A rapid and selective carbon composite platinum coated electrode for determination of copper ion in real samples
Abdolkarim
Abbaspour
*,
Fatemeh
Norouz-Sarvestani
,
Hashem
Sharghi
and
Fatemeh
Moeini
Chemistry Department, College of Sciences, Shiraz University, Shiraz, 7145685464, Iran. Tel: +98-711-2284822; Fax: +98-711-2286008; E-mail: Abbaspour@chem.susc.ac.ir
First published on 2nd September 2010
Abstract
A Pt wire coated with pyridine-2,6-dicarboxamide derivative–carbon composite in a poly(vinyl chloride) membrane was used for detection of copper. The sensor has a Nernstian slope of 29.50 ± 0.17 mV per decade over a wide range concentration, of 8.7 × 10−7 to 1.0 × 10−1 mol L−1 for Cu2+ ions. The detection limit is 1.1 × 10−7 mol L−1 and the electrode is applicable in the pH range of 3.0–7.0. The response time of the proposed electrode is 6 s, its life time is at least 3 months and it has good selectivity. The practical analytical utility of the electrode is demonstrated by measurement of Cu2+ ions in multivitamin, orange juice, river water and as indicator electrode in potentiometric titration of copper ion with EDTA. From the obtained data it is seen that there is satisfactory agreement between the results obtain by the proposed electrode and those determined by atomic absorption spectroscopy (AAS), and perfect stoichiometry is observed in titration plot.
Introduction
The Trace metal determination is important for environmental protection, food and agricultural chemistry. Copper is an essential trace element and is present in all land and marine organisms. It is widely used in industry, agriculture and for domestic purposes, therefore it is one of the most widely distributed elements of industrialized countries. It is well known that copper plays an important role in many biological processes, such as blood formation and the functioning of various enzymes.1,2 The maximum tolerable level for copper is 2.0 mg L−1 and excessive intake of this element manifests certain diseases in humans such as, Menke's syndrome and Wilson's disease.3,4 Thus, the determination of copper is important in view of its utility as well as its toxicity. A number of instrumental methods such as atomic absorption spectroscopy (AAS), inductively coupled plasma (ICP), stripping voltammetry and flame photometry are employed for the determination of copper at low concentration levels. These methods generally require sample pretreatment and infrastructure backup and are not very convenient for routine analysis of large numbers of environmental samples.Due to the many applications of copper in industry, biological and medical systems,5 and the need for potentiometric monitoring of Cu2+ ions in these areas, a variety of ion selective electrodes (ISEs) of copper has been constructed. In these copper sensors, small size thia-crown ethers,6,7 non-cyclic neutral ionophores,8,9 calyx arenes10,11 and Schiff bases12 have been used as ion carriers. In addition, solid-state electrodes based on CuS–Ag2S are well-established,13 but cations such as Hg22+,Hg2+, and Fe3+ give serious interference.13,14
The specific metal–ligand interaction is the most important recognition mechanism that can be utilized in the development of potentiometric sensors.15 The copper(II)-nitrogen-sulfur ligands frame provides a remarkable contribution to determine copper ions in various samples.16 Successful attempts have also been made in the design and synthesis of highly selective ionophores as sensory molecules for Cu2+ ion ISEs, but they reveal a poor detection limit17–19 narrow concentration range20,21 and serious interference by other ions.22 Most of these electrodes require internal solution.
The development of miniaturized and micro sized ISE probes continues to be an active topic of research, because most commercial ISEs, with tip diameters of the order of 3–15 mm, are regarded as macro in size. Such large sensors such as IS(internal solution)-ISEs are not suitable for measurements in small volumes of sample or for the desired in vivo applications of ISEs that biomedical researchers have long awaited. This quest for miniaturization has resulted in the development of coated wire electrodes (CWEs).23 Although long-term stability may be a problem, CWEs have been found to be quite useful for many direct determinations if the electrodes are calibrated often. The first report on CWEs was published by Cattrall and Freiser,24 since then a variety have been reported.25,26
Carbon-based materials have been used in electrochemistry because of their many advantages, for example low background, low cost, high stability, and resistance to passivation.27
We previously reported, for the first time, use of a PVC–carbon composite on platinum wire as a CWE for analysis of Ag(I), Pb(II), Cr(III) and Al(III). In comparison with most commercially available electrodes, these electrodes were readily prepared and had high selectivity and low detection limits.28–32
The object of this work is to demonstrate use of a new carbon–PVC– pyridine-2,6-dicarboxamide composite electrode on platinum wire for rapid and selective determination of copper over a wide concentration range. The significance of this membrane composition is twofold use of carbon to increasing sensitivity, conductivity and also use of ionophore, an important aspect of increasing the selectivity of this composition. Although complex and expensive methods are used to detect copper with low detection limits, as already mentioned, our method has many advantages, such as good selectivity, sensitivity, stability, easy preparation and requirement of simple and inexpensive potentiometric instruments.
Experimental
Reagents and chemicals
Analytical-reagent grade diethyl phthalate (DEP), dibutyl sebacate (DBS), diethyl sebacate (DES), and o-nitrophenyloctylether (o-NPOE) were obtained from Fluka. Poly (vinylchloride) (PVC), carbon powder and tetrahydrofuran (THF) were purchased from Merck. All solutions were prepared from analytical reagents with deionized water.Ionophore preparation
N,N′-bis(2-hydroxyphenyl)-pyridine-2,6-dicarboxamide (ionophore I), N,N′-bis(2-methoxyphenyl)-pyridine-2,6-dicarboxamide (ionophore II), 2,6-bis(N-phenylcarbamoyl)pyridine (ionophore III) were used as ionophores (Fig. 1) Typical procedure for synthesis of ionophore I is reported in literature.33 | ||
Fig. 1 Chemical structure of the three ionophores: Ionophore I, R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH; ionophore II, R OH; ionophore II, R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OCH3; ionophore III, R OCH3; ionophore III, R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H. H. | ||
Membrane preparation
The schematic setup and general protocol for preparation of this sensing electrode, which was used in this work was similar to our previous studies31–37 except that this electrode was a carbon composite PVC coated wire electrode with a copper selective ionophore. This cocktail mixture contained by weight: ionophore (2.2%); carbon powder (2%); DES (63.9%) and PVC (31.9%). A total weight of 150 mg of the mixture was dissolved in 1.5 cm3 of dry freshly distilled THF and the mixture was homogenized by use of ultrasound. The coating process of this carbon composite platinum wire electrode was performed by dipping Pt wire several times into this mixture. After coating the membrane, as in our previous work,32 it was air-dried for 12 h until a thin film was formed. The electrode was finally conditioned for 3 h in a 10−3 M of Cu (NO3)2 solution.Apparatus
All measurements of emf were made at 25 °C by use of a Metrohm pH meter (Model 654, Swiss, Zurich) with the following cell assembly:| Ag|AgCl, KCl (sat'd.) ‖Cu2+ solution|membrane|Pt |
Activities were calculated in accordance with the Debye–Hückel procedure.8
Results and discussion
Electrode response characteristics
Prior to any optimization process the potential responses of platinum coated wire electrodes for solutions containing each cation separately were obtained and the results are shown in Fig. 2. As this figure exhibits the sensor has a better response to the copper cation than to the other species. The Pt coated wire electrode with optimum composition (at pH value of 4.2) showed a linear Nernstian response with a slope of 29.50 ± 0.17 mV per decade over the range of 8.7 × 10−7 to 1.0 × 10−1 M for Cu(NO3)2 and a correlation coefficient of 0.999(n = 5).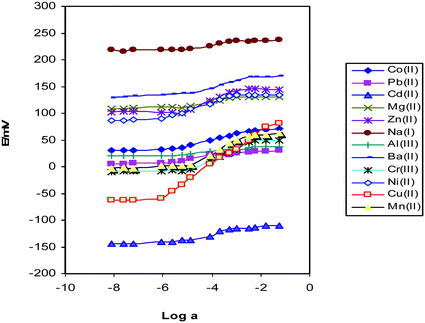 | ||
| Fig. 2 The potential response of various cations on CWE. | ||
Optimization of membrane composition
Studies on the response characteristics of copper ion selective electrodes for ionophores I–III, show that ionophore number I has the best behavior with a good Nernstian response over a wide linear concentration range (8.7 × 10−7–1.0 × 10−1M).The results are given in Table 1 (electrodes 1-3). Therefore ionophore I was chosen to fabricate copper selective electrode. From spectroscopic characterization as reported in literature,36 it can be concluded that the ligand acts as an ambidentate ligand towards copper metal ions under experimental conditions. The ligand binds to the copper ion through the pyridine nitrogen, two amide oxygens and two deprotonated phenolic oxygens.38
| Coating composition (%) | Slope (mV/decade) | R2 | Concentration range/M | ||||
|---|---|---|---|---|---|---|---|
| No. | Plastisizer | PVC | C | Ionophore | |||
| 1 | DES | 31.9 | 2 | I(2.2) | 29.45 | 0.998 | 8.7 × 10−7 to 1.0 × 10−1 |
| 2 | DES | 31.9 | 2 | II(2.2) | 19.12 | 0.994 | 3.2 × 10−6 to 1.4 × 10−3 |
| 3 | DES | 31.9 | 2 | III(2.2) | 18.74 | 0.994 | 3.2 × 10−6 to 4.0 × 10−4 |
Several solvent mediators (o-NPOE, DES, DBS, DEP) were tested. Ion selective electrode based on DES exhibits a better Nernstian slope (29.50 ± 0.17 mV/decade) than o-NPOE, DBS and DEP as shown in Fig. 3 and Table 2. For this reason DES was chosen as plasticizer in the rest of the experiments.
| No | DES | DBS | DEP | o-NPOE | Ionophore | PVC | Ca | Slope (mV/decade) | R2 | Concentration range/M |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63.9 | — | — | — | 2.2 | 31.9 | 2.0 | 29.5 | 0.9995 | 8.7 × 10−7−1 × 10−1 |
| 2 | — | 63.9 | — | — | 2.2 | 31.9 | 2.0 | 23.7 | 0.9901 | 2.5 × 10−6−1.7 × 10−2 |
| 3 | — | — | 63.9 | — | 2.2 | 31.9 | 2.0 | 20.4 | 0.9890 | 2.5 × 10−6-1.7 × 10−2 |
| 4 | 63.9 | — | — | — | 2.2 | 31.9 | 2.0 | 14.3 | 0.9930 | 6.3 × 10−6−1.7 × 10−2 |
| 5 | 63.9 | — | — | — | 2.2 | 31.6 | 3.0 | 35.9 | 0.9966 | 2.5 × 10−6−2.5 × 10−2 |
| 6 | 63.9 | — | — | — | 2.2 | 32.3 | 1.0 | 30.8 | 0.9927 | 2.3 × 10−6–2.5 × 10−2 |
| 7 | 63.9 | — | — | — | 3.3 | 31.6 | 2.0 | 27.1 | 0.9800 | 2.3 × 10−6–2.5 × 10−2 |
| 8 | 63.9 | — | — | — | — | 32.7 | 2.0 | 21.6 | 0.9946 | 3.6 × 10−5−3.6 × 10−3 |
| 9 | 63.9 | — | — | — | 2.2 | 32.6 | - | 23.6 | 0.9961 | 1.0 × 10−6–2.5 × 10−2 |
| 10 | 63.9 | — | — | — | 1.5 | 32.2 | 2.0 | 31.5 | 0.9923 | 6.0 × 10−6−3.6 × 10−3 |
 | ||
| Fig. 3 Effect of several plastisizers on the response of the composite electrode under optimized conditions. | ||
The performance of membranes of different composition was investigated; the results are given in Table 2. It is apparent from the table that the response of the electrode coated with the composite containing no ligand (no. 8) has a near Nernstian slope of 21.59 mV per decade over a short range of concentration and that the electrode coated with the composite containing 0.0% carbon powder (no. 9) has also a near Nernstian slope of 23.55 mV per decade in the concentration range 1.0 × 10−6 2.5 × 10−2 M whereas at the optimum composition of ionophore (2.2%), carbon powder (2.0%), DES (63.9%), and PVC (31.9.%) the slope obtained was 29.50 mV per decade in the concentration range 8.7 × 10−7−1.0 × 10−1 M (no. 1). Table 2 also shows that an electrode with the optimum amount of ionophore but with more carbon than its optimum value (no. 5) has a super Nernstian slope. Changing the composition of the electrode by reducing the amount of carbon to lower than its optimum value (no. 6) results again in a super Nernstian response. From this Table it is clear that the detection limits and linear dynamic ranges for this CWE are not only influenced by carbon, but also by the amount of the ionophore. The results obtained in this study indicate that the electrodes based on both the N,N′-bis(2-hydroxyphenyl)-pyridine-2,6-dicarboxamide and the carbon powder show a high sensitivity and selectivity for copper ions.
Effect of pH
The pH dependence of the potentials of the proposed electrode for 1.0 × 10−3 and 1.0 × 10−5 M copper solutions were investigated over the pH range of 2.0–10.0 (HCl or NaOH solutions were used to adjust the pH) and the results are depicted in Fig. 4. The potential responses remained constant over the pH ranges 3.0–6.7 and 3.0–7.0 for the concentration 1.0 × 10−3, and 1.0 × 10−5 M of Cu(NO3)2, respectively. Drift observed at low pH which could be because of the response of the electrode to H+ ions whereas at higher pH the formation of hydroxy complexes of Cu2+ ions may cause a decrease in potential response.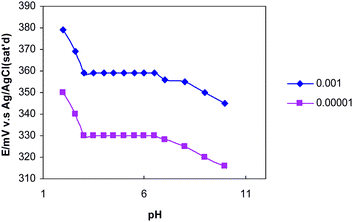 | ||
| Fig. 4 pH effect on proposed composite electrode in two different concentrations of copper ion in the pH range of 2.0–10.0. | ||
Detection limit, reproducibility, repeatability response time and life time of the copper electrode
The limit of detection, which is evaluated according to IUPAC recommendations39 was 1.1 × 10−7 M of Cu (NO3)2.The reproducibility was investigated by preparing seven similar electrodes at optimum membrane composition, then the slope of each electrode was determined and the average slope with standard deviation was 29.78 ± 0.58 mV/decade.
In the repeatability study, the calibration curves of one electrode were obtained five times over ten days, the calibration curves were taken every other day. The average slope with standard deviation was 29.50 ± 0.17 mV/decade.
The response time of the electrode was evaluated (according to IUPAC definition) by measuring the time required to achieve a 90% value of steady potential for a copper solution. A response time of 6 s was obtained for this carbon-PVC membrane.
To investigate the life time of the electrode, the calibration curves of copper electrode at its optimized composition were periodically obtained for three months. The results showed that this sensor is stable at this period of time.
Selectivity coefficients of copper selective electrode
The selectivity is clearly one of the most important characteristics of a sensor, as it often determined whether a reliable measurement in the target sample is possible. The selectivity coefficients for the membrane electrode were measured by using both the mixed solution method (MSM)40 and the matched potential method (MPM).41,42 In the mixed solution method, selectivity coefficients of the copper sensor were evaluated graphically with a fixed concentration of the Cu2+ ions (7.4 × 10−3 M) and varying amounts of the interfering ions (Mn+).The resulting values of the selectivity coefficients are summarized in Table 3. As this Table shows these interfering cations could not affect the selectivity of the copper electrode.| Interfering ion | log Kij | |
|---|---|---|
| FIM | MPM | |
| Mg2+ | −3.24 | −2.08 |
| Co2+ | −3.60 | −2.08 |
| Cd2+ | −3.18 | −2.06 |
| Ba2+ | −3.13 | −2.05 |
| Ni2+ | −2.53 | −1.86 |
| Zn2+ | −3.09 | −2.05 |
| Pb2+ | −2.02 | −1.66 |
| Ca2+ | −3.59 | −2.03 |
| K+ | −1.08 | −1.96 |
| Na+ | −1.15 | −1.84 |
| NH4+ | −2.17 | −1.89 |
| Hg2+ | −1.18 | −1.73 |
| Mn2+ | −3.54 | −2.06 |
| Al3+ | −1.66 | −1.29 |
| Cr3+ | −1.82 | −1.34 |
| Li+ | −1.97 | −1.79 |
In cases where the ions of different charges are present, the validity of the MSM based on the semi-empirical Nikolskii–Eisenman equation is in question, therefore, the MPM was also applied to these ions. In MPM, the selectivity coefficient is defined as the activity ratio of the primary ion and the interfering ion that gives the same potential change in a reference solution. The concentration of Cu2+ ions used as the primary ion in this study was 1.0 × 10−5 M. The resulting values of the selectivity coefficient for MPM are also summarized in Table 3. As this table shows the interfering cations do not significantly affect the selectivity of the proposed electrode.
Analytical applications
Determination of copper in river water and orange juice samples
The electrode was successfully applied to determine copper ions directly in river water and orange juice solution. The water sample was acidified with HCl to adjust stable pH at 4.5. The results obtained from the triplicate measurement of proposed copper sensor (for both river water and orange juice) are compared with those determined by atomic adsorption spectroscopy (AAS) and are summarized in Table 4. From the obtained data it is seen that there is satisfactory agreement between the results obtain by the proposed electrode and those determined by AAS.| Sample | Potentiometry with Pt/CWE/mg L−1 | AAS method/mg L−1 |
|---|---|---|
| River water | 1.70 ±.06 | 1.80 ±.05 |
| Orange juice | 0.21 ±.07 | 0.22 ±.02 |
| Multivitamina | 0.79 ±.05 | 0.80 ±.03 |
| Vitamin | Quantity |
|---|---|
| a Components of Theragran-M high potency multivitamin (SQUIBB Company,USA). | |
| Vitamin A | 5000 I.U. |
| Vitamin B1 | 3 mg |
| Vitamin B2 | 3.4 mg |
| Vitamin B6 | 3 mg |
| Vitamin B12 | 9 mcg |
| Vitamin C | 90 mg |
| Vitamin D | 400 I.U. |
| Vitamin E | 30 I.U. |
| Niacin | 20 mg |
| Folic acid | 400 mcg |
| Pantothenic acid | 10 mg |
| Biotin | 30 mcg |
| Electrolytes | |
| Chloride | 7.5 mg |
| Potassium | 7.5 mg |
| Minerals | |
| Iron | 27 mg |
| Copper | 2 mg |
| Iodine | 150 mcg |
| Zinc | 15 mg |
| Magnesium | 100 mg |
| Calcium | 40 mg |
| Phosphorus | 31 mg |
| Chromium | 15 mcg |
| Molybdenum | 10 mcg |
| Selenium | 10 mcg |
| Manganese | 5 mg |
Potentiometric titration
The proposed Cu2+ ion membrane sensor was found to work well under laboratory conditions and it was successfully applied to the titration of Cu2+ with EDTA. A 20 mL buffer solution of 1.0 × 10−3 M Cu2+ ions at pH 4.2 was titrated against a 1.0 × 10−2 M EDTA solution. The resulting titration curve is shown in Fig. 5. As shown in this figure, perfect stoichiometry is observed in the titration plot. | ||
| Fig. 5 Potentiometric titration curve of 20.0 mL 1 × 10−3 M of Cu2+ ions. | ||
Estimation of copper(II) in multivitamin
The proposed electrode was applied to determine copper in Theragran-M high potency multivitamin (SQUIBB Company, USA). Two tablets of multivitamin were subjected to heating in a muffle furnace in a silica crucible at about 600 °C for 6-7 h; the ash was then dissolved in 10 mL 1-1 HCl and diluted with 100 mL distillate water. This solution was used for Cu2+ ions determination by the CWE as an indicator electrode using the standard addition method and the results are summarized in Table 4. As this Table shows the result is comparable with the value obtained by AAS.Conclusions
We have shown that electrode prepared from a composite of carbon, and poly(vinyl chloride) can be used for analytical applications. This composite electrode is sufficiently stable and has a long lifetime. The proposed electrode also has many other advantages; it is inexpensive, easy to prepare, and requires simple potentiometric equipment. By this method larger “real surface area/apparent electrode area” ratios are readily obtained25,34 and by using ultrasound a homogenized mixture was obtained. Coating this material was simply preformed by dipping the platinum wire into the mixture. A comparison between the present coated copper wire selective electrode and the previous reported copper electrodes are given in Table 5. As this Table shows the proposed sensor is superior to most reported sensors.| Type of electrode | Carrier name | Linear range/M | Detection limit/M | Slope (mV/decade) | Response time/s | Ref. No. |
|---|---|---|---|---|---|---|
| CWE | N,N′-bis(2-hydroxyphenyl)-pyridine-2,6-dicarboxamide benzyl | 8.7 × 10−7to 1.0 × 10−1 | 1.1 × 10−7 | 29.5 | 6 | This work |
| IS-ISE* | Schiff-base | 1.9 × 10−to 1.0 × 10−1 | — | 30.0 | 12 | 43 |
| IS-ISE | 3-(2-Pyridinyl)-2H-pyrido[1,2,-a]-1,3,5-triazine-2,4(3H)-dithione | 5.0 × 10−8 to 1.0 × 10−2 | 4.0 × 10−8 | 29.5 | 12 | 2 |
| IS-ISE | Schiff's base | 8.0 × 10 −6to 1.0 × 10−1 | 3.0 × 10−6 | 29.5 | 15 | 18 |
| IS-ISE | Ethambutol–Cu2+ complex | 7.9 × 10−6 to 1.0 × 10−1 | 7.0 × 10−6 | 29.9 | 11 | 20 |
| IS-ISE | o-Vanilin | 5.0 × 10−6 to 1.0 × 10−1 | 8.0 × 10−7 | 28.5 | 30 | 19 |
| IS-ISE | Salens | 1.0 × 10−5 to 1.0 × 10−1 | 3.1 × 10−6 | 29.7 | 10 | 22 |
| IS-ISE | Porphyrin | 4.0 × 10−6 to 1.0 × 10−1 | 4.4 × 10−6 | 29.3 | 8 | 44 |
| IS-ISE | Cyclic tetrapeptide derivative | 1.0 × 10−5 to 1.0 × 10−2 | 2.1 × 10−7 | 25.9 | 15 | 45 |
| CWE | Phenylglyoxal-_-monoxime | 1.0 × 10−6 to 1.0 × 10−1 | 5.0 × 10−7 | 28.2 | 10 | 46 |
| IS-ISE | Napthol-derivative Schiff's base | 5.0 × 10−8 to 2.0 × 10−2 | 3.1 × 10−6 | 29.8 | 5 | 47 |
| IS-ISE | o-Xylene bis(dithiocarbamates) | 10−6 to 10−1 | 1.4 × 10−7 | 29.0 | 9 | 48 |
Notes and references
- E. E. Tyrala, E. L. Brodsky and V. Auerbach, Am. J. Clin. Nutr., 1982, 35, 542 CAS.
- A. K. Singh and S. Mehtab, Anal. Chim. Acta, 2006, 572, 25 CrossRef.
- P. C. Bull and D. W. Cox, Trends Genet., 1994, 10, 246 CrossRef CAS.
- M. Schaefer and G. D. Gitlin, Am. J. Physiol., 1999, 276, 311.
- S. Dadfarnia and M. Shamsipur, J. Membr. Sci., 1992, 75, 61 CrossRef CAS.
- S. Kamata, Y. Yamasaki, M. Higo, A. Bhale and Y. Fukanaga, Analyst, 1988, 113, 45 RSC.
- J. casabo, L. Mestres, L. Escriche, F. Texidor and C. perez-Jimenez, J. Chem. Soc., Dalton Trans., 1991, 1969 RSC.
- S. Kamata, A. Bhale, y. Fukunaga and H. Murata, Anal. Chem., 1988, 60, 2464 CrossRef CAS.
- Z. Brzozka, Analyst, 1988, 113, 1803 RSC.
- P. Buhlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593 CrossRef.
- P. L. H. M. Cobben, R. J. M. Egberivk, J. B. Bomer, P. Bergveld, W. Verboon and D. N. Reinhoudt, J. Am. Chem. Soc., 1992, 114, 10573 CrossRef CAS.
- K. Ren, Talanta, 1989, 36, 767 CrossRef CAS.
- Y. Umezawa (ED), Handbook of Ion- selective electrodes: Selectivity Coefficients, CRC Press, Boca Raton, FL, USA ( 1990) Search PubMed.
- D. A. Roman, Mar. Chem., 1992, 38, 165 CrossRef CAS.
- R. S. Hutchins and L. G. Bachas, Anal. Chem., 1995, 67, 1654 CrossRef CAS.
- P. K. Dhara, S. Pramanik, T.-H. Lu, M. G. B. Drew and P. Cattopadhayay, Polyhedron, 2004, 23, 2457 CrossRef CAS.
- J. Kouljenovic, V. Martinac and N. Radic, Anal. Chim. Acta, 1990, 231, 137 CrossRef CAS.
- S. Sadeghi, M. Eslahi, M. A. Naseri, H. Sharghi and A. Shmeli, Electroanalysis, 2003, 15, 1327 CrossRef CAS.
- L. P. Singh and J. M. Bhatnagar, Talanta, 2004, 64, 313 CrossRef CAS.
- V. K. gupta, R. Prasad and A. Kumar, Talanta, 2003, 60, 149 CrossRef CAS.
- A. K. jain, V. K. Gupta, L. P. Singh and j. R. Raisoni, Talanta, 2005, 66, 1355 CrossRef CAS.
- A. R. Fakhari, T. A. Raji and H. Naeimi, Sens. Actuators, B, 2005, 104, 317 CrossRef.
- A. M. Arnold and M. E. Meyerhoff, Anal. Chem., 1984, 56, 20 CrossRef.
- R. W. Cattrall and H. Freiser, Anal. Chem., 1971, 43, 1905 CrossRef CAS.
- K. Stulik, V. Pocakova and B. Starkova, J. Chromatogr., A, 1981, 213, 41 CrossRef CAS.
- F. Albertus, A. J. Alpizar, V. Cerda, M. Luque, A. Riso and M. Valcarcel, Anal. Chim. Acta, 1997, 355, 23 CrossRef CAS.
- S. N. Tan and L. Hua, Anal. Chim. Acta, 2001, 450, 263 CrossRef CAS.
- A. Abbaspour and A. Izadyar, Anal. Bioanal. Chem., 2006, 386, 1559 CrossRef CAS.
- A. Abbaspour., A. Izadyar and H. Sharghi, Anal. Chim. Acta, 2004, 525, 91 CrossRef CAS.
- A. Abbaspour, E. Mirahmadi, A. Khalafi-nejad and S. Babamohammadi, J. Hazard. Mater., 2009, 174, 656.
- A. Abbaspour and A. Izadyar, Talanta, 2001, 53, 1009 CrossRef CAS.
- A. Abbaspour, M. Refahi, Anal. Chim. Acta, in press Search PubMed.
- H. Sharghi, M. Hosseini-Sarvari and F. moeini, Can. J. Chem., 2008, 86, 1044 CrossRef CAS.
- A. Abbaspour and M. A. Kamyabi, Talanta, 2002, 57, 859 CrossRef CAS.
- A. Abbaspour and F. Tavakol, Anal. Chim. Acta, 1999, 378, 145 CrossRef CAS.
- A. Abbaspour and M. A. Kamyabi, Anal. Chim. Acta, 2002, 455, 225 CrossRef CAS.
- A. Abbaspour and A. Izadyar, Microchem. J., 2001, 69, 7 CrossRef CAS.
- Kalagouda B. Gudasi, Ramesh S. Vadavi, Rashmi V. Shenoy, Manjula S. Patil and Siddappa A. Patil, Transition Met. Chem., 2005, 30, 569 CrossRef.
- P. Y. Gadzekpo and G. D. Christain, Anal. Chim. Acta, 1984, 167, 279 CrossRef.
- K. Srinivasan and G. A. Rechnitz, Anal. Chem., 1969, 41(1), 203.
- E. Bakker, Electroanalysis, 1997, 9, 7 CAS.
- E. Bakker, P. Pretsch and Buhlmann, Anal. Chem., 2000, 72, 1127 CrossRef CAS.
- A. K. Jain, R. K. Singh and S. Jain, Transition Met. Chem., 2008, 33, 243 CrossRef CAS.
- S. S. M. Hassan, E. M. Elnemma and A. H. K. Mohamed, Talanta, 2005, 66, 1034 CrossRef CAS.
- N. Alizadeh, S. Ershad, H. Naeimi, H. Sharghi and M. Shamsipur, Fresenius J. Anal. Chem., 1999, 365, 511 CrossRef CAS.
- A. R. Firooz, M. Mazloum, J. Safari and M. K. Amini, Anal. Bioanal. Chem., 2002, 372, 718 CrossRef CAS.
- M. R. Ganjali, M. Emami and M. Salavati-Niasari, Bull. Korean Chem. Soc., 2002, 23, 1394 CrossRef CAS.
- O. Marcin, M. Agata and M. Krzysztof, Electrochim. Acta, 2006, 51, 2298 CrossRef.
| This journal is © The Royal Society of Chemistry 2010 |
