An immobilized dehydrohalogenase based potentiometric biosensor for the detection of chlorinated pesticides†
Mahadevu Rajashekara
Murthy
,
Iychettira Machaiah
Mandappa‡
,
Rangachary
Latha
,
Aaydha Chidambara
Vinayaka
,
Munna Singh
Thakur
* and
Haravey Krishnan
Manonmani
Fermentation Technology and Bioengineering Department, Central Food Technological Research Institute, Mysore, 570 020, India. E-mail: msthakur@cftri.res.in; msthakur@yahoo.com; Fax: +91 821 2517233; Tel: +91 821 2515792
First published on 10th August 2010
Abstract
Among the organochlorine pesticides, DDT and HCH are the major cause for food and environmental contamination. To detect them effectively in water samples an immobilized dehydrohalogenase based potentiometric biosensor was developed. The chloride ion released as a result of dehalogenation by immobilized dehydrohalogenase during the degradation of DDT and HCH, respectively, was detected using an ion selective electrode. The voltage response had a direct linear relationship with the concentration of chloride (Cl−) released by DDT and HCH, respectively. The immobilization was advantageous in improving the enzyme property and could be used repeatedly without losing activity.
Introduction
Worldwide, there has been a 44% increase in the usage of pesticides over the past decade. Since insecticides account for 70% of the total pesticide used, it is likely that insecticide residues will continue to be an issue for at least another decade.1Over the past few years, the counter productive effects of pesticide such as serious health hazards has become apparent. The long persistence of organochlorine pesticides in the environment has introduced a series of undesirable effects in the food chain.2,3 Hence, bioaccumulation of pesticides and their biomagnification processes have become the weak links in the food chain.4
Among the organochlorine pesticides that have acquired notoriety,1,1,1- trichloro-2,2-bis (ρ- chlorophenyl) ethane (DDT) and hexachlorocyclohexane (HCH) take the upper position with regard to their usage, toxicity and persistence. In several countries, DDT and HCH have been the two major chemicals used in agriculture, cosmetics, animal husbandry and public health programs. Although, now restricted they are still very much in use because of their wide spectrum of activity, especially as no low cost alternatives are readily available for public health programmes. Our biggest concern is that these molecules are stable in the environment for long periods of time. Worldwide, more than 600,000 tonnes of HCH and 270,000 tonnes of DDT have been added to the environment since their respective introductions in 1949 and 1952. It is suspected that most of our water bodies and soils are contaminated with these chemicals and their degradation products.5,6 DDT persists with a half life of about 10 years. The uptake and accumulation of DDT and its metabolites in different plants and animal species is also well documented.7
Toxic effects attributed to these pesticides include neurotoxic, nephrotoxic, mutagenic, carcinogenic, teratogenic and immunosuppressiveness.8 Due to these toxic effects, these pesticides have been either banned or allowed for restricted applications in agriculture and public health programmes.
The persistence of these pesticide residues in the environment and their entry into the food chain has made it mandatory to develop new, simple, specific, rapid, sensitive and portable techniques for their detection. Therefore an efficient qualitative/quantitative method for the rapid estimation of DDT and HCH is highly desirable. Conventional methods are time consuming; require expensive instrumentation, pre-treatment and extensive clean up of the samples. Most of the reports available in literature to date are based on conventional analytical methods such as immunological methods, GC, GCMS and HPLC etc.9 An optical immunosensor system was reported10 for the sensitive detection of DDT in water samples. The assay sensitivity against DDT was 15 ng L−1 due to the use of monoclonal antibodies (MAbs). Further, Hirano et al. (2008)11 reported an enzyme immunoassay for the detection of DDT using polyclonal antibodies with a quantification limit of 0.41 ng mL−1. In this manuscript we report a novel approach for the detection of DDT and HCH using an immobilized microbial enzyme-based biosensor system. The application of ion selective electrode for the qualitative and quantitative detection of organochlorine pesticides in environmental samples is also discussed.
Experimental
Materials and apparatus
DDT, HCH and other metabolites used in these studies were 99% pure and were purchased from Sigma-Aldrich, USA. The cellophane membrane was procured from Spectra/Por, USA. The ion selective electrode (Model-LI 126) was supplied by ELICO, Ltd. Hyderabad, India. Sepharose 6B was purchased from Pharmacia LKB Biotechnology Inc., Sweden. Nutrient broth, polyacrylamide was procured from Hi-Media Laboratories, Mumbai, India.The buffer salts and other chemicals used in these studies were of analytical grade and were purchased from standard chemical companies. Instruments used were an UV-visible spectrophotometer (UV-1601, Shimadzu) and an ion selective electrode-based detection system (Model-LI 126) from ELICO, Ltd. Hyderabad, India.
Isolation and purification of DDT-dehydrohalogenase and HCH-dehydrohalogenase enzymes
Both DDT-dehydrohalogenase and HCH-dehydrohalogenase enzymes were isolated from bacterial isolates, Pseudomonas putida T5 and Burkholderia pseudomallei T4, respectively, by long-term enrichment of contaminated soil and sewage.4,7Pseudomonas putida T5 was acclimated to 10 ppm DDT in minimal medium for 72 h after the initial growth in nutrient broth and incubated for 72 h at 28 °C. The cultures were harvested by centrifugation at 10,000 g for 20 min at 4 °C followed by thorough washing with 0.1 M phosphate buffer, pH 7.0. The cells were macerated vigorously with sterile sand in ice-cold phosphate buffer in an ice bath. The homogenate was centrifuged at 10,000 g for 20 min at 4 °C and the clear supernatant was concentrated and dialyzed against phosphate buffer pH 7.0 and designated as crude extract. Crude enzyme extract was loaded on Sepharose 6B column (1.1 cm × 100 cm) previously equilibrated with phosphate buffer (50 mM, pH 7.0) and then eluted with the same buffer. Fractions were collected (3 mL each) at a flow rate of 15 mL h−1. Active fractions were pooled, dialyzed against distilled water and lyophilised. The enzyme assays of these fractions were carried out. The lyophilised active enzyme powders were stored at 4 °C until further use.Similarly Burkholderia pseudomallei T4 was acclimated to 10 ppm HCH in minimal medium for 72 h after the initial growth in nutrient broth and incubated for 72 h at 28 °C. The cultures were harvested by centrifugation at 10,000 g for 20 min at 4 °C followed by thorough washing with 0.1 M phosphate buffer, pH 7.0. The cells were macerated vigorously with sterile sand in ice-cold phosphate buffer in an ice bath. The homogenate was centrifuged at 10,000 g for 20 min at 4 °C and the clear supernatant was concentrated and dialyzed against phosphate buffer pH 7.0 and designated as crude extract. Crude enzyme extract was loaded on Sepharose 6B column (1.1 cm × 100 cm) previously equilibrated with phosphate buffer (50 mM, pH 7.0) and then eluted with the same buffer. Fractions were collected (3 mL each) at a flow rate of 15 mL h−1. Active fractions were pooled, dialyzed against distilled water and lyophilised. The enzyme assays of these fractions were carried out. The lyophilised active enzymes powders were stored at 4 °C until further use.
Enzyme assay
For the enzyme assay, a known amount of enzyme containing 2 μM of p,p′-DDT in dimethylformamide (DMF) as the substrate in 0.1M phosphate buffer, pH 7.0 was taken and incubated at 28 °C for 1 h with intermittent shaking. The reaction was stopped by lowering the pH to 2.0 by adding HNO3; halide (Cl−) production was determined spectrophotometrically at 460 nm with mercuric thiocyanate and ferric ammonium sulfate as previously described by Bergman and Sainik.12 The protein concentration was determined according to Lowry et al. with bovine serum albumin (BSA) as standard.13Similarly γ-HCH-dehydrohalogenase was estimated with γ-HCH as the substrate. Other reaction conditions were same as described above.
Polyacrylamide gel electrophoresis (PAGE)
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed for fractionated enzyme extracts according to Laemmli.14 The molecular weight of denatured enzyme proteins was determined by SDS-PAGE according to Laemmli.14 Bovine serum albumin (Mol. Wt. 66,000 D), carbonic anhydrase (Mol. Wt. 29,000 D), alcohol dehydrogenase (Mol. Wt. 1, 50,000 D) and cytochrome C (Mol. Wt. 12,400 D) were used as reference standards. Proteins were stained with Coomassie Brilliant Blue R 250 and also with silver staining.Immobilization of enzymes
DDT-dehydrohalogenase and HCH-dehydrohalogenase enzymes were immobilized separately by cross-linking with glutaraldehyde on cellophane membranes. The enzymes (22.46 U/mg of each) were taken in sodium phosphate buffer (pH 7.2, 50 mM). BSA (30 mg) was dissolved in 1 mL sodium phosphate buffer (pH 7.2, 50 mM). Both BSA and respective enzymes were spread on 2 cm × 2 cm cellophane membrane separately, along with 30 μL of glutaraldehyde solution (1% v/v). The reactants were mixed thoroughly so that enzyme and stabilizing agents were distributed evenly over the membrane surface. The membrane was partially dried for 1 h then washed five times with 0.1 M phosphate buffer, pH 7.2 to remove any excess glutaraldehyde solution.15Extraction of DDT and HCH from water samples
The local domestic water supply samples were taken and spiked with DDT and HCH separately for the experiments. These spiked samples were acidified to pH 2.0 by the addition of concentrated HNO3. DDT and HCH were extracted from acidified water samples using dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and repeated three times. Solvent fractions were pooled and passed through anhydrous sodium sulfate to remove traces of water. Solvent fractions obtained were then concentrated and resuspended in required quantity of buffer and DMF mixture (1
1 v/v) and repeated three times. Solvent fractions were pooled and passed through anhydrous sodium sulfate to remove traces of water. Solvent fractions obtained were then concentrated and resuspended in required quantity of buffer and DMF mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for further analysis.
1 v/v) for further analysis.
Fabrication of biosensor system
An immobilized enzyme-based electrode was used for the detection of chloride released by enzymatic reactions by the respective enzymes. The sensing device comprised of an ion-selective electrode, enzyme immobilized membrane system consisting of Teflon tape to avoid leakage of the electrolyte and a hydrophilic polymer (cellophane membrane) with respective immobilized dehydrohalogenases (Fig. 1). The immobilized enzyme was secured to the electrode using an “O” ring. The ion-selective electrode was immersed in the reaction cell-containing phosphate buffer (50 mM, pH 7.2) and DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). A commercially available signal-conditioning unit consisting of a current-to-voltage converter circuit was used to process the electrode signals. Respective substrates (DDT/HCH) were introduced into the reaction cell using the sample injector and continuously agitated using a magnetic stirrer. Chloride ions released in the vicinity of the enzyme membrane as a result of the biochemical reaction yielded an electrochemical signal (decreasing voltage) was detected. This signal was suitably conditioned using a commercial signal-conditioning unit obtained from ELICO Ltd, which displays signals in mV in response to the chloride ions released (Fig. 1).
1 v/v). A commercially available signal-conditioning unit consisting of a current-to-voltage converter circuit was used to process the electrode signals. Respective substrates (DDT/HCH) were introduced into the reaction cell using the sample injector and continuously agitated using a magnetic stirrer. Chloride ions released in the vicinity of the enzyme membrane as a result of the biochemical reaction yielded an electrochemical signal (decreasing voltage) was detected. This signal was suitably conditioned using a commercial signal-conditioning unit obtained from ELICO Ltd, which displays signals in mV in response to the chloride ions released (Fig. 1).
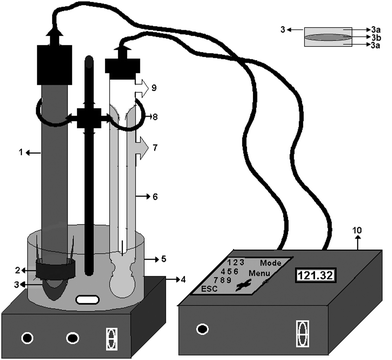 | ||
| Fig. 1 Schematic representation of the biosensor system and enzyme immobilized membrane. (1) Chloride electrode, (2) “O” Ring, (3) Immobilized membrane, (3a) Semipermiable membrane layer, (3b) Immobilized enzyme layer (4) Magnetic stirrer, (5) Sample Cell, (6) Reference electrode, (7) Outer solution inlet, (8) Clamp, (9) Inner solution inlet, (10) Data analyzer. | ||
Different concentrations of potassium chloride (KCl) from 3.55 ppm to 3550 ppm were used for calibration of the electrode. A standard graph was obtained with a regression of R2 = 0.9943 (Fig. 2). Further, the biosensor was used for analyzing both DDT and HCH in separate experiments. Substrates were added both continuously and in batches. In the batch method, fresh substrate was added after each change of contents of the sample cell. The continuous method was studied by adding substrate to the sample cell continuously without washing the enzyme immobilized electrode to determine the efficacy of immobilization of respective enzymes.
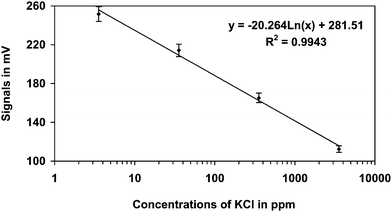 | ||
| Fig. 2 Standard graph obtained for KCl in the concentration range 3.55 ppm to 3550 ppm in the ion selective electrode. | ||
Results and discussion
Isolation and purification of dehydrohalogenase enzymes
The crude extracts of dehydrohalogenase enzymes isolated from Pseudomonas putida T5 and Burkholderia pseudomallei T4 showed the dehalogenation activity with DDT and HCH, respectively. After gel exclusion chromatography using sepharose 6B the DDT dehydrohalogenase and HCH dehydrohalogenase enzymes could be purified by 7.12- and 4.8-fold, respectively. SDS-PAGE for the active fractions collected from the column showed two non-identical subunits. The presence of a single band under non-reducing conditions on the native gel confirmed the purity of both dehydrohalogenase enzymes.The SDS-PAGE showed that enzyme could be a heterodimer with two bands of ∼32 kDa mass (Fig. S1†). The difference in molecular weight may be due to the rate of glycosylation. It is reported that dehydrohalogenase of Pseudomonas spp. is capable of degrading 4-chlorobenzoicacid (4-CBA) which was found to be a heterodimer of 57 and 30 kDa components.16
It was found that DDT-dehydrohalogenase and HCH-dehydrohalogenase enzymes extracted and purified from Pseudomonas putida T5 and Burkholderia pseudomallei T4 are very specific towards DDT and HCH, respectively. Trnkova et al. (2008)17 reported quantification of Cl− ions in NaCl amperometrically using carbon paste electrode (CPE) modified with solid AgNO3 with a detection limit of 100 μM. Trnkova suggested using enzyme activity for the detection of halogenated pesticides with the release of Cl−. In a study involving glutathione-S transferase (GST) having dehydrochlorinase activity from the mosquito Aedes aegypti, the detection of DDT based on pH change was carried out.18 The reaction was monitored potentiometrically or colorimetrically in the presence of pH marker.18
Therefore, as DDT-dehydrohalogenase and HCH-dehydrohalogenase enzymes have novel properties to act on specific chlorinated pesticides, an attempt was made to use these enzymes for biosensor applications.
Optimisation of conditions for enzyme activity
Dehydrohalogenase enzyme activity is defined as the amount of enzyme that catalyses the release of 1 μmol halide (Cl−) per min at optimum pH and temperature and expressed as units per mg protein. DDT-dehydrohalogenase enzyme showed maximum activity at pH 7.0 and HCH-dehydrohalogenase enzyme showed maximum activity at pH 6.0 (Fig. 2S and 3S†). In our study it was observed that the enzyme from Pseudomonas putida T5 at acid and alkaline ranges showed 95.7% and 95.3% lower activity, respectively.Temperature is also an important factor responsible for enzyme activity and stability. Both DDT and HCH dehydrohalogenase enzymes showed a classical Gaussian curve for relative enzyme activity against temperature (Fig. 4S and 5S†). Maximum activity was observed at 37 °C when purified DDT-dehydrohalogenase was incubated from 20 °C to 50 °C in 0.1 M phosphate buffer at pH 7.0, in presence of DDT as substrate. HCH-dehydrohalogenase showed maximum activity at 26 °C in presence of HCH as a substrate.
Immobilization of dehydrohalogenase enzymes for potentiometric biosensor system: Effect of immobilization on enzyme activity
Dehydrohalogenases were key enzymes responsible for the initial dehalogenation of DDT and HCH, respectively. The enzyme reacts with respective substrates by removing Cl− from the DDT/HCH molecule. The chloride ions released in the form of HCl molecule (H+ and Cl−) during the degradation of DDT or HCH were detected using an ion selective electrode. As for the anion, a negative slope is expected and therefore the voltage will be inversely proportional to the concentration of chloride ions released. The performance of enzyme activities for the degradation of both DDT and HCH, respectively, were monitored before and after immobilization. It was observed that the activity of both dehydrohalogenase enzymes increased as a result of immobilization. The difference in the response obtained in terms of mV has indicated the increased concentration of Cl− released from DDT and HCH, respectively. Both the immobilized enzymes showed better Cl− release compared to that of their respective free enzyme (Fig. 3a).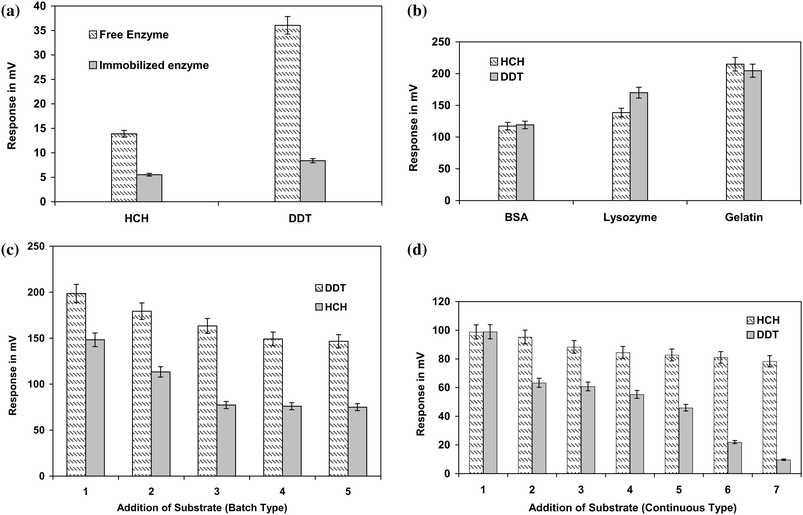 | ||
| Fig. 3 (a) Studies on immobilization on enzyme activity. (b) Effect of different stabilizers on immobilized enzyme. (c) Studies of the effect of addition of substrate (batch type) on enzyme activity. (d) Studies on effect of addition of substrate (continuous type) on enzyme activity. | ||
It is reported that immobilization will enhance the stability of an enzyme by retaining its natural catalytic surroundings. It also enables easy conversion of batch processes into a continuous mode without washout conditions.15,16 Immobilization of respective dehydrohalogenase enzymes has thus provided an opportunity for repeated use of enzymes making the process economical.
Glutaraldehyde is an ideal crosslinking-based immobilizing agent for biosensor applications.19 As glutaraldehyde is a strong bifunctional reagent, enzymes may undergo conformational changes due to immobilization leading to loss of activity. Therefore, protein-based stabilizers are of importance during immobilization of enzymes to overcome the deleterious effects of crosslinking agents and in turn to retain enzyme activity.20 BSA, lysozyme and gelatin were tested for their efficacy as protein-based stabilizing agents for immobilization of dehydrohalogenase enzymes. These protein-based stabilizing agents protect the denaturation of enzymes under harsh conditions enhancing intermolecular linkages rather than intramolecular linkages. Among the three protein-based stabilizing agents used, BSA was the best followed by lysozyme and gelatin (Fig. 3b). Probably the durability of BSA as a carrier protein along with its complimentary surface stabilizing nature has played an important role in stabilizing immobilized dehydrohalogenase enzymes and in turn their activity.21–23 The variation observed in Fig. 3b for immobilized dehydrohalogenase enzymes activity in presence of different stabilizers further supports the theory of durability and complimentary surface stabilizing nature of different proteins as stabilizing agents during immobilization.
Studies of the effect of substrate addition on enzyme activity
Release of Cl− was studied in both batch and continuous type substrate additions. In batch type additions, the immobilized HCH-dehydrohalogenase enzyme and immobilized DDT-dehydrohalogenase enzymes could be used for four to five cycles without any loss of activity (Fig. 3c). Whereas in continuous type additions, both immobilized HCH-dehydrohalogenase and DDT-dehydrohalogenase enzymes were used for more than 7 cycles (Fig. 3d). The decrease in the number of cycles of substrate analysis during batch type addition may be due to loss of enzyme activity. This could be due to the loss of stabilizer from the enzyme surface during the extensive washing steps involved in the batch type analysis.Conclusions
Dehydrohalogenase enzymes play an important role in the degradation of chlorinated molecules as monitored by an enzyme-based ion selective electrode system. The enzymes were highly specific for DDT and HCH, respectively. With the immobilized enzyme method, detection of DDT and HCH was possible with specificity. The analysis can be completed within 10 to 15 min. The present invention is economical as the immobilized enzyme can be reused. DDT/HCH can be detected easily by injecting extracted samples and the detection showed high specificity. This technique possesses several advantages in terms of rapidity, specificity and cost effectiveness and could be used for on-site testing of pesticides.Acknowledgements
The authors thank the Director, CFTRI, and CSIR for providing facilities. The authors are grateful to the Department of Biotechnology (DBT), India, for providing funds and supporting the research work.References
- N. G. K. Karanth, P. G. Deo and N. K. Veena Nadig, Curr Sci., 1999, 77, 126.
- A. C. Vinayaka, S. Basheer and M. S. Thakur, Biosens. Bioelectron., 2009, 24, 1615 CrossRef CAS.
- M. Lisa, R. S. Chouhan, A. C. Vinayaka, H. K. Manonmani and M. S. Thakur, Biosens. Bioelectron., 2009, 25, 224 CrossRef.
- B. Rajkumar and H. K. Manonmani, Process Biochem., 2002, 38, 49 CrossRef.
- C. R. Krishnamurthi, Pesticide Residues in Food and Biological Tissues. New Delhi, Indian National Science Academy Indian National Science Academy., 1984, pp. 1 Search PubMed.
- J. F. Garcia-Reyes, B. Gilbert-Lopez and A. Molina-Diaz, Anal. Chem., 2008, 80, 8966 CrossRef CAS.
- B. Rajkumar, A. Mohammed and H. K. Manonmani, Chemosphere, 2004, 56, 803 CrossRef.
- U.S. Department of Health and Human Services, Public Health Service Agency for Toxic, Substances and Disease Registry, Atlanta, GA 30333, 2002, http://www.atsdr.cdc.gov/toxfaq.html Search PubMed.
- J. S. S. Dikshit, residues of DDT and HCH in major sources of drinking water in Bhopal, India, Bull. Environ. Contam. Toxicol., 1990, 45, 389 CrossRef.
- E. Mauriz, A. Calle, J. J. Manclus, A. Mtoya, A. Hilderbrandt, D. Barcelo and L. M. Lechuga, Biosens. Bioelectron., 2007, 22, 1410 CrossRef CAS.
- M. Hirano, K. Kitamura, I. Kato, C. Yanaihara, K. Iwamoto, M. Sekiyama, C. Watanabe, T. Nakamoto, N. Miyamoto, Y. Onishi and K. Arizono, J. Environ. Sci. Health, Part B, 2008, 43, 44 CrossRef CAS.
- J. G. Bergmann and J. Sainik Jr, Anal. Chem., 1957, 29, 241 CrossRef CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265 CAS.
- U. K. Laemmli, Nature, 1970, 227, 680 CAS.
- K. S. Abhijith, P. V. Sujith Kumar, M. A. Kumar and M. S. Thakur, Anal. Bioanal. Chem., 2007, 389, 2227 CrossRef CAS.
- J. D. Sholten, K. H. Chand, P. C. Babbitt, H. Charest, M. Sylvestre and M. D. Dunaway, Science, 1991, 253, 182 CrossRef.
- L. Trnkova, V. Adam, J. Hubalek, P. Babula and R. Kizek, Sensors, 2008, 8, 5619 CrossRef CAS.
- E. Morou, H. M. Ismail, A. J. Dowd, J. Hemingway and N. Labrou, Anal. Biochem., 2008, 378, 60 CrossRef CAS.
- C. Webb, Aust. J. Biotechnol., 1989, 3, 50 Search PubMed.
- M. D. Gouda, M. A. Kumar, M. S. Thakur and N. G. Karanth, Biosens. Bioelectron., 2002, 17, 503 CrossRef CAS.
- D. Gabel, P. Vretblad, R. Axen and J. Porath, Biochem. Biophys. Acta, 1970, 214, 561 CrossRef CAS.
- D. Gabel, Eur. J. Biochem., 1973, 33, 348 CrossRef CAS.
- S. B. Chang and R. R. Mahoney, Biotechnol. Appl. Biochem., 1995, 22, 203.
Footnotes |
| † Electronic supplementary information (ESI) available: SDS-PAGE, temperature and pH studies of DDT and HCH dehydrohalogenase enzymes. See DOI: 10.1039/c0ay00272k |
| ‡ Food Safety and Analytical Quality Control Laboratory, Central Food Technological Research Institute (A constituent laboratory of Council of Scientific and Industrial Research, New Delhi), Mysore 570 020, India. |
| This journal is © The Royal Society of Chemistry 2010 |
