHG-44 cells culturing time from 48 h to 72 h. This phenomenon suggests that Cu2+ induced interaction of Aβ1–16(biotin) with living cells is cell cycle related and 48 h culture time could be a check point of the interaction. At the check point, the SHG-44 cells may express the highest level of Aβ related membrane components (e.g., APP, tau protein and cellular produced Aβ peptides).34,35 This finding is also consistent with previous results that indicate a directly causal relation between extracellular Aβ level and neuron growth cycle or neuronal activity.36–39 Research efforts toward the exact mechanism of this process are in progress, for instance, using gene expression profiling approach to identify gene differences of SHG-44 cells at different culture time. Taken together, these experimental results indicate that our assay could provide a simple and visual method to investigate the metallic ions induced interaction of Aβ1–16(biotin) with cells. | ||
| Fig. 5 Time-dependent study of SHG-44 cells co-cultured with 4.1 μM Aβ1–16(biotin) and 5 μM of Cu2+. The cell culture time is 12 (a), 24 (b), 36 (c), 48 (d), 60 (e), or 72 (f) h, respectively. The scale bars indicate 10 μm. Corresponding UV-visible spectra of the streptavidin–GNP stained cells (g). Inset of (g) shows the maximum absorption changes of streptavidin–GNP stained cells with culture time. | ||
Inhibition by chelators
The Cu2+ chelators (EDTA, EGTA, histidine and clioquinol) were added into the culture medium to investigate their inhibitory abilities on the Cu2+ induced interactions of Aβ1–16(biotin) with living cells. The inhibition level of the chelators can also be visualized by conventional microscope and quantitatively measured by UV-visible spectroscopy because more efficient chelator (inhibitor) leads to higher level of disassembling Aβ1–16(biotin) with living cells, which in turn gives a significantly decreased absorption of cells. As shown in Fig. 6 and 7, the streptavidin–GNP stained level of cells was decreased by increasing the concentrations of chelators. This phenomenon suggests that the Cu2+ induced interaction of Aβ1–16(biotin) with living cells is inhibited by the chelators. In particular, we found that the inhibition efficiencies of these chelators are dependent on their coordination abilities with Cu2+, i.e., following the order, EGTA ≅ EDTA > clioquinol > histidine. This finding suggests that the approach could be used to screen and design new inhibitors of Cu2+ induced interaction of Aβ with cells.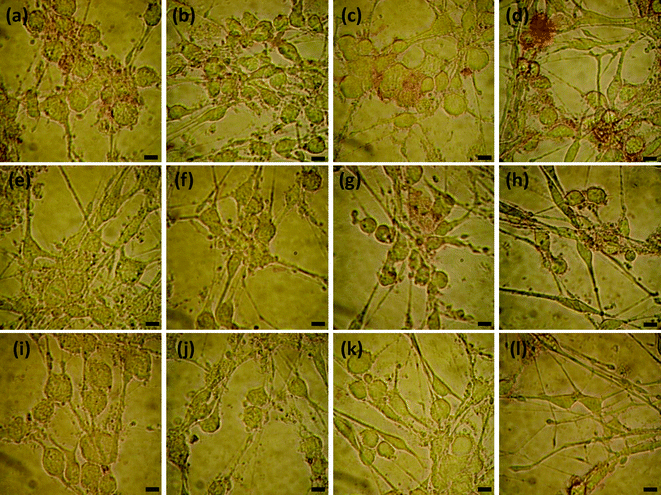 | ||
| Fig. 6 Effects of chelators on Cu2+ induced interaction of Aβ1–16(biotin) with SHG-44 cells (2.25 × 105 cells mL−1). The images represent: cells co-cultured with Aβ1–16(biotin), 5 μM Cu2+ and 0.5 (a), 5 (e), 50 (i) μM EDTA; 0.5 (b), 5 (f), 50 (j) μM EGTA; 2.5 (c), 25 (g), 250 (k) μM histidine; 0.5 (d), 5 (h), and 50 (l) μM clioquinol, respectively. The scale bars indicate 10 μm. | ||
 | ||
| Fig. 7 UV-visible spectral data analysis on inhibitory abilities of chelators. SHG-44 cells were co-cultured with 4.1 μM Aβ1–16(biotin), 5 μM Cu2+ and various concentrations of chelators, respectively. | ||
Conclusions
In summary, combining the unique optical properties of GNPs and high affinity of biotin and streptavidin, streptavidin–GNPs are used as optical probes for studying the Cu2+ induced Aβ interaction with living cells. As proof-of-principle experiment, the interactions of Cu2+, biotin modified Aβ1–16, and SHG-44 cell line have been carefully studied. The experimental results suggest that our assay could provide a simple and visual method to evaluate the role of Cu2+ in Aβ aggregation on living neural cell surfaces. Furthermore, the results also indicate that the assay is able to use as an effective tool to discover and screen novel compounds for inhibiting Aβ interaction with living neural cells.Acknowledgements
The authors would like to thank the NSFC (Grant No. 20675080, 20875087), Chinese Academy of Sciences (Grant No. KJCX2-YW-H11) and CAS-Bayer Start-up Fund (2008) for financial support.References
- http://www.alz.org/alzheimers_disease_facts_figures.asp .
- F. M. LaFerla, K. N. Green and S. Oddo, Nat. Rev. Neurosci., 2007, 8, 499–509 CrossRef CAS.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS.
- W. Qiu and M. F. Folstein, Neurobiol. Aging, 2006, 27, 190–198 CrossRef CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS.
- A. Rauk, Chem. Soc. Rev., 2009, 38, 2698–2715 RSC.
- P. Faller and C. Hureau, Dalton Trans., 2009, 1080–1094 RSC.
- D. Noy, I. Solomonov, O. Sinkevich, T. Arad, K. Kjaer and I. Sagi, J. Am. Chem. Soc., 2008, 130, 1376–1383 CrossRef CAS.
- M. J. Kogan, N. G. Bastus, R. Amigo, D. Grillo-Bosch, E. Araya, A. Turiel, A. Labarta, E. Giralt and V. F. Puntes, Nano Lett., 2005, 6, 110–115.
- K. Pagel, T. Seri, H. von Berlepsch, J. Griebel, R. Kirmse, C. Böttcher and B. Koksch, ChemBioChem, 2008, 9, 531–536 CrossRef CAS.
- H. Yu, J. Ren and X. Qu, ChemBioChem, 2008, 9, 879–882 CrossRef CAS.
- J. Shearer and V. A. Szalai, J. Am. Chem. Soc., 2008, 130, 17826–17835 CrossRef CAS.
- T. Miura, K. Suzuki, N. Kohata and H. Takeuchi, Biochemistry, 2000, 39, 7024–7031 CrossRef CAS.
- B.-k. Shin and S. Saxena, Biochemistry, 2008, 47, 9117–9123 CrossRef CAS.
- Q. F. Ma, J. Hu, W. H. Wu, H. D. Liu, J. T. Du, Y. Fu, Y. W. Wu, P. Lei, Y. F. Zhao and Y. M. Li, Biopolymers, 2006, 83, 20–31 CrossRef CAS.
- P. Inbar, M. R. Bautista, S. A. Takayama and J. Yang, Anal. Chem., 2008, 80, 3502–3506 CrossRef CAS.
- A. M. Mancino, S. S. Hindo, A. Kochi and M. H. Lim, Inorg. Chem., 2009, 48, 9596–9598 CrossRef CAS.
- S. S. Hindo, A. M. Mancino, J. J. Braymer, Y. Liu, S. Vivekanandan, A. Ramamoorthy and M. H. Lim, J. Am. Chem. Soc., 2009, 131, 16663–16665 CrossRef CAS.
- Giordano F. Z. da Silva and L.-J. Ming, Angew. Chem., Int. Ed., 2007, 46, 3337–3341 CrossRef.
- C. M. Niemeyer, Angew. Chem., Int. Ed., 2001, 40, 4128–4158 CrossRef CAS.
- E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS.
- N. Rosi and C. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS.
- M. De, P. Ghosh and V. Rotello, Adv. Mater., 2008, 20, 4225–4241 CrossRef CAS.
- L. Wang, J. Li, S. Song, D. Li and C. Fan, J. Phys. D: Appl. Phys., 2009, 42, 203001 CrossRef.
- B. Sepućlveda, P. C. Angelomeć, L. M. Lechuga and L. M. Liz-Marzaćn, Nano Today, 2009, 4, 244–251 CrossRef CAS.
- Z. Wang and L. Ma, Coord. Chem. Rev., 2009, 253, 1607–1618 CrossRef CAS.
- J. Liu, Z. Cao and Y. Lu, Chem. Rev., 2009, 109, 1948–1998 CrossRef CAS.
- J. Turkevich, P. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- G. Frens, Nature: Phys. Sci., 1973, 241, 20–22.
- C. Wang, J. Wang, D. Liu and Z. Wang, Talanta, 2010, 80, 1626–1631 CrossRef CAS.
- M. A. Hahn, P. C. Keng and T. D. Krauss, Anal. Chem., 2008, 80, 864–872 CrossRef CAS.
- N. Miyashita, J. E. Straub and D. Thirumalai, J. Am. Chem. Soc., 2009, 131, 17843–17852 CrossRef CAS.
- B. Bulic, M. Pickhardt, I. Khlistunova, J. Biernat, E.-M. Mandelkow, E. Mandelkow and H. Waldmann, Angew. Chem., Int. Ed., 2007, 46, 9215–9219 CrossRef.
- W. M. Brooks, P. J. Lynch, C. C. Ingle, A. Hatton, P. C. Emson, R. L. M. Faull and M. P. Starkey, Brain Res., 2007, 1127, 127–135 CrossRef CAS.
- T. Dunckley, T. G. Beach, K. E. Ramsey, A. Grover, D. Mastroeni, D. G. Walker, B. J. LaFleur, K. D. Coon, K. M. Brown, R. Caselli, W. Kukull, R. Higdon, D. McKeel, J. C. Morris, C. Hulette, D. Schmechel, E. M. Reiman, J. Rogers and D. A. Stephan, Neurobiol. Aging, 2006, 27, 1359–1371 CrossRef CAS.
- R. J. Bateman, G. Wen, J. C. Morris and D. M. Holtzman, Neurology, 2007, 68, 666–669 CrossRef CAS.
- D. L. Brody, S. Magnoni, K. E. Schwetye, M. L. Spinner, T. J. Esparza, N. Stocchetti, G. J. Zipfel and D. M. Holtzman, Science, 2008, 321, 1221–1224 CrossRef CAS.
- K. M. Webber, A. K. Raina, M. W. Marlatt, X. Zhu, M. I. Prat, L. Morelli, G. Casadesus, G. Perry and M. A. Smith, Mech. Ageing Dev., 2005, 126, 1019–1025 CrossRef CAS.
- J. Woods, M. Snape and M. A. Smith, Biochim. Biophys. Acta, Mol. Basis Dis., 2007, 1772, 503–508 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-visible spectrum and TEM image of the streptavidin–GNPs. See DOI: 10.1039/c0ay00438c |
| This journal is © The Royal Society of Chemistry 2010 |
