Qualitative and quantitative analysis of retinol, retinyl esters, tocopherols and selected carotenoids out of various internal organs from different species by HPLC
Michael W.
Schäffer
a,
Somdutta Sinha
Roy
a,
Shyamali
Mukherjee
a,
Donatus
Nohr
b,
Michael
Wolter
b,
Hans K.
Biesalski
b,
David E.
Ong
c and
Salil K.
Das
*a
aMeharry Medical College, Dept. of Biochemistry and Cancer Biology, 1005, Dr D.B. Todd, Jr. Blvd., Nashville, TN 37208, U.S.A. E-mail: sdas@mmc.edu; Fax: +1 615- 327- 6442; Tel: +1 615-327-6988
bDepartment of Biological Chemistry and Nutrition, University of Hohenheim, Garbenstraße 30, 70593, Stuttgart, Germany
cDepartment of Biochemistry, Vanderbilt University Medical Center, Nashville, TN 37232, U.S.A
First published on 30th July 2010
Abstract
We report the validation of a reversed-phase gradient HPLC method allowing simultaneous quantification of retinol, retinyl esters, tocopherols and selected carotenoids in lung, liver and plasma of mouse, rat and guinea pig (gp) using a diode array detector. A significant species difference was observed regarding the distribution of retinol and retinyl esters. The levels of total retinol in lung, liver and plasma were in the following order: mouse ≫ rat > gp; rat >mouse > gp; and gp ≫ rat > mouse, respectively. Furthermore, comparison studies revealed similarities between the vitamin A profiles of human and gp lung samples.
Introduction
Vitamin A (retinol) and its derivatives are known to have a wide range of functions ranging from their role in embryonic development to the prevention of cancer. The active form of vitamin A, retinoic acid, affects many downstream target genes, some of which are up-regulated in cancers. There is always a fine balance maintained between the various forms of vitamin A available in the body, therefore it is critical to have an accurate profile of different retinoids under different experimental conditions. Similarly, vitamin E (tocopherols; α, γ and δ) and various carotenoids (lutein and lycopene) are important antioxidants and part of cellular defense mechanisms.High Performance Liquid Chromatography (HPLC) is a well-established tool for separation of various retinoids, tocopherols, and carotenoids. Analyses have been done on normal phase and reverse phase columns using mostly single wavelength detection method. Most of the methods described previously dealt with either polar1 or non-polar retinoids/vitamin A.2 Analysis of samples containing both forms resulted in either very long run times3 or restricted the analysis to one or two wavelengths.4 Barua and Olson5 however described a method using photodiode array detection where they could separate various retinoids, carotenoids and tocopherols within 30 min of run time. Unfortunately, this method uses buffers and methanol as solvents resulting in a high column back pressure. Alternatively a run conducted at lower flow rates increased the run time to almost an hour. Thus, it seemed important to design a new separation method, which would combine a high flow rate, a lower column pressure and a shorter separation time, to give a clean separation of retinoids, vitamin A, carotenoids, and tocopherol. In this study we report and validate a versatile reverse-phased gradient HPLC method that allows the simultaneous detection of retinol, retinyl esters (retinyl palmitate, stearate, laurate, oleate), tocopherol isomers (α, γ, δ), selected carotenoids (lutein, lycopene, β-carotene), and possibly retinoic acid (RA) isomers (13-cis, 9-cis, all-trans-RA), using a diode array detector within a total run time of 26 min.
The starting point for the development of this gradient method was an isocratic determination for polar retinoids,1 combined with a gradient method that allowed the detection of more lipophilic analytes like retinyl esters and carotenoids.2 Some modifications were required to achieve the best results. Since this method allows detection over a wide range of the UV-spectrum at the same time, tocopherol isomers (α,γ,δ) and selected carotenoids (lutein, lycopene, β-carotene) can also be determined with reasonable sensitivity. It should be noted that we reported earlier6 the identification of lutein in guinea pig lungs, using the same HPLC-conditions as described in this paper. However, here we report for the first time the separation of vitamin A, its metabolites and tocopherols, as well as the validation of this HPLC-method.
We tested the validity of the method in quantifying the above referred antioxidant analytes in tissue samples (lung, liver, and plasma) of different rodent species (rat, mouse and guinea pig). In addition, we included in our study the analysis of human lung specimens obtained from the Lung Tissue Research Consortium, Bethesda, MD.
Experimental
Chemicals and reagents
HPLC-grade methanol, n-hexane, tetrahydrofurane (THF), and acetic acid (glacial) were purchased from Fisher Scientific (Pittsburgh, PA). HPLC-grade acetonitrile and ethanol were purchased from Pharmaco-AAPER (Shelbyville, KY). Standards (9-cis-, 13-cis-, all-trans-retinoic acid, retinol, retinyl acetate, retinyl palmitate, (+)-α, (+)-γ, rac-β-and (+)-δ-tocopherol were obtained from Sigma-Aldrich (St. Louis, MO). All-trans-lutein (isolated, 94%), all-trans-zeaxanthin (synth. 97%), all-trans-lycopene (synth. 95%) and β-carotene (synth. 96%) were purchased from Carotenature GmbH (Lupsingen Switzerland). Retinyl esters (retinyl laurate, -palmitate, -oleate, and –stearate) were a kind gift from Dr A. Catherine Ross (Department of Nutritional Sciences, Pennsylvania State University, University Park, PA). Standard solutions were prepared and handled under protection from light and stored at −80 °C. Fetal bovine serum (FBS) was purchased from Sigma-Aldrich, St. Louis MO.Equipment
The HPLC system (Shimadzu, Norcross, GA USA) was equipped with an autoinjector SIL-10ADvp (with 20 μL injection loop), an online solvent degasser DGU-14A, a system controller CBM-10AWvp, a low pressure gradient unit FCV-10ALvp, a solvent delivery unit LC-10ADvp, a column oven CTO-10Avp and a photodiode-array detector SPD-M10Avp, controlled by EZStart v7.3 software.Chromatographic conditions
Chromatographic analysis was performed using an analytical scale (Dimensions 300 mm × 4 mm) Nucleosil column C18, with a particle size of 3 μm and a pore size of 120 Å (ES industries, West Berlin, NJ). A ODS C18 guard column (4 mm × 3 mm) (Phenomenex, Torrance, CA) preceded the running column. Chromatographic conditions were as follows: mobile phase A: acetonitrile/H2O/glacial acetic acid - 90/10/2; mobile phase B: acetonitrile/methanol - 90/10; mobile phase C: 100% THF). All mobile phases were filtered through nylon membrane filters with 0.45 μm pore size (Whatman® International Ltd., Maidstone, England) before use. Over a total run time of 26 min, the following gradient program was applied: 0–4.5 min 100% A; 4.5–6.5 min to 100% B; 6.5–12 min to 60% B, 40% C; 12–15 min holding at 60% B, 40% C; 15–20 min to 100% B; 20–26 min back to 100% A with a constant flow rate of 1 mL/min and a constant column temperature of 22 °CAnimal housing
Guinea pigs (Hartley Strain, Cavia porcellus, 7–8 weeks), rats (F344, >12 weeks) and mice (C57BL6, 8 weeks), were obtained from Harlan (Indianapolis, IN) and Charles River (Wilmington, MA). Animals received a standard diet (guinea pig: #7006; Harland Teklad, Madison, WI: rats and mice: Purina Laboratory Rodent Diet #5001, PMI, St. Louis, MO, each with 15 IU/g of vitamin A), drinking water ad libitum and were housed under standard conditions. Animals were sacrificed by decapitation using a guillotine. Organs were taken out, flushed in ice-chilled phosphate buffered saline (PBS) and flash-frozen in liquid nitrogen. Plasma was obtained by centrifugation of fresh blood at 10,000 g at 4 °C for 10 min. Samples were stored at −80 °C away from light until analysis. FBS was stored at −20 °C until analysis.Human lung samples were obtained from the Lung Tissue Research Consortium (LTRC) of the National Heart, Lung, and Blood Institute, Bethesda, MD.
Standards and quantification
Results
Sample preparation
To determine optimal sample amounts, preliminary assays were performed with different amounts of guinea pig liver sample with different volumes of n-hexane (2 × 4 mL, 3 × 4 mL, 4 × 4 mL). Considering the high abundance of retinyl esters in this tissue, extraction efficiencies were optimal with sample weights between 100–150 mg.Identification of retinoids, tocopherols and carotenoids
For determination of retention times, the reference standards were injected both individually and as a mixture. Typical chromatograms at specific wavelengths of a standard mixture are shown in Fig. 1.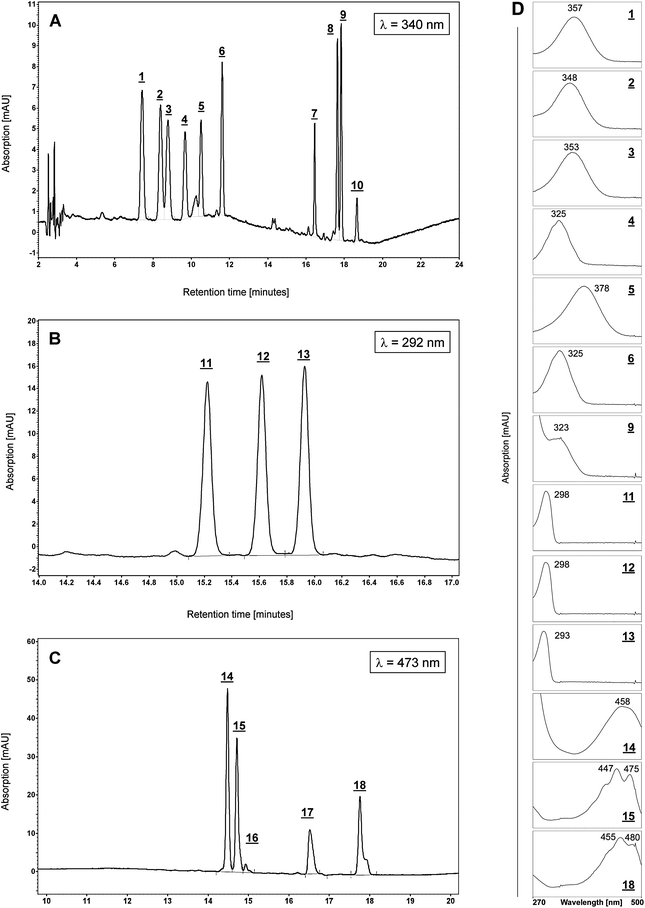 | ||
| Fig. 1 Standard Chromatogram. A: Chromatogram of standard substances at λ = 340 nm: 1- 13-cis RA, 2- 9-cis RA, 3- all-trans RA, 4- all-trans retinol, 5- all-trans retinal, 6- all-trans retinyl acetate (internal standard, IS), 7- all-trans retinyl laurate, 8- all-trans retinyl oleate, 9- all-trans retinyl palmitate, 10- all-trans retinyl stearate. 1–3 were monitored at λ =350 nm, and 4, 7–10 were monitored at λ = 325 nm. B: Chromatogram (retention time range 14–17 min) of standard substances at λ = 292 nm, 11- δ-tocopherol, 12- γ-tocopherol, 13- α-tocopherol. C: Chromatogram (retention time range 10–20 min) of standard substances at λ = 473 nm, 14- β-apo-8′-carotenal, 15- lutein, 16- zeaxanthin, 17- lycopene, 18- β-carotene (monitored at λ = 450 nm). D: Panel of UV-spectra of selected analytes (1–6, 9, 11–15, 18) recorded at their corresponding peaks. Measured maxima are indicated near peaks. Chromatographic conditions as described in the experimental section. | ||
Each analyte was assigned a number as shown in the corresponding figure legend and in the listing of Table 1.
| Analytea | Retention time [min] | ε/L mol−1 cm−1 | |
|---|---|---|---|
| a bold numbers refer to corresponding peaks in the standard chromatograms (Fig. 1). Molar extinction coefficients ε were applied according to 7 and 8. b not included in the validation. c rac-β-tocopherol has the same retention time as γ-tocopherol. | |||
| 1 | 13-cis RA | 7.71 ± 0.04 | 39750 |
| 2 | 9-cis RA | 8.61 ± 0.00 | 36900 |
| 3 | all-trans RA | 8.84 ± 0.04 | 45300 |
| 4 | all-trans retinol | 9.76 ± 0.04 | 52700 |
| 5 | all-trans retinal | 10.25b | 42880 |
| 7 | all-trans retinyl laurate | 16.29 ± 0.03 | 49260 |
| 8 | all-trans retinyl oleate | 17.45 ± 0.04 | 49260 |
| 9 | all-trans retinyl palmitate | 17.64 ± 0.06 | 49260 |
| 10 | all-trans retinyl stearate | 18.35 ± 0.05 | 49260 |
| 11 | δ-tocopherol | 15.36 ± 0.00 | 3750 |
| 12 | γ-tocopherolc | 15.75 ± 0.00 | 3672 |
| 13 | α-tocopherol | 16.10 ± 0.03 | 3015 |
| 14 | β-apo-8′-carotenal | 14.01b | 109800 |
| 15 | lutein | 14.05 ± 0.02 | 145100 |
| 16 | zeaxanthin | 14.10b | 133400 |
| 17 | lycopene | 16.15 ± 0.01 | 185200 |
| 18 | β-carotene | 17.23 ± 0.01 | 139060 |
In addition, to ensure a successful identification each peak was identified by assessing its UV-spectrum as a feature of the diode array detector and the controlling software and each sequence of analysis was accompanied by runs of external standard analytes. Furthermore, where still in doubt, samples were spiked with standard analytes for a further confirmation. The resulting UV-spectra of the used reference standards are shown in Fig. 1D.
All-trans retinol (Fig.2A), all-trans retinyl laurate, all-trans retinyl oleate, all-trans retinyl palmitate, all-trans retinyl stearate (Fig. 2B), (±) α-tocopherol (Fig. 2C) and all-trans-lutein (Fig. 2D) were identified in organic gp liver extracts by the comparison of retention times (tr) and UV absorption spectra with those obtained for the corresponding standards. In addition, a cis-isomer of retinol (tr20 = 9.39 ± 0.04 min, Fig. 2A), and at least five additional retinyl esters (tr21 = 16.8 ± 0.03 min, tr22 = 17.31 ± 0.04 min, tr23 = 17.98 ± 0.05 min, tr24 = 18.75 ± 0.06 min, tr25 = 19.15 ± 0.06 min, Fig. 2B) were identified by their characteristic absorption maximum of λ = 325 nm of the corresponding UV-spectra. However, RA-isomers, all-trans retinal, γ-tocopherol, δ-tocopherol, lycopene and β-carotene were not detected in guinea pig liver extracts under the described analytic conditions.
 | ||
| Fig. 2 Chromatograms of guinea pig liver sample. A: Chromatogram of liver at λ = 325 nm, within the retention time range of 0–13 min: 4- all-trans retinol, 6- all-trans retinyl acetate (internal standard, IS). In addition, a cis-retinol isomer (20) and a peak for the added antioxidant BHT (19) could be detected. B: Chromatogram of liver at λ = 325 nm, within the retention time range of 15.5–19.5 min: Apart from the retinyl ester identified by comparison with external standards (7- all-trans retinyl laurate, 8- all-trans retinyl oleate, 9- all-trans retinyl palmitate, 10- all-trans retinyl stearate), additional retinyl esters were found in guinea pigliver samples, of which three could be identified as retinyl esters by their characteristic UV-spectra and subsequent comparison with published chromatograms11 (21- retinyl linoleate, 22- retinyl pentadecanoate, 23- retinyl heptadecanoate, 24- unknown retinyl ester 1, 25-unknown retinyl ester 2). C: Chromatogram of liver at λ = 292 nm, within the retention time range of 14–16.4 min.; amongst the tocopherols, only α-tocopherol (13) could be detected. D: Chromatogram of liver at λ = 450 nm: 15- all-trans lutein. Chromatographic conditions as described in the experimental section. | ||
Method validation
This study was conducted with guinea pig liver. The linearity of standard curves (Table 2) was expressed in terms of the determination coefficient (r2) from plots of the integrated peak area versus concentration of the standards (μg/mL). The equations were obtained over a wide concentration range encompassing 3–4 orders of magnitude. The ranges were chosen to ensure that the concentration of each found analyte in liver extracts falls within this range (all-trans retinol: 1.6 μg/mL, all-trans retinyl palmitate: 79 μg/mL, -stearate: 35 μg/mL, α-tocopherol: 4 μg/mL and all-trans lutein 0.1 μg/mL). All data were based on HPLC runs of at least four to six dilutions of the standards. Linear relationships between the peak area and the concentration were found for all compounds with coefficients of determination ranging from 0.9711 (all-trans lutein) to 0.9996 (α-tocopherol) as listed in Table 2. Fig. 3 shows the standard curves with linear regression for the tested analytes.| Analyte | Range [μg/mL] | Equation | r 2 | |
|---|---|---|---|---|
| 1 | 13-cis RA | 0.023–22.562 | y = 115100x + 6212 | 0.9981 |
| 2 | 9-cis RA | 0.022–21.617 | y = 105000x + 10750 | 0.9974 |
| 3 | all-trans RA | 0.018–17.609 | y = 123600x + 17900 | 0.9964 |
| 4 | all-trans retinol | 0.144–14.355 | y = 90770x − 42100 | 0.9916 |
| 6 | all-trans retinyl acetate (IS) | 0.017–16.977 | y = 118300x − 22920 | 0.9981 |
| 9 | all-trans retinyl palmitate | 0.259–259.450 | y = 73540x − 153900 | 0.9975 |
| 11 | δ – tocopherol | 0.246–245.866 | y = 18230x − 88900 | 0.9873 |
| 12 | γ – tocopherol | 0.279–302.927 | y = 8787x − 11130 | 0.9979 |
| 13 | α – tocopherol | 0.368–367.857 | y = 7512x − 2991 | 0.9996 |
| 15 | all-trans lutein | 0.009–9.273 | y = 214400x − 60540 | 0.9711 |
| 17 | all-trans lycopene | 0.074–7.363 | y = 116100x − 13610 | 0.9992 |
| 18 | all-trans-β-carotene | 0.102–10.202 | y = 171300x − 92360 | 0.9758 |
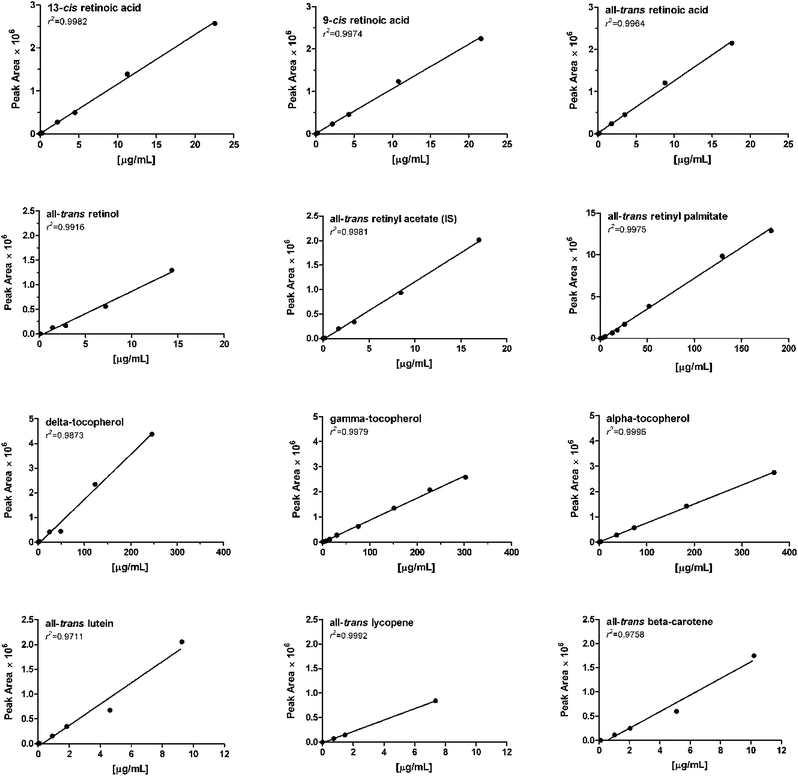 | ||
| Fig. 3 Linearity for analytes demonstrated by the standard curves for the analytes. The characteristics of these curves are given in Table 2. | ||
Limits of detection (LOD) and the limits of quantification (LOQ) were determined on the basis of the signal-to-noise ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for LOD and 10
1 for LOD and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for LOQ) as a result of an injection of the pure and defined analyte onto the HPLC system according to the guidelines of the American Chemical Society.9 In order to distinguish between the detection limitations of the instrument and the method, we determined the instrument detection limit (IDL) and the method detection limit (MDL). IDL was determined by the sole injection of a low concentration of working standard solutions to produce a signal that was about three times the signal-to-noise ratio. The MDL was assessed by spiking a blank sample, followed by the subsequent application of the extraction method. In order to find the concentration of the analyte that produced a peak at least three times the signal-to-noise ratio, for each standard four different spiking levels were analyzed in triplicate. Since most of the tested analytes are endogenous substances, finding a blank sample is difficult. Therefore, we used FBS in the determination of the MDL since the batch of this product we used contained only retinol amongst the tested analytes. Therefore, the related ester retinyl acetate was used to estimate the MDL for retinol. Results for LOD (expressed as IDL, MDL) and LOQ are summarized in Table 3. Fig. 4 shows a representative example of 13-cis RA for the IDL (0.7 pmol), MDL (1.9 pmol) and LOQ (3.1 pmol).
1 for LOQ) as a result of an injection of the pure and defined analyte onto the HPLC system according to the guidelines of the American Chemical Society.9 In order to distinguish between the detection limitations of the instrument and the method, we determined the instrument detection limit (IDL) and the method detection limit (MDL). IDL was determined by the sole injection of a low concentration of working standard solutions to produce a signal that was about three times the signal-to-noise ratio. The MDL was assessed by spiking a blank sample, followed by the subsequent application of the extraction method. In order to find the concentration of the analyte that produced a peak at least three times the signal-to-noise ratio, for each standard four different spiking levels were analyzed in triplicate. Since most of the tested analytes are endogenous substances, finding a blank sample is difficult. Therefore, we used FBS in the determination of the MDL since the batch of this product we used contained only retinol amongst the tested analytes. Therefore, the related ester retinyl acetate was used to estimate the MDL for retinol. Results for LOD (expressed as IDL, MDL) and LOQ are summarized in Table 3. Fig. 4 shows a representative example of 13-cis RA for the IDL (0.7 pmol), MDL (1.9 pmol) and LOQ (3.1 pmol).
| Analyte | IDL [pmol] | MDL [pmol] | LOQ [pmol] |
|---|---|---|---|
| a n/a – not applicable. | |||
| 1 13-cis RA | 0.7 | 1.9 | 3.1 |
| 2 9-cis RA | 0.7 | 2.2 | 4.1 |
| 3 all-trans RA | 0.6 | 1.4 | 3.5 |
| 4 all-trans retinol | 0.3 | n/a | 3.6 |
| 6 all-trans retinyl acetate | 0.5 | 0.6 | 1.5 |
| 9 all-trans retinyl palmitate | 0.4 | 0.5 | 0.9 |
| 11 δ – tocopherol | 5.3 | 6.6 | 32.9 |
| 12 γ – tocopherol | 5.4 | 9.3 | 37.8 |
| 13 α – tocopherol | 8.1 | 8.5 | 34.2 |
| 15 all-trans lutein | 0.9 | 0.8 | 2.4 |
| 17 all-trans lycopene | 6.9 | 10.2 | 11.8 |
| 18 all-trans-β-carotene | 1.4 | 2.7 | 2.9 |
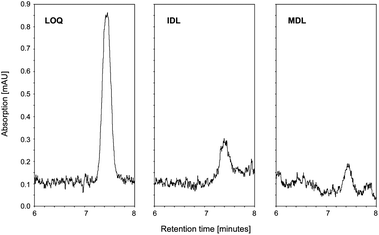 | ||
| Fig. 4 Representative data showing LOQ (3.1 pmol), IDL (0.7 pmol) and MDL (1.9 pmol) for 13-cis RA. | ||
The precision study was comprised of both repeatability and reproducibility studies using guinea pig liver as the tissue sample. A total of eight replicate determinations of a sample were performed under optimal conditions to determine repeatability. Three replicate analyses of the same sample were made on five different days to determine reproducibility (Table 4).
| Compound | Repeatability (n = 8) | Reproducibility (n = 3) | ||
|---|---|---|---|---|
| Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | |
| a Results are expressed in μg/g wet weight, RSD = relative standard deviation. | ||||
| all-trans retinol | 5.80 ± 0.30 | 5.20 | 6.76 ± 0.81 | 11.94 |
| retinylpalmitate | 313.31 ± 11.84 | 3.80 | 319.66 ± 21.84 | 6.83 |
| α–tocopherol | 15.93 ± 0.63 | 3.94 | 14.81 ± 1.23 | 8.28 |
| lutein | 0.51 ± 0.07 | 14.38 | 0.60 ± 0.03 | 4.40 |
Only the metabolites all-trans retinol, retinyl palmitate, α-tocopherol and all-trans lutein were present in guinea pig liver.
Accuracy was estimated by means of recovery assays, which was done as described by Strobel et al.10 First, the slope of an external calibration curve for each standard was determined. These calibration curves were used to quantify each analyte in guinea pig liver samples. Using the method of standard addition a second set of calibration curves were prepared by assaying four spiked levels in quintuplicates. In order to determine the recovery rate, the external calibration and the calibration using the method of standard addition were compared. For this purpose, the measured absorption of each value of the standard addition method was inserted into the equation of the external calibration curve and the x values for the concentration were calculated. Plotting these found values against the added standards to the samples provided the recovery curve. The slope of this curve (mr = cfound/cadded) yields information about proportional systematic variations, and is equivalent to a recovery rate (rr [%] = mr·100). The samples were subjected to the entire extraction and determination process. The equations for the recovery curves are shown in Table 5.
| Analytea | mr | b | r2 | |
|---|---|---|---|---|
| a Recovery was tested in liver samples. It should be noted that recoveries for all-trans retinyl palmitate and β-carotene were assessed in FBS. | ||||
| 1 | 13-cis RA | 0.8115 ± 0.0224 | −9.78 ± 9.39 | 0.9977 |
| 2 | 9-cis RA | 0.8053 ± 0.0212 | −14.28 ± 8.49 | 0.9979 |
| 3 | all-trans RA | 0.8849 ± 0.0270 | −10.29 ± 7.77 | 0.9916 |
| 4 | all-trans retinol | 0.7389 ± 0.1481 | 643.70 ± 34.02 | 0.8924 |
| 9 | all-trans retinyl palmitate | 0.8037 ± 0.024 | 20.73 ± 11.73 | 0.9974 |
| 11 | δ – tocopherol | 0.8562 ± 0.0191 | −118.40 ± 88.45 | 0.9985 |
| 12 | γ – tocopherol | 0.8741 ± 0.0238 | −151.40 ± 123.5 | 0.9978 |
| 13 | α – tocopherol | 0.9237 ± 0.0449 | 1554.00 ± 306.1 | 0.9930 |
| 15 | all-trans lutein | 0.9695 ± 0.1122 | 59.46 ± 19.72 | 0.9613 |
| 17 | all-trans lycopene | 0.6834 ± 0.0529 | −10.74 ± 7.22 | 0.9824 |
| 18 | all-trans-β-carotene | 0.9979 ± 0.0780 | 2.62 ± 14.81 | 0.9820 |
The overall recovery percentages obtained for each analyte as well as the applied spiking levels are shown in Table 6. A representative recovery curve for lutein is shown in Fig. 5
| Analytes | Spiked concentration levels [ng/sample] | Recovery (n = 5) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| c1 | c2 | c3 | c4 | Mean [%] | |||||
| RSD (%) | RSD (%) | RSD (%) | RSD (%) | ||||||
| a Recoveries for all-trans retinyl palmitate and all-trans β-carotene were assessed in FBS samples (sample size was 2 mL each). | |||||||||
| 1 13-cis RA | 101.53 | 4.23 | 203.05 | 3.26 | 406.11 | 2.45 | 812.21 | 1.60 | 81.15 |
| 2 9-cis RA | 97.28 | 2.40 | 194.55 | 4.54 | 389.11 | 1.64 | 778.21 | 1.82 | 80.53 |
| 3 all-trans RA | 69.69 | 3.67 | 139.38 | 4.68 | 274.76 | 1.25 | 557.51 | 1.34 | 88.49 |
| 4 all-trans retinol | 55.70 | 14.31 | 111.39 | 12.82 | 222.79 | 23.47 | 445.57 | 10.79 | 73.89 |
| 9 retinyl palmitatea | 120.83 | 5.21 | 241.66 | 3.33 | 483.32 | 9.24 | 966.64 | 1.40 | 80.37 |
| 11 δ–tocopherol | 1125.72 | 5.37 | 2251.44 | 4.83 | 4502.88 | 2.22 | 9005.76 | 1.56 | 85.62 |
| 12 γ–tocopherol | 1258.51 | 4.39 | 2517.02 | 5.14 | 5034.03 | 2.63 | 10068.07 | 1.49 | 87.41 |
| 13 α–tocopherol | 1655.36 | 6.11 | 3310.71 | 5.95 | 6621.43 | 5.30 | 13242.85 | 3.29 | 92.37 |
| 15 lutein | 42.61 | 9.01 | 85.22 | 10.12 | 170.43 | 22.04 | 340.87 | 4.06 | 96.95 |
| 17 lycopene | 33.13 | 12.34 | 66.27 | 6.31 | 132.53 | 13.14 | 265.07 | 6.09 | 68.34 |
| 18 β-carotenea | 46.04 | 6.66 | 92.09 | 11.56 | 184.18 | 5.58 | 368.35 | 6.09 | 99.79 |
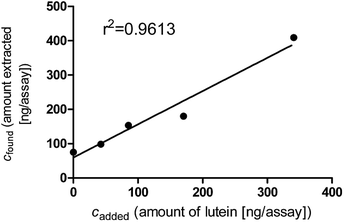 | ||
| Fig. 5 Representative recovery curve for the analyte lutein. The characteristics of this recovery curve are given in Table 5. | ||
In liver, the mean recovery for retinyl palmitate was 162%. In order to find out, if the high abundance of retinyl palmitate and other retinyl esters might influence its extraction efficiency and in effect its recovery, increasing amounts of liver (25 mg, 50 mg, 75 mg, 100 mg and 125 mg) were extracted and analyzed for their retinyl palmitate concentration. Fig. 6 indicates that the presented method is suitable to extract high abundance-retinyl palmitate from liver in a near perfect linear relation (r2 = 0.9955) within the tested range.
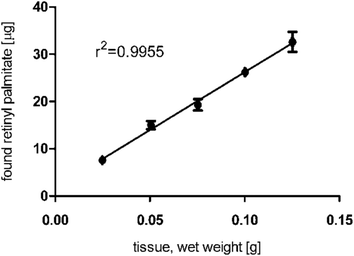 | ||
| Fig. 6 Concentration and linearity of the main retinyl ester, all-trans retinyl palmitate, from guinea pig liver extracts. | ||
However, the spiking range of the analyte (120 ng/sample to 967/ng sample) may be too small [and thus and fall within the standard deviation of the endogenous liver concentration of retinyl palmitate (313.31 μg/g ± 11.84 μg/g)] in order to detect differences in concentration due to the added standard. To rule this out, the recovery assay was repeated using FBS samples, which did not contain this analyte. Here, the mean recovery for all-trans retinyl palmitate was found to be 80.37% (Table 6). The recovery assay was also repeated for β-carotene. Although both sample matrices (liver and FBS) do not contain any endogenous all-trans β-carotene, the recovery rate improved from 63.76% to 99.79%.
For lycopene, the recovery rate could not be improved by using FBS matrices. In order to test if lycopene is affected by the extraction procedure, a FBS sample was spiked with a high amount of lycopene (3 μg) and analyzed after the normal extraction procedure. A comparison with a typical standard chromatogram revealed the occurrences of four major peaks in addition to the lycopene peak (data not shown).
Their retention times (trL1 = 12.75 min, trL2 = 14.07 min trL3 = 14.38 min, trL4 = 14.54 min) indicate the formation of oxidation and cleavage products rather than isomerisation of lycopene, despite the presence of the antioxidant BHT. If the detection and quantification of lycopene, its isomers and oxidation products should be the focus of an analysis, we would recommend choosing a more suitable method with a C30 HPLC-column.
Comparative tissue analysis
Rodent species like mouse, rat and guinea pig are often the models of choice in research examining perturbations in the vitamin A pathway. Levels for retinol, its metabolites and tocopherol have been analyzed under various experimental conditions in those species; however there is no single study that compares the levels of these nutrients in these rodents in order to relate the values to those seen in humans. Therefore, we analyzed selected tissues collected from these often-used experimental animals by applying our hereby described and validated method. Organs were taken from normal, typical laboratory animals, which were not previously subjected to any experimental procedures. Tissues (i.e. lung, liver, and plasma) were chosen based on their relevance and function toward vitamin A metabolism (target, storage, and transport, respectively). Table 7 summarizes their retinoid and tocopherol levels. However, no retinoic acid isomer was found. As reported earlier, we could identify the presence of lutein in several tissues from guinea pig,6 but not in tissues from rat and mouse.| Tissue | Analytes [ng/g] | Human (n = 3) | Guinea Pig (n = 3) | Rat (n = 2) | Mouse (n = 8) |
|---|---|---|---|---|---|
| a Data represents mean values in [ng/g wet weight] ± SEM; n.d. = not detected, n.a. = not assessed. b Plasma values are given in [ng/mL] ± SEM. | |||||
| Lung | 9-cis retinol | n.d. | 11.0 ± 0.6 | 32.7 ± 2.4 | 600.0 ± 42.1 |
| all-trans retinol | 168.2 ± 18.9 | 233.7 ± 8.4 | 498.0 ± 48.6 | 8,948.1 ± 436.4 | |
| retinyl linoleate | n.d. | 7.9 ± 0.7 | 456.3 ± 110.9 | 14,644.8 ± 468.1 | |
| retinyl oleate | 45.9 ± 8.4 | 13.4 ± 1.0 | 696.1 ± 178.8 | 15,944.1 ± 534.4 | |
| retinyl palmitate | 57.8 ± 0.9 | 31.5 ± 4.5 | 2,803.8 ± 447.9 | 213,889.8 ± 6,459.6 | |
| retinyl stearate | 106.5 ± 11.4 | 85.4 ± 2.0 | 1,525.7 ± 354.8 | 110,126.2 ± 3,291.2 | |
| α–tocopherol | 15,453.5 ± 1,770.6 | 11,130.9 ± 495.0 | 60,775.7 ± 6,126.5 | 35,312.1 ± 1,573.5 | |
| δ–tocopherol | 231.4 ± 135.7 | 49.4 ± 8.6 | 127.0 ± 15.6 | 721.2 ± 43.4 | |
| γ/β–tocopherol | 1,329.1 ± 765.9 | 538.6 ± 44.5 | 1,582.9 ± 354.8 | 1,510.2 ± 116.0 | |
| lutein 6 | 118.2 ± 9.9 | 140.2 ± 8.9 | n.d. | n.d. | |
| β-carotene | 109.3 ± 55.2 | n.d. | n.d. | n.d. | |
| Liver | 9-cis retinol | n.a. | 365.3 ± 70.6 | 3,702.5 ± 474.9 | 785.5 ± 89.1 |
| all-trans retinol | n.a. | 5,593.4 ± 452.8 | 117,126.2 ± 15,925.6 | 28,171.7 ± 2,469.2 | |
| retinyl linoleate | n.a. | 26,517.5 ± 1,617.7 | 253,876.5 ± 7,202.0 | 24,557.4 ± 1,328.0 | |
| retinyl oleate | n.a. | 40,242.0 ± 3,013.5 | 243,338.7 ± 11,994.1 | 19,063.4 ± 1,137.4 | |
| retinyl palmitate | n.a. | 300,536.4 ± 20,283.3 | 4,149,895.5 ± 85,269.1 | 449,180.6 ± 21,979.1 | |
| retinyl stearate | n.a. | 133,121.9 ± 9,538.6 | 797,903.3 ± 17,515.6 | 33,277.6 ± 1,870.2 | |
| α–tocopherol | n.a. | 15,878.3 ± 920.5 | 270,294.1 ± 6,850.7 | 37,563.2 ± 1,647.8 | |
| δ–tocopherol | n.a. | n. d. | 4,284.5 ± 2,246.0 | 1,144.5 ± 268.0 | |
| γ/β–tocopherol | n.a. | n. d. | 11,938.5 ± 1,748.3 | 6,742.7 ± 1,772.4 | |
| lutein6 | n.a. | 709.4 ± 57.8 | n.d. | n.d. | |
| Plasmab | 9-cis retinol | n.a. | 89.7 ± 17.5 | 20.2 ± 9.1 | 0 |
| all-trans retinol | n.a. | 517.6 ± 14.7 | 164.2 ± 31.7 | 33.92 ± 8.17 | |
| retinyl linoleate | n.a. | n.d. | n.d. | n.d. | |
| retinyl oleate | n.a. | n.d. | n.d. | n.d. | |
| retinyl palmitate | n.a. | 32.9 ± 19.2 | 84.9 ± 7.0 | 9.60 ± 1.85 | |
| retinyl stearate | n.a. | 40.6 ± 30.7 | 25.2 ± 6.9 | 3.97 ± 1.09 | |
| α–tocopherol | n.a. | 2,019.7 ± 818.2 | 19,096.5 ± 862.3 | 207.36 ± 39.37 | |
| δ–tocopherol | n.a. | 77.5 ± 46.2 | n.d. | n.d. | |
| γ/β–tocopherol | n.a. | 296.1 ± 229.6 | 297.6 ± 80.3 | 2.95 ± 2.76 | |
| lutein 6 | n.a. | 18.4 ± 1.0 | n.d. | n.d. |
The levels of all-trans retinol differed between the species and organs: mouse lung levels (8.95 μg/g ± 0.46 μg/g) were 18-fold higher compared to rat and 38.3-fold higher compared to guinea pig levels. In liver, retinol levels in rat (117.13 μg/g ± 15.93 μg/g) were 4.2-fold higher compared to mouse and 21-fold higher compared to guinea pig liver levels. In plasma, retinol levels in guinea pig (518 ng/mL ± 15 ng/mL) were 14.3-fold higher compared to mouse and 3.1-fold higher compared to rat plasma values.
Kane et al.4 reported the presence of the 9-cis isomer of retinol in mouse liver extracts, and showed that it was not formed as a result of the extraction procedure. Therefore we quantified 9-cis retinol levels in lung, liver and plasma of guinea pig, mouse and rat. Due to a lack of a commercially available 9-cis retinol standard, the isomer-peak was checked for its maximum at a wavelength of λ = 325 nm in its UV- spectrum. Readings of 9-cis levels were quantified using the molar absorbance for all-trans retinol and finally adjusted by applying a quotient of the molar extinction coefficients7 for all-trans retinol (ε = 52770 L cm−1 mol−1) and 9-cis retinol (ε = 42300 L cm−1 mol−1). It is striking, that both mouse and rat lung had a similar percent of 9-cis retinol compared to the total retinol content (6.3% and 6.2%, respectively), as do their liver samples (2.7% and 3.1%, respectively), The pattern is distinct from what we observe in guinea pig tissues where 9-cis retinol concentrations represent 4.7% and 7.2%, respectively, of total retinol content in lung and liver tissues. While mouse plasma had undetectable amounts of 9-cis retinol, rat and guinea pig plasma sample had 9-cis retinol levels that represented 11% and 14.8%, respectively, of their total retinol content.
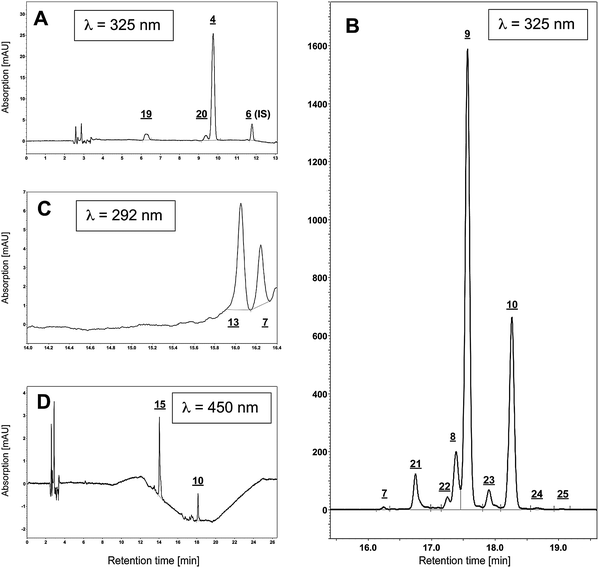
Liver samples from guinea pig, rat and mouse show a similar profile of nine retinyl esters, however the found values differ over several orders of magnitude. Apart from the retinyl esters identified by comparison with external standards (all-trans retinyl laurate, all-trans retinyl oleate, all-trans retinyl palmitate, all-trans retinyl stearate), five additional retinyl esters were found in liver samples. Three were identified as retinyl esters by their characteristic UV-spectra and subsequent comparison with published chromatograms11 (retinyl linoleate, retinyl pentadecanoate, retinyl heptadecanoate, unknown retinyl ester 1, unknown retinyl ester 2; Fig. 2B). Retinyl palmitate was the most abundant retinyl ester found in all rodent liver samples tested.
Retinyl palmitate levels in rat (4.15 mg/g ± 0.09 mg/g) were 9.1-fold higher compared to mouse and 13.8-fold higher compared to guinea pig liver levels. The comparison of the ratios of the four most abundant retinyl esters retinyl linoleate, retinyl oleate, retinyl palmitate and retinyl stearate reveals no significant differences between these three species.
In lung samples from guinea pig, rat and mouse, the retinyl ester profiles differed significantly. Retinyl palmitate levels in mouse (213.90 μg/g ± 6.46 μg/g) were 76.3-fold higher compared to rat (2.80 μg/g ± 0.45 μg/g) and 6785-fold higher compared to guinea pig (31.5 ng/g ± 4.5 ng/g). The levels of retinyl linoleate (14.65 μg/g ± 0.47 μg/g), retinyl oleate (15.94 μg/g ± 0.53 μg/g) and retinyl stearate (1.10 mg/g ± 0.003 mg/g) in mouse were 32.1-, 22.9-, and 72.2-fold, respectively higher than those in rat. In addition, the levels of retinyl linoleate, retinyl oleate and retinyl stearate in rats were 58-, 52.1-, and 17.9-fold, respectively higher than those in guinea pig. It is noteworthy that retinyl stearate was the most abundant retinyl ester found in guinea pig lung.
Only retinyl palmitate and stearate were found in plasma of all three species. In contrast to mouse and rat, retinyl stearate was the predominant ester in guinea pig plasma samples. Its level was 10-fold higher compared to mouse and 1.6-fold higher compared to rat plasma.
The described method was also used to quantify tocopherols in lung, liver and plasma of mouse, rat and guinea pig origin (Table 7). In all tissues, α-tocopherol (αT) was the most abundant tocopherol. Furthermore, the αT-levels only differed slightly between species. In rat lung, αT values were highest with 60.8 μg/g ± 6.13 μg/g, a value which was 1.7-fold higher than that seen in mouse and 5.5-fold more than guinea pig (35.31 μg/g ± 1.57 μg/g, and 11.13 μg/g ± 0.50 μg/g, respectively).
Comparison to human lung samples
All three species show in part a great variation among the detected analytes. In order to determine, which animal model most resembles humans, we analyzed three human lung samples that were provided by the LTRC. We found that the values for the analytes in human lung, listed in Table 7, are in the same order of magnitude as those found for guinea pig samples of lung. In contrast the vitamin A and E profiles of rat are more similar to the mouse. Fig. 7 represents comparative overlays of chromatograms (at λ = 325 nm) of a human and a guinea pig sample (Fig. 7A) and an overlay of rat and mouse sample chromatogram (Fig. 7B). It should be noted that absorption values of mouse, rat and human were normalized to the corresponding weight of the guinea pig sample in order to allow for a direct comparison between these chromatograms.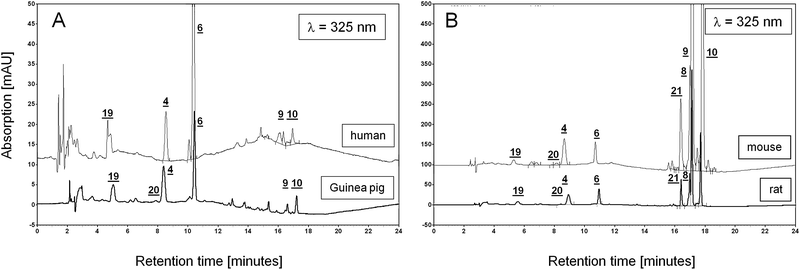 | ||
| Fig. 7 Comparison of chromatograms of lung samples from different species. A: Overlay of chromatograms of lung extracts from guinea pig and human at λ = 325 nm, y-axis range: −5 to 50 mV. B: Overlay of chromatograms of lung extracts from mouse and rats at λ = 325 nm, y-axis range: −50 to 500 mV. All chromatograms were normalized to the weight of the guinea pig lung sample. Numbers refer to each corresponding peak in both overlaid chromatograms. Note the order of magnitude difference between y-axes in A and B. Peak assignment: 4- retinol, 6- retinyl acetate (IS), 8- retinyl oleate, 9- retinyl palmitate, 10- retinyl stearate, 19- BHT, 20- 9-cis retinol, 21- retinyl linoleate. | ||
Like in guinea pig lung samples, similar amounts of lutein were found in human lung samples. In addition to other unidentified carotenoids (as judged by their UV-spectra), the analyzed human lung samples do contain β-carotene (109.3 ng/g ± 55.2 ng/g).
In contrast to the three animal species, neither 9-cis retinol nor retinyl linoleate were detected in the human lung samples.
Two reports12,13 suggest that αT influences tissue vitamin A stores by modulating the rate of retinyl ester hydrolysis. Therefore, we compared the ratios of the sum of found tocopherols to the sum of found retinyl esters (Fig. 8). Ratios for human and guinea pig were similar (81 and 85 respectively) in contrast to the ratios for rat and mouse (11 and 0.1, respectively).
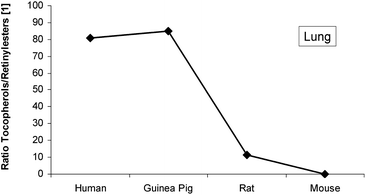 | ||
| Fig. 8 Ratio of the sum of found tocopherols to the sum of the four major retinyl esters in lung samples from human, guinea pig, rat and mouse. | ||
Discussion
The analytical method
The method described in the present paper is satisfactory with regards to its analytical characteristics with respect to its precision and recovery. This method allows detection over a wide range of the UV-spectrum at the same time resulting in a simultaneous detection of various retinoids or vitamin A compounds (such as retinol and retinyl esters), vitamin E or tocopherol isomers (α,γ/β,δ) and selected carotenoids (all-trans lutein, all-trans lycopene, all-trans β-carotene) in a single run. All these metabolites can be measured with reasonable sensitivity, with values ranging several orders of magnitude. Limits of detections are in a comparable range to similar methods.4,7 Since our method focuses on the analysis of natural, endogenous substances in tissue and fluids, it is difficult to find a suitable matrix that can serve as field blanks for all analytes. The most suitable blank matrix that could be used in this regard was FBS, which only contained retinol amongst the analytes. Therefore, we estimated the MDL value for retinol using retinyl acetate. Because retinyl acetate is not a naturally occurring metabolite of retinol, it was also chosen as an internal standard in our method. The similar values for IDL and MDL (0.18 pmol and 0.2 pmol, respectively) justify its choice, in addition to its similar properties. It is noteworthy that all-trans lycopene showed a MDL and LOQ, an order of magnitude higher than the related analytes all-trans lutein and all-trans β-carotene. This seems to be attributed to absorbance changes dependent on the solvent. Approximately at the retention time for lycopene, the mobile phase consists of a mixture of B (i.e. acetonitrile/methanol - 90/10) and C (THF) at a ratio of 60/40. A spectroscopic comparison of similar amounts of lycopene dissolved in hexane and the mobile-phase mix showed a reduction in absorbance of 28.9% at the maximum of λ = 470 nm. This observation is in agreement with the report of Zang et al.14 Apart from the solvent-dependent absorbance for lycopene, possible autoxidative degradation during extraction might also account for the reduction in sensitivity. However, we have not observed a similar degradation with any of the other tested standards.Concentration of all the metabolites were calculated based on their standards except for the retinyl esters which were calculated based on the values of retinyl palmitate. According to Ross15 retinyl esters have equal molar absorbances and thus retinyl palmitate may be used as a standard to quantify the other esters.
Using the present method we also detected a cis-isomer of retinol in liver extracts. This lesser known isomer of retinol is not an artifact during extraction as reported by Kane et al.4 in mouse liver extracts.
Known limitations
Because of the presence of very low levels of retinoic acid isomers in biological samples, simultaneous analysis of these isomers with retinol, retinyl esters, tocopherols and carotenoids by the present method may not always be ideal. However, if endogenous retinoic acid levels are of interest, an increase in sample size and a reduction of the volume of the final extract may be helpful to overcome this limitation. Our presented method gives values for LOD and LOQ comparable to those obtained by Kane et al.4 using a comparable HPLC-method with UV detection. However, in another publication by the same authors,16 a LOD of 10 fmol for RA was achieved by HPLC-separation coupled with Triple-quadropole LC/MS/MS detection. As result, 10 pmol/g tissue of all-trans RA was found in mouse liver. In order to detect such a signal with a signal to noise ratio of at least 3 in extracts with our method under the described parameters, at least 3.7 g mouse liver would need to be extracted, which is approximately the weight of a whole mouse liver. However, this method is suitable for the determination of RA-isomers since we reported very recently the uptake of an all-trans RA aerosol to guinea pig lungs at detectable levels.17It should be noted, that it is not possible to separate the two isomers γ- and β-tocopherol with the presented method.
If a more accurate determination of a geometric carotenoid isomer profile of a biological sample is of interest, the application of a polymeric C30 RP stationary phases may be a better choice, but requires significantly increased separation time.18 Despite these known limitations, that not all geometrical isomers of the tested analytes can be distinguished with this method and given the fact that not all possible isomers have the same biological relevance, our method provides a useful screening approach.
Although not included in the validation, this method can potentially be used for the detection of more polar retinoids (4-oxo retinoic acid, 18-hydroxy retinoic acid),1 9,13-dicis RA (presumably between peaks 1 and 2, Fig. 1A),19 retinal (i.e. retinal-(O-ethyl) oxime derivates),4,20 cryptoxanthin, zeaxanthin, and different apo-carotenals. As shown in Fig. 1, our method is able to detect β-apo-8′-carotenal (peak14), and all-trans retinal (peak 5), which correspondents to a “β-apo-15′-carotenal”. The time between both peaks is ca. 3.75 min, which would leave enough potential to separate other carotenals like β-apo- 10′-, 12′-, 14′-carotenal, since they differ significantly in their masses, polarity and UV-absorption maxima. Hence, we expect these substances to migrate in distinct peaks between 10.5 min–14 min.
From all aspects this method is robust; however care should be taken in performing several crucial steps during tissue extraction. We found it important to use glass or polyethylene terephthalate (PET)- vials in the course of tissue handling. The organic solvent n-hexane was able to extract chemicals in high amounts out of the plastic tube wall of polypropylene (PP)-tubes, which co-eluted at a retention time similar to 13- and 9-cis RA. Our pretests showed that PET-plastic ware is inert to our extraction method, in contrast to PP.
Comparative tissue analysis
Using this method we demonstrated that vitamin A composition varies considerably among rodent lung, liver and plasma samples. For instance, the mouse lung contains 38-fold more retinol and 6785-fold more retinyl palmitate (RP) than the guinea pig lung. Furthermore, in guinea pig lungs retinyl stearate (RS) is the major retinyl ester, in contrast to mouse and rat, but this finding is similar to that seen in the human. Interestingly, in plasma, the highest amount of retinol was found in guinea pig samples.Since the target tissue like the lung receive their supply of retinol bound to the plasma protein RBP (retinol binding protein), the observed esterification patterns are solely due to the enzymatic activities present in the target tissue. It is now well accepted, that one enzyme, the lecithin: retinol acyltransferase (LRAT) is capable of esterifying retinol in various tissues. LRAT catalyzes the transfer of the sn-1 fatty acid from membrane-associated phosphatidyl choline to retinol. Although other enzymes are known to perform this reaction, only LRAT is capable of esterifying retinol bound to the cytosolic retinol binding proteins, CRBP-I and CRBP-II.21 The other substrate for this reaction is phosphatidyl choline (PC, or lecithin), an acyl-donor with the sn-1 position as the determining factor for the formation of a specific retinyl ester. LRAT is a membrane-bound protein and co-localizes with the site of PC synthesis, in the smooth endoplasmic reticulum. Since retinyl esters in liver samples of the guinea pig, mouse and rat show the same profile of retinyl esters (with retinyl palmitate being the most abundant), the reason for the species-differences seen in lung must originate in this peripheral tissue. Retinyl stearate is the predominant retinyl ester in the lung of guinea pigs and humans, indicating that PC with stearic acid in the sn-1 position (PC-S) might be the main PC in these tissues available for this reaction. PC is not only a general component of the cellular membrane, in lung it is also a major component of pulmonary surfactant which is formed and secreted only by alveolar type II cells.22 It is not known at this time whether guinea pig lung is very rich in elongases activity, responsible for the presence of high stearic acid in PC. Furthermore, pulmonary surfactant contains several surfactant proteins including SP-A, -B, -C, and -D that contribute to its function. Interestingly, the human SP-B gene is also regulated by retinoic acids (RA) and their receptors (RARs).23
The different retinyl ester profiles in human, guinea pig, mouse and rat are a new finding and have not been reported before, however the reason why PC-S is the preferred substrate for the LRAT-catalyzed retinol esterification in human and guinea pig lungs is still unknown and warrants further investigation.
The retinyl ester composition of guinea pig, rat and mice diets are the same in IU units (15 IU/g). Though, a point of interest is if the actual amount of diet consumption and/or absorption of vitamin A vary, and if these differences affect the amount of vitamin A found in these assays.
Moreover, guinea pig6 and humans2,24 both have vegetables included in their diets which provide carotenoids and can be considered additional source of vitamin A.24 Comparison of HPLC chromatograms (Fig. 7) show similarities between human and guinea pig retinoid profiles that are very distinct from rat/mouse profile comparisons. These differences in the retinol/retinyl ester patterns may also be the result of the tissue specific balance between LRAT and retinyl ester hydrolase (REH) activities. The LTRC human lung samples were derived from patients with mild emphysema and we do not expect the impact of this mild pathological condition to cause any dramatic changes in vitamin A lung levels.
We are comparing analyte-levels in organs obtained from typical normal and healthy laboratory animals, receiving standard diets that have not been subjected to any experimental procedure. Under these standardized conditions, it is unlikely that vitamin A levels in lung would vary within individuals of one species as it does between species (guinea pig vs. rat, mouse) as we have shown in this manuscript, since uptake and storage are tightly controlled. In the light of these data, we were interested in the vitamin A-profile in samples from human lung in order to determine which animal model resembles best the situation in humans. Since human lung tissue is difficult to obtain, not much is known about the composition of the human vitamin A & E profile. A report from Schmitz et al.25 found a large variation in lung samples from human individuals obtained by autopsy. Only the total vitamin A contents after saponification is given, which varies from 0.7–405 nmol/g tissue, but also in their age (from 29–86 years) and different causes of death. However, no information on the composition of retinyl esters or vitamin E is given. In comparison to the common laboratory animal species like guinea pig, rat and mouse, a group of human individuals may vary due to many complex factors like health and lifestyle. Changes in lung vitamin A levels caused by pathological conditions like COPD and smoking is a current research focus in our laboratory.
Retinyl esters play an important role as a “acute reserve” during development, especially at the late phase of gestation. The fetal lungs can accumulate retinyl palmitate. Before birth, these vitamin A stores (retinol and retinyl plamitate) are lowered dramatically, indicating a high vitamin A demand.26 Postnatally, levels start to increase again. Interestingly, only retinyl palmitate but no retinyl stearate is found in the perinatal lung of mice.27 A similar alteration was observed for retinol during lung development before and after the hatching of chicks.28 However, no retinyl esters were detected in lung tissue of the embryonic and posthatch chicks, nor even in adult hens.28 The kinetics and the exact compostion of vitamin A compounds in the perinatal human lung is not know, however the low vitamin A reserves and vitamin A plasma levels are critical, especially in premature neonates with very low birthweight,29 resulting in complications like bronchopulmonary displasia, pneumonia or even death.30
Since vitamin A deficiency is a global health problem,31 animals like mouse, rat and guinea pig have been intensively studied as models for vitamin A deficiency. In rat32 and guinea pig,33 vitamin A deficiency can be induced after 8–12 weeks, respectively, on a vitamin A-free diet, whereas mice are more difficult to deplete. Thus, the most reliable method to rapidly induce vitamin A deficiency in mice is to feed the mother and the offspring the vitamin A-deficient diet from birth of the experimental animals.34 However, the most rapid method is to use a mouse retinol-binding protein (RBP) knockout mouse, that was been generated by a targeted disruption of the genomic locus.35
α-Tocopherol levels may also contribute to this delicate balance between retinol and retinyl esters as tocopherol has been described as an inhibitor of REH.12,13 Increased levels of vitamin E might be a critical parameter for the accumulation of retinyl ester. Interestingly, the tocopherol/RE ratio (Fig. 8) is similar for GP/human and rat/mouse, which supports this hypothesis.
Our observations on 9-cis retinol in GP, rat and mouse is significant because it has been reported that it can give rise to 9-cis RA through cis retinol dehydrogenase36 and 9-cis RA is an active form of RA and binds to both retinoic acid receptors (RARs) and retinoid X receptors (RXRs). However, using our method, we were unable to detect any endogenous 9-cis RA in the analyzed samples. Paik et al.37 reported that Hep G2 hepatocytes and HSC-T6 stellate cells readily take up and esterify 9-cis retinol. Furthermore, in Hep G2 cells, 9-cis-retinoic acid synthesis was strongly inhibited by high concentrations of 9-cis-retinol. Since liver is the predominant storage organ for vitamin A in the body, an quantitative esterification of 9-cis retinol and a controlled hydrolysis with subsequent oxidation to 9-cis RA and its release at a very low concentration (under the detection limit) seems to be more probable, since reports by Schmidt et al.38 and Heyman et al.39 confirmed the physiological occurrences of 9-cis RA in human and mouse liver.
Conclusion
This paper provides important insights into the retinoid composition of the major organs of retinoid action in important animal models. We can conclude that with reference to studies on lung and retinoids, guinea pig is a more suitable animal model compared to the rat and mouse, as it appears to be closest to human lung with respect to its retinoid profile. Moreover, it has been suggested earlier that guinea pig is not a rodent40 and a previous study from our laboratory showed that developmentally guinea pig lung is closer to humans41 and the present study supports this observation.Acknowledgements
This work was supported by NIH grant 1R03 HL095419-01A1, FAMRI clinical investigator award #062415, and US Army Grant W81XWH-06-2-0044. We thank Dr A. Catherine Ross for providing the retinyl esters. This study utilized human lung specimens provided by the Lung Tissue Research Consortium (LTRC) supported by the National Heart, Lung, and Blood Institute (NHLBI).Notes and references
- P. I. Francz, J. Conrad and H. K. Biesalski, Biol. Chem., 1998, 379, 1263 CrossRef CAS.
- G. R. Chichili, D. Nohr, M. Schäffer, J. von Lintig and H. K. Invest, Invest. Ophthalmol. Visual Sci., 2005, 46, 3562 CrossRef.
- S. Kang, E. A. Duell, G. J. Fisher, S. C. Datta, Z. Wang, A. P. Reddy, A. Tavakkol, J. Y. Yi, C. E. M. Griffits, J. T. Elder and J. J. Voorhees, J. Invest. Dermatol., 1995, 105, 549 CrossRef CAS.
- M. A. Kane, E. A. Folias and J. L. Napoli, Anal. Biochem., 2008, 378, 71 CrossRef CAS.
- A. B. Barua and J. A. Olson, J. Chromatogr., B: Biomed. Sci. Appl., 1998, 707, 69 CrossRef CAS.
- M. W. Schäffer, S. Sinha Roy, S. Mukherjee and S. K. Das, Biochem. Biophys. Res. Commun., 2008, 374, 378 CrossRef.
- A. B. Barua, H. C. Furr, J. A. Olson and R. B. van Breemen, in Modern Chromatographic Analysis of Vitamins, ed. A. P. De Lenheer, W. E. Lambert and J. F. Van Bocxlaer, Marcel Dekker, New York, 3rd edn, 2000, pp 1–74 Search PubMed.
- H. J. Nelis, E. D'Haese and K. Vermis, in: Modern Chromatographic Analysis of Vitamins, ed. A. P. De Lenheer, W. E. Lambert and J. F. Van Bocxlaer, Marcel Dekker, New York, 3rd edn, 2000, pp 143–228 Search PubMed.
- American Chemical Society (ACS), Subcommittee of Environmental Analytical Chemistry, Anal. Chem., 1980, 52, 2242 CrossRef.
- M. Strobel, F. Heinrich and H. K. Biesalski, J. Chromatogr., A, 2000, 898, 179 CrossRef CAS.
- H. C. Furr, Methods Enzymol., 1990, 189, 85 CAS.
- J. L. Napoli and C. D. Beck, Biochem J., 1984, 223, 267 CAS.
- J. L. Napoli, A. M. McCormick, B. O'Meara and E. A. Dratz, Arch. Biochem. Biophys., 1984, 230, 194 CrossRef CAS.
- L. Y. Zang, O. Sommerburg and F. J. G. M. van Kuijk, Free Radical Biol. Med., 1997, 23, 1086 CrossRef CAS.
- A. C. Ross, Methods Enzymol., 1986, 123, 68 Search PubMed.
- M. A. Kane, N. Chen, S. Sparks and J. L. Napoli, Biochem. J., 2005, 388, 363 CrossRef CAS.
- M. W. Schäffer, S. Sinha Roy, S. Mukherjee, D. E. Ong and S. K. Das, Exp. Lung Res., 2010 Search PubMed , in press.
- C. Emenhiser, L. C. Sander and S. J. Schwartz, J. Chromatogr., A, 1995, 707, 205 CrossRef CAS.
- G. Tzimas, J. O. Sass, W. Wittfoth, M. M. A. Elmazar, K. Ehlers and H. Nau, Drug Metab. Dispos., 1994, 22, 928 CAS.
- F. J. G. M. van Kuijk, G. J. Handelman and E. A. Dratz, J. Chromatogr., A, 1985, 348, 241 CrossRef CAS.
- A. C. Ross and R. Zolfaghari, J. Nutr., 2004, 134, 269S CAS.
- R. Burghardt and L. M. G. van Golde, in Lung Cell Biology, ed. D. Massaro, Marcel Dekker, Inc., New York, 1989, pp 591–654 Search PubMed.
- A. Naltner, M. Ghaffari, J. A. Whitsett and C. Yan, J. Biol. Chem., 2000, 275, 56 CrossRef CAS.
- C. Carlier, J. Coste, M. Etchepare, B. Périquet and O. Amédée-Manesme, Br. Med. J., 1993, 307, 1106 CrossRef CAS.
- H. H. Schmitz, C. L. Poor, R. B. Wellman and J. W. Erdman, Jr., J. Nutr., 1991, 121, 1613 CAS.
- S. K. Geevarghese and F. Chytil, Biochem. Biophys. Res. Commun., 1994, 200, 529 CrossRef CAS.
- M. Schäffer, S. Rubenbauer, S. Cvek, M. Langer, D. Nohr and H. K. Biesalski, Faseb J., 2005, 19, 292.
- S. Takase and T. Goda, Comp. Biochem. Physiol., 1990, 96B, 415 CAS.
- J. P. Shenai, K. A. Kennedy, F. Chytil and M. T. Stahlman, J. Pediatr., 1987, 111, 269 CAS.
- J. H. Humphrey, T. Agoestina, L. Wu, A. Usman, M. Nurachim, D. Subardja, S. Hidayat, J. Tielsch, K. P. West Jr. and A. Sommer, J. Pediatr., 1996, 128, 489 CrossRef CAS.
- A. Sommer, J. Nutr., 2008, 138, 1835 CAS.
- M. J. Koch, H. K. Biesalski, E. Stofft, H. Weiser, H. E. Gabbert, H. P. Dienes, B. Schulz-Dobrick and K. H. Bässler, Cell Tissue Res., 1990, 260, 625 CrossRef CAS.
- T. Nayyar, S. Mukherjee and S. K. Das, Mol. Cell. Biochem., 2000, 211, 47 CrossRef CAS.
- L. M. De Luca, R. L. Shores, E. F. Spangler and M. L. Wenk, Cancer Res., 1989, 49, 5400 CAS.
- L. Quadro, W. S. Blaner, D. J. Salchow, S. Vogel, R. Piantedosi, P. Gouras, S. Freeman, M. P. Cosma, V. Colantuoni and M. E. Gottesman, EMBO J., 1999, 18, 4633 CrossRef CAS.
- M. V. Gamble, E. Shang, R. P. Zott, J. R. Mertz, D. J. Wolgemuth and W. S. Blaner, J. Lipid Res., 1999, 40, 2279 CAS.
- J. Paik, S. Vogel, R. Piantedosi, A. Sykes, W. S. Blaner and K. Swisshelm, Biochemistry, 2000, 39, 8073 CrossRef CAS.
- C. K. Schmidt, A. Brouwer and H. Nau, Anal. Biochem., 2003, 315, 36 CrossRef CAS.
- R. A. Heyman, D. J. Mangelsdorf, J. A. Dyck, R. B. Stein, G. Eichele, R. M. Evans and C. Thaller, Cell, 1992, 68, 397 CrossRef CAS.
- A. M. D'Erchia, C. Gissi, G. Pesole, C. Saccone and U. Arnason, Nature, 1996, 381, 597 CrossRef CAS.
- I. E. Stith and S. K. Das, Biochim. Biophys. Acta, Gen. Subj., 1982, 714, 250 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
