DOI:
10.1039/C0AY00303D
(Paper)
Anal. Methods, 2010,
2, 1316-1319
Sensitive assay for picogram levels of sudan I in chilli foodstuffs by flow injection chemiluminescence
Received
7th May 2010
, Accepted 21st June 2010
First published on
29th July 2010
Abstract
The determination of Sudan I in contaminated hot chilli products by luminol–myoglobin chemiluminescence was proposed. It was found that the Sudan dyes (Sudan I, II, III and IV) could bind to myoglobin and remarkably enhance the chemiluminescence signal from luminol–myoglobin system, and the increment of chemiluminescence intensity was proportional to the concentration of Sudan I, II, III and IV in the range of 0.1–300 pg mL−1, 5.0–1000 pg mL−1, 10.0–1000 pg mL−1 and 1.0–300 pg mL−1, respectively. At the flow rate of 2.0 mL min−1, a typical analytical procedure for Sudan dyes including sampling and washing was finished within 0.5 min with the relative standard deviation less than 3.0%. The present CL method was successfully applied to the determination of Sudan I in contaminated soy sauces and agreed well with HPLC data. The possible mechanism of the CL reaction was given.
Introduction
The red dyes of Sudan I, II, III and IV are oil soluble, meaning they were forbidden for use in food as additives due to their toxicity. The International Agency for Research on Cancer (IARC) has assessed the Sudan dyes as Group 3 genotoxic carcinogens.1 In recent years, Sudan I has been found as a contaminant in chilli powder and some foodstuffs prepared with it, such as sauce, soup, and ready meals.2 Several general procedures have been employed for the measurements of Sudan dyes, such as high performance liquid chromatography-mass spectroscopy (HPLC-MS),3,4 HPLC-ultraviolet-visible (HPLC-UV)5–7 and HPLC-chemiluminescence (HPLC-CL),8 capillary electrophoresis (CE),9 electrochemical (EC) determination10 and molecularly imprinted solid phase extraction (MISPE).11 CL detection combined with flow injection (FI) system, offering high sensitivity, instrumental simplicity, sampling efficiency and reduced consumption, is an attractive alternative for food analysis. We have recently reported the luminol–KIO412 and luminol–H2O213 CL systems for the determination of Sudan I in contaminated hot chilli sauce with the limit of detection (LOD) of 0.03 and 3.0 pg mL−1, respectively.
It was reported that CL intensity of luminol with Mb in alkaline medium could be enhanced or inhibited evidently with small molecular ligands.14 For example, nitrite, formaldehyde and carbon monoxide could enhance the luminol–Mb CL intensity,15–17 melamine18 could inhibit the luminol–Mb CL intensity. In this work, based on the specific binding of Mb with Sudan I to catalyze the luminol–Mb CL reaction, the determination of picogram levels Sudan I was proposed. Under the optimum conditions, the increment of CL intensity was proportional to the concentration of Sudan I, II, III and IV in the range of 0.1–300 pg mL−1, 5.0–1000 pg mL−1, 10.0–1000 pg mL−1 and 1.0–300 pg mL−1, respectively, and the LODs for them were 0.03 pg mL−1, 1.5 pg mL−1, 3.4 pg mL−1 and 0.4 pg mL−1, respectively. With the flow rate of 2.0 mL min−1, the whole procedure including sampling and washing was accomplished within 0.5 min and the throughput was 120 h−1. Chilli foodstuff samples confirmed as contaminant were analyzed by the proposed procedure with results obtained in a good agreement with that of the reference method.19
Materials and methods
Apparatus
The schematic profile of equipment in the flow system was described in Fig. 1 with conditions stated. The flow system consisted of four lines of luminol, Mb, sample and NaOH solution. A peristaltic pump of the IFFM-E Luminescence Analyzer (Xi'an Remax Electronic Science-Tech. Co. Ltd., Xi'an, China) propelled all streams at a flow rate (per tube) of 2.0 mL min−1. PTFE tubing (1.0 mm i.d.) was used throughout the manifold for connecting all components and carrying the CL reagents. A six-way valve with a loop of 100 μL was used for sampling. The flow cell was made by coiling a 15 cm length of glass tube (1.0 mm i.d.) into a spiral disk shape with a diameter of 2 cm in order to produce a large surface area exposed close to photomultiplier tubes (PMT). The CL signal induced in the flow cell was detected without wavelength discrimination, and the output of PMT was recorded by a computer with an IFFE-E client system (Remax, Xi'an, China).
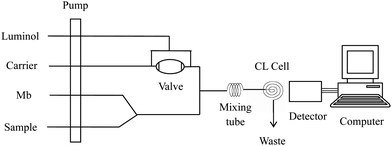 |
| | Fig. 1 Schematic diagram of the FI-CL system for the determination of Sudan dyes. Luminol: 1.0 × 10−5 mol L−1; Mb: 5.0 × 10−8 mol L−1; NaOH: 0.05 mol L−1; flow rate: 2.0 mL min−1; high voltage: −700 V. | |
Reagents
All reagents were of analytical-reagent grade; doubly deionized water purified in a Milli-Q system (Millipore, Bedford, MA, USA) was used for the preparation of solutions in the whole procedure. Luminol (Fluka, Biochemika) obtained from Xi'an Medicine Purchasing and Supply Station, China, was solved in 0.5 mol L−1 sodium hydroxide to prepare the stock solution (2.5 × 10−2 mol L−1) in a brown calibrated flask. Horse heart Mb (Sigma) was purchased from local market and used as received without further purification. All working strength solutions in the experiment were prepared freshly.
General procedure
The solutions (luminol, Mb, carrier and sample) were propelled at a flow rate of 2.0 mL min−1 on each line as Fig. 1 shown. The peristaltic pump was started to wash the whole flow system until a stable baseline was recorded. Luminol (100 μL) was quantitatively injected into the mixed solutions of Mb and samples by a six-way valve. The final solution was delivered to the flow cell producing CL signal in the alkaline medium. The peak height of CL was detected by PMT (negative voltage set as 700 V) and displayed on the computer. The concentration of sample could be quantified by the accompanied increment of CL intensity, ΔI = Is − Io, where Is and Io was the CL signal in the presence and in the absence of sample solution, respectively.
The samples of three soy sauces (Golden mark satay sauce, Pixian bean sauce, and Golden mark Guilin chilli sauce confirmed as contaminated samples by Sudan I, supplied by Entry–Exit Inspection and Quarantine Bureau in Shaanxi, China) were used in the experiment. The three samples were pretreated according to the reference method,19 which were ground to fine powder firstly, approximate 3.0 g was weighted and dissolved with the mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetonitrile and pure water in a digester. The solution was homogenized ultrasonically and the digested sample was centrifuged and the supernatant solution was used for assay.
1 (v/v) acetonitrile and pure water in a digester. The solution was homogenized ultrasonically and the digested sample was centrifuged and the supernatant solution was used for assay.
Results and discussion
Intensity of different CL systems
Intensity of different CL systems in the flow injection system was showed in Fig. 2. For curve a and b at luminol system, it is clear that the time of maximum CL intensity in the absence and presence of Sudan I is around 8.2 (±0.2) s with the corresponding intensity around 66 (±4); as to curve c and d, the CL intensity of luminol–Mb reached the maximum changed from 7.8 s to 6 s with its relative intensity increased from 211 to 456 in the absence and presence of Sudan I.
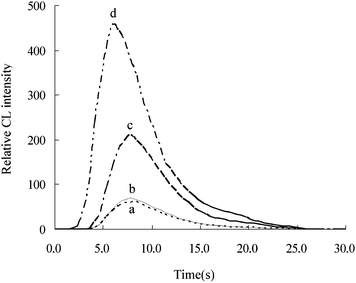 |
| | Fig. 2 Intensity of different CL systems in the flow injection system. a: luminol CL system; b: luminol–Sudan I CL system; c: luminol–Mb CL system; d: luminol–Mb–Sudan I CL system. The concentrations of Mb, Sudan I and luminol were 5.0 × 10−8 mol L−1, 100 pg mL−1 and 1.0 × 10−5 mol L−1, respectively. | |
Effect of luminol, Mb and NaOH concentrations
Influences of the concentrations of luminol and Mb on the CL intensity were examined by testing a series of standard solutions from 5.0 × 10−7 to 1.0 × 10−4 mol L−1 and from 1.0 × 10−9 to 2.0 × 10−7 mol L−1 for luminol and Mb, respectively. The CL signal reached steady state with the concentrations of luminol up to 1.0 × 10−5 mol L−1. As to Mb, the CL intensity approached a maximum when its concentration reached 5.0 × 10−8 mol L−1. Thus 1.0 × 10−5 mol L−1 luminol and 5.0 × 10−8 mol L−1 Mb were used in subsequent experiments.
Because the luminol reaction is more favored under basic conditions, NaOH was introduced to improve the sensitivity of the system. A series of NaOH solutions with different concentrations, such as 0.005 mol L−1, 0.01 mol L−1, 0.03 mol L−1, 0.05 mol L−1, 0.07 mol L−1 and 0.1 mol L−1 were tested. The CL intensity arrived at a peak with the concentration of NaOH arriving at about 0.05 mol L−1, so this concentration was employed in subsequent experiments.
Effect of pH on Mb solution
Mb exists mainly in the state of MbO2 in aqueous solutions, and MbO2 is easily autooxidized to Mb(FeIII) below pH 6.5, whereas above pH 8.0, MbO2 is dominant in Mb(FeII).20 It was found that the CL intensity increased steeply with the pH of Mb up to 6.5, above which it decreased. Thus, the Mb solution was prepared directly in water at pH 6.5.
Effect of reaction tube length and flow rate
The effect of the mixing tube length on CL intensity was tested from 7.0 to 19.0 cm. It could be observed that the CL intensity was much stronger using 16.0 cm mixing tube than that of others in the presence of 5.0 pg mL−1 Sudan I. The influence of flow rate was tested, and the flow rate of 2.0 mL min−1 offering highest ratio of signal to noise (S/N) was chosen as suitable condition considering analytical precision.
Performance of Sudan I-IV measurements
A series of Sudan I, II, III and IV standard solutions were injected into the manifold depicted in Fig. 1. The CL intensity was measured by detector and recorded by a computer with an IFFE-E client system. The concentration of Sudan dyes could be quantified by the accompanied increment of CL intensity, ΔI = Is − Io, where Is and Io was the CL signal in the presence and in the absence of Sudan dye, and the results were listed in Table 1. Compared with the determination of Sudan II, III and IV, Sudan I showed the highest sensitivity and the widest calibration graph linear over the concentration from 0.1 pg mL−1 to 300 pg mL−1 with the lowest LOD 0.03 pg mL−1 (3σ).
Table 1 Regression equations, linear ranges and limits of detection of Sudan dyes (n = 7)
| Sudan dyes |
Regression equation/R2 |
Linear range pg mL−1 |
RSD % Slope/intercept |
LOD (3σ) pg mL−1 |
| I |
ΔICL = 2.22CSudanI + 25.55 |
0.1–300 |
1.4/0.5 |
0.03 |
| 0.9963 |
| II |
ΔICL = 0.41CSudanII + 33.32 |
5.0–1000 |
4.7/0.8 |
1.5 |
| 0.9927 |
| III |
ΔICL = 0.24CSudanIII + 10.55 |
10.0–1000 |
5.4/1.2 |
3.4 |
| 0.9922 |
| IV |
ΔICL = 1.31CSudanIV + 9.81 |
1.0–300 |
1.8/3.4 |
0.4 |
| 0.9921 |
Interference studies
Considering the effect of the matrix for the determination of Sudan I in hot chilli samples, the influence of foreign substances was tested. Under optimized conditions, increasing amounts of interfering species were added to Sudan I standard solutions (10.0 pg mL−1). The tolerable limit of foreign substance was taken as the relative error less than 5%. The results were shown in Table 2, from which it could be seen that most of the species studied did not interfere with the determination.
Table 2 Tolerable ratio of foreign species with respect to 10.0 pg mL−1 Sudan I (< 5% error)
| Species |
Tolerable ratio |
Species |
Tolerable ratio |
| NO3−, Br−, PO43−, SO42− |
5.0 × 105 |
citric acid, ascorbic acid, tartaric acid, malic acid, Co2+, Cu2+, Pb2+, Fe3+/Fe2+ |
5.0 × 103 |
| Mg2+, Ca2+, Zn2+, Ni2+, Cr3+ |
| barbiturate, albumin, amylum, glucose, borate, oxalate, benzoic acid |
7.0 × 103 |
Determination of Sudan I in soy sauce samples
The proposed method was applied to the assay of Sudan I in three contaminated hot chilli samples. Following the Sample preparation section, Golden mark satay sauce, Pixian bean sauce, and Golden mark Guilin chilli sauce were prepared for the determinations. In order to verify the validity of the present method, HPLC was employed to detect the content of Sudan I in Golden mark satay sauce sample.19 The other two samples undetectable with HPLC were divided into two groups: one was spiked into Sudan I standard solutions and the other was not spiked. The results for the determination of Sudan I in the three contaminated soy sauces were summarized in Table 3, from which it can be seen that the RSDs were less than 3.0% and recoveries were ranging from 92.9% to 113.3%. The contents of Sudan I measured by the proposed method was in a good agreement with that of comparison.
Table 3 Results of Sudan I in chilli productsa
| Sample No. |
Added pg mL−1 |
Found pg mL−1 |
Recovery (%) |
RSD (%) |
Content in sauce μg g−1 |
| By proposed method |
By HPLC |
|
The average of five determinations.
Golden mark satay sauce.
Pixian bean sauce.
Golden mark Guilin chilli sauce.
|
| 1b |
0 |
4.1 |
97.3 |
2.3 |
1.37 |
1.34 |
| 10 |
13.8 |
1.4 |
| 2b |
0 |
11.3 |
96.6 |
3.0 |
1.32 |
| 20 |
30.6 |
1.6 |
| 3c |
0 |
5.5 |
113.3 |
1.8 |
0.55 |
undetectable |
| 10 |
16.8 |
1.7 |
| 4c |
0 |
3.1 |
106.3 |
1.5 |
0.56 |
undetectable |
| 10 |
13.8 |
1.4 |
| 5d |
0 |
4.8 |
92.9 |
1.8 |
1.20 |
undetectable |
| 10 |
14.1 |
1.2 |
| 6d |
0 |
11.4 |
97.4 |
1.9 |
1.11 |
undetectable |
| 20 |
26.3 |
1.2 |
Possible mechanism of the CL reaction
The possible CL mechanism of Sudan I enhancing luminol–Mb reaction was discussed using UV and CL method and the results were listed in Table 4. It was found that the absorption of Mb at 409 nm, which was the characteristic absorption wavelength of Mb(FeIII),14 was subdued from 0.2120 to 0.1735 in the presence of Sudan I, which suggested that Mb interact with Sudan I. According to the CL results, it could be seen that the time of the maximum CL intensity of luminol–Mb shifted from 7.8 s to 6 s in the presence of Sudan I with the corresponding intensity increased from 211 to 456. Thus, it was supposed that Sudan I could bind to Mb, forming a complex, which accelerate the electron transfer of luminol and resulted in the CL signal of luminal–Mb remarkably increased.
Table 4 Results of different reaction systems by UVa and CLb
| System |
A409 nm |
CL (T1/s)/ICL |
CL (T2/s) |
|
The concentrations of Mb, Sudan I and luminol were 1.0 × 10−6, 2.0 × 10−6 and 1.0 × 10−5 mol L−1, respectively.
The concentrations of Mb, Sudan I and luminol were 5.0 × 10−8 mol L−1, 100 pg mL−1 and 1.0 × 10−5 mol L−1, respectively. T1 and T2 represent the corresponding time of CL signal reaching the maximum and vanishing, respectively.
|
| Mb/luminol/Sudan I |
0.2120/0.0370/0.0437 |
— |
— |
| Mb–Sudan I |
0.1735 |
— |
— |
| luminol–Sudan I |
0.0454 |
8.2/70 |
29.0 |
| luminol–Mb |
0.0502 |
7.8/211 |
26.0 |
| luminol–Mb–Sudan I |
0.0507 |
6.0/456 |
24.0 |
Conclusion
The comparison of the proposed procedure with other methods is presented in Table 5. The proposed CL method shows the advantages of simplicity of apparatus, less reagent consumption, the highest sensitivity and the lowest LOD for the determination of Sudan I. Maybe it is a good pathway for the simultaneous determination of the Sudan dyes (I, II, III, IV) with the present sensitive CL detection and HPLC separation.
Table 5 Comparison of different methods for determination of Sudan I
| Methods |
Linear range/ng mL−1 |
LOD/ng mL−1 |
Refs |
| HPLC-MS |
5 ∼ 18 |
5 |
4
|
| HPLC-UV |
20 ∼ 100 |
6 |
5
|
| HPLC-CL |
1 ∼ 2000 |
— |
8
|
| CE |
96 ∼ 610 |
96.5 |
9
|
| EC |
0.2 ∼ 4 |
0.08 |
10
|
| Proposed method |
1 × 10−4 ∼ 0.3 |
3 × 10−5 |
this work |
Acknowledgements
The authors gratefully acknowledge the financial support from Shaanxi Province Nature Science Foundation, the Foundation of Ministry of Education, the NWU Graduate Innovation and Creativity Funds and NWU Graduate Experimental Research Funds, China, Grant No. 2006B05, No. 07JK395, No. 09YZZ45 and No. 09YSY18.
References
-
IARC, World Health Organisation. International Agency for Research on Cancer. IARC Monographs on the evaluation of the carcinogenic risk of chemicals to man: Some aromatic azo compounds. Lyon, 1975, 8, 225 Search PubMed.
-
Food Standards Agency, Sudan dyes in chilli imported from India: Guidance notes, http://www.food.gov.uk/foodindustry/guidancenotes/foodguid/sudanguidance. Accessed 18 February 2004.
- F. Calbiani, M. Careri, L. Elviri, A. Mangia, L. Pistarà and I. Zagnoni, J. Chromatogr., A, 2004, 1042, 123–130 CrossRef CAS.
- M. R. V. S. Murty, N. Sridhara Chary, S. Prabhakar, N. Prasada Raju and M. Vairamani, Food Chem., 2009, 115, 1556–1562 CrossRef CAS.
- Y. Ye, B. R. Xiang, W. Zhang and E. X. Shang, Phys. Lett. A, 2006, 359, 620–623 CrossRef CAS.
- M. Mazzetti, R. Fascioli, I. Mazzoncini, G. Spinelli, I. Morelli and A. Bertoli, Food Addit. Contam., Part A, 2004, 21, 935–941 Search PubMed.
- V. Cornet, Y. Govaert, G. Moens, J. Van Loco and J. M. Degroodt, J. Agric. Food Chem., 2006, 54, 639–644 CrossRef CAS.
- Y. T. Zhang, Z. J. Zhang and Y. H. Sun, J. Chromatogr., A, 2006, 1129, 34–40 CrossRef CAS.
- E. Mejia, Y. S. Ding, M. F. Mora and C. D. Garcia, Food Chem., 2007, 102, 1027–1033 CrossRef CAS.
- H. G. Lin, G. Li and K. B. Wu, Food Chem., 2008, 107, 531–536 CrossRef CAS.
- F. Puoci, C. Garreffa, F. Iemma, R. Muzzalupo, U. G. Spizzirri and N. Picci, Food Chem., 2005, 93, 349–353 CrossRef CAS.
- X. F. Gao, H. Y. Liu, Z. H. Song, X. L. He and F. X. Dong, Spectroscopy, 2007, 21, 135–141 Search PubMed.
- Y. H. Liu, Z. H. Song, F. X. Dong and L. Zhang, J. Agric. Food Chem., 2007, 55, 614–617 CrossRef CAS.
- Z. H. Song, L. Wang and S. Hou, Anal. Bioanal. Chem., 2004, 378, 529–535 CrossRef CAS.
- Q. L. Yue and Z. H. Song, Microchem. J., 2006, 84, 10–13 CrossRef CAS.
- X. F. Xie, Z. H. Song and X. D. Shao, Int. J. Environ. Anal. Chem., 2007, 87, 149–157 CrossRef CAS.
- X. F. Xie, X. L. He and Z. H. Song, Appl. Spectrosc., 2007, 61, 706–710 CrossRef CAS.
- Z. M. Wang, D. H. Chen, X. J. Tan, X. Gao and Z. H. Song, J. Agric. Food Chem., 2009, 57, 3464–3469 CrossRef CAS.
- GB/T 19681-2005, The method for the determination of sudan dyes in foods-high performance liquid chromatography. Meat Hygiene, 2005, Vol. 8 Search PubMed.
- H. D. Pojahn, C. H. Dreher and R. V. Eldik, J. Am. Chem. Soc., 1990, 112, 6304–6309 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetonitrile and pure water in a digester. The solution was homogenized ultrasonically and the digested sample was centrifuged and the supernatant solution was used for assay.
1 (v/v) acetonitrile and pure water in a digester. The solution was homogenized ultrasonically and the digested sample was centrifuged and the supernatant solution was used for assay.

