A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent
Kadriye Işıl
Berker
a,
Kubilay
Güçlü
b,
Birsen
Demirata
a and
Reşat
Apak
*b
aDepartment of Chemistry, Faculty of Science and Letters, Istanbul Technical University, Ayazaga Maslak, 34469, Istanbul, Turkey
bDepartment of Chemistry, Faculty of Engineering, Istanbul University, Avcilar, 34320, Istanbul, Turkey. E-mail: rapak@istanbul.edu.tr; Fax: +90-212-4737180; Tel: +90-212-4737028
First published on 21st September 2010
Abstract
Since antioxidants are health-beneficial compounds capable of removing reactive species, assay of total antioxidant capacity (TAC) by simple and low-cost methods is important. The magenta-coloured iron(II)-ferrozine (Fe(II)-FZ) complex showing an absorbance maximum at 562 nm has previously been utilized for iron-binding assays, but not for antioxidant determination. Ferrozine is a highly ferrous-stabilizing ligand such that ferric ion in the presence of ferrozine easily oxidizes antioxidants and is itself reduced to Fe(II)-FZ, yielding a very high molar absorptivity and thus enhanced sensitivity for most antioxidants. The hierarchic order of antioxidant power for common antioxidants was in accordance with known structure–activity relationships. The Fe(III)-FZ assay was applied to synthetic antioxidant mixtures to yield additive absorbance values, which is a prerequisite for precise determination of antioxidant capacity of complex mixtures. The calibration curves (lines) of trolox and quercetin individually and in herbal infusions—by using the method of standard additions—were parallel, confirming that the herbal antioxidants and trolox did not chemically interact among each other so as to cause apparent deviations from Beer's law. The proposed method was applied to medicinal plant infusions for total antioxidant capacity assay as trolox-equivalents, and the results were compared to those found with CUPRAC (cupric reducing antioxidant capacity), FRAP (ferric reducing antioxidant power) and Folin total phenols assays, the highest correlation being achieved with CUPRAC. In short, a novel ferric reducing assay for food antioxidants was introduced, which was superior to FRAP in regard to its realistic pH, enhanced sensitivity, faster kinetics, and absence of free Fe(II)—which can cause Fenton-type oxidations—in the reaction products.
Introduction
Reactive oxygen species (ROS) such as superoxide anion (O2˙−), hydrogen peroxide (H2O2), and hydroxyl radical (˙OH) are produced in organisms and also can be induced by exogenous sources like tobacco smoke, ionizing radiation, certain pollutants, organic solvents and pesticides.1–3 ROS may attack biological macromolecules, giving rise to tissue injury and a number of oxidative stress-originated diseases. Antioxidants are believed to have a significant role in preventing the hazards of ROS in the organism.4 Therefore consumption of antioxidants in food or as dietary supplements are believed to have a protective role against various physiological disorders.A variety of assays have been used to measure the total antioxidant capacity (TAC) of pure substances, food extracts, and beverages.4 Some representative assays are 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)/trolox-equivalent antioxidant capacity (TEAC),5,6 Folin-Ciocalteu total phenols,7 ferric reducing antioxidant power (FRAP),8–10 ferricyanide/Prussian blue method,11 cupric reducing antioxidant capacity (CUPRAC),12–14 cerium(IV) reducing antioxidant capacity,15 and 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity (DPPH)16 methods. These methods are either ET (electron transfer)—based or a mixture of ET—and hydrogen atom transfer (HAT)-based assays in mechanism, measuring the absorbance difference of an oxidant reagent (at a prespecified wavelength) during the course of the oxidation of an antioxidant.
Stookey used the disodium salt of 3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine (known as ferrozine: FZ) for determination of iron(II) in acetic acid/sodium acetate buffer medium at pH 5.5.17 The molar absorptivity found by Stookey17 for the Fe(FZ)34− chelate was ε = 2.8 × 104 L mol−1cm−1 at 562 nm, with notable interference from only oxalate, nitrite, and cyanide. Pascual-Ruguera et al. used ferrozine for the sensitive and reproducible flow-injection (FI) spectrophotometric analysis of iron.18 Molina-Diaz et al. used ferrozine in hexamethylenetetramine–buffered medium at pH 5.5 as a reagent for indirect FI-determination of ascorbic acid after its oxidation with Fe(III) in acid solution.19 Giokas et al. used ferrozine for speciation of Fe(II) and Fe(III) by using FI-spectrophotometry, and flame-AAS after cloud-point extraction.20 Ferrozine has generally been used in literature in the assay of iron or ‘iron binding capacity’ of food and human serum.21,22 Among many examples of Fe(II) binding measurement, ferrozine has been used by Gülçin et al. (2003),23 Gülçin (2007),24 Ak and Gülçin (2008)25 as a reagent for determining ‘ferrous ion chelating activity’ of phenolic compounds and plant extracts via measuring the percentage inhibition of ferrozine-Fe2+ complex, in accordance with the modified method of Dinis et al. (1994).26
A variety of ligands have been used in iron(III)-based assays in the determination of TAC or reducing capacity. These are 1,10-phenanthroline, 4,7-diphenyl-1,10-phenanthroline (batho-phenanthroline), 2,4,6-tris(2-pyridyl)-1,3,5-triazine(TPTZ),8,27 and ferricyanide.11 The most widely used ferric reducing antioxidant assay, FRAP, was extensively criticised for its unrealistic pH of 3.6 (where most phenolics would not dissociate their protons, and thus would be less susceptible to oxidative attack by the assay reagent), relatively slow kinetics which do not enable the completion of oxidation of certain hydroxycinnamic acids and thiols within the protocol time period of the assay, and higher affinity toward hydrophilic antioxidants than hydrophobic ones.28 Moreover, since the (Fe(III)/TPTZ) mole ratio in the FRAP reagent exceeds the stoichiometric value of complex formation, the associated redox reaction with antioxidants may produce unbound (free) Fe(II) which is suspected to cause redox cycling of antioxidants during the assay as a result of Fe(II)-mediated Fenton-type reactions.29 No assay has been reported in literature capable of measuring TAC through iron(III) reduction in the presence of ferrozine. Ferrozine is a partially selective and very sensitive reagent for Fe(II) which may emerge as a result of reduction of ferric ion by antioxidants, and the stoichiometric reaction ratio between Fe(II) and ferrozine is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.17,19
3.17,19
In this study, we measured TAC with the use of ferric-ferrozine reagent. The visible absorption spectrum of the ferrous complex of ferrozine exhibits a single sharp absorption peak with maximum absorbance at λmax = 562 nm. The TAC assay proceeds via reduction of Fe(III) to Fe(II), and subsequent determination of the formed Fe(II) with ferrozine using spectrophotometric absorbance measurement at λmax. The advantages of ferrozine over other iron-based TAC assays are higher molar absorptivity,17 relatively lower interference from foreign ions,17 wide pH tolerance,17 complex stability constant as high as β3 = 3.4 × 1015,30 water solubility, and low viscosity.19 The molar absorptivity is so high (at the order of 2.8 × 104 L mol−1cm−1)17 that quite low concentrations of antioxidants can be determined. The maximum absorption wavelength is quite different from those characteristic of plant pigments, enabling the interference-free measurement of TAC of plant food. By forming a stable iron(II)-chelate, a plant extract with a high antioxidant power strongly reduces the free ferrous ion concentration with the aid of ferrozine thereby decreasing the probability of a Fe(II)-based Fenton reaction31 which is common to many iron–based assays32 The standard redox potential of the Fe3+/Fe2+ couple at the order of 0.77 V should be shifted to distinctly more positive potentials due to selective complexation of ferrous ions in preference to the ferric state, enabling the oxidation of antioxidants acting as weak reductants. Another advantage of the proposed method is that incubation (at a higher temperature) is not required. Gibbs suggested that in an aqueous solution with a pH of 3–6 and a temperature range of 10°–45 °C, a 5-min colour development would normally be adequate for ± 2% accuracy in iron determination.30 Since use of hexamethylenetetramine buffer at pH 5.5 causes precipitation, acetic acid/sodium acetate buffer is preferred.
The proposed assay depends on the reduction of a ferric-ferrozine reagent with antioxidants to the stable ferrous-ferrozine chelate in buffered medium. The apparent molar absorptivity, linear concentration range and TEAC (trolox equivalent antioxidant capacity) values of the studied antioxidants were found in the proposed assay. Additivity of TAC values of individual antioxidants in the proposed method were tested for synthetic mixtures, and the found antioxidant capacities of certain medicinal plant extracts were compared with those of other similar assays.
Experimental
Chemicals
3-(2-Pyridyl)-5,6-di(4-phenylsulfonicacid)-1,2,4-triazine monosodium salt (ferrozine: FZ), quercetin, ellagic acid, catechin were purchased from Fluka; caffeic acid, ferulic acid, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), rosmarinic acid, and ascorbic acid were from Aldrich; glutathione, cysteine, FeCl3·6H2O, CH3COONa, NH4Fe(SO4)2·12H2O, Na2CO3, NaKC4H4O6, CuSO4, CuCl2 and CH3COONH4 were from E. Merck; rutin and sodium dodecyl sulfate were from Sigma Chemical Co.; concd. HCl, concd. NH3, NaOH, CH3COOH, and C2H5OH (96%, by wt.) were purchased Riedel-de Haën. The real sample matrices containing a mixture of antioxidants were as follows: Commercial samples of tea bags and herbal teas were supplied from the local food market in Istanbul-Turkey, namely as green tea bags (Camellia sinensis) from Doğadan Company Co., linden tea bags (Tilia), nettle herbal tea bags (Urtica diocia/urens), mint (Mentha piperita), sage herbal tea bags (Salvia officinalis) from Doğa Company Co., chamomile (Matricaria chamomilla L.), ceylon tea (Camellia sinensis) from Lipton Company Co. All polyphenolic compounds and vitamin solutions were freshly prepared in 96% ethanol at 1 mM concentration and were further diluted to 3.0 × 10−4 M as required. Glutathione and cysteine solutions were freshly prepared in water at 1 mM concentration, and were further diluted to 7.0 × 10−4 M as required.Instruments
All spectrophotometric measurements were made with a pair of matched quartz cuvettes placed in a CARY 100 Bio UV-Vis spectrophotometer. The pH measurements were made with the aid of a E512 Metrohm Herisau pH-meter using a glass electrode.Procedures
Preparation of solutions. An aqueous solution containing 0.024 g of NH4Fe (SO4)2·12H2O (molecular weight = 482.2) and 1 mL of 1 M HCl was mixed with a separate solution containing 0.123 g ferrozine (molecular weight = 492.47) in water. These two solutions were mixed and the mixture diluted to 25 mL with distilled water so as to make the final iron(III) concentration 2.0 × 10−3 M and ferrozine concentration 1.0 × 10−2 M. This ferric-ferrozine complex solution, when kept in a stoppered, dark-coloured bottle and protected from sunlight, was shown to be stable for a day.
Procedure. To (x) mL antioxidant solution—where x is variable volume—was added (0.5 − x) mL EtOH (96%), 1.5 mL of ferric-ferrozine solution, 2.0 mL of pH 5.5 buffer solution (0.2 M CH3COOH/CH3COONa), and 0.5 mL water so as to make the final volume 4.5 mL. The absorbance against a reagent blank was measured at 562 nm after 30 min standing at room temperature.
Preparation of solutions. Copper(II) chloride stock solution (10−2 M) was prepared by dissolving 0.4262 g dihydrate salt in distilled water, and diluting to a final volume of 250 mL. Ammonium acetate (NH4Ac) buffer at pH 7 was prepared by dissolving 19.27 g NH4Ac in water and diluting to 250 mL. Neocuproine solution (7.5 × 10−3 M) was prepared by dissolving 0.039 g neocuproine (free base) in 96% ethanol, and diluting to 25 mL with the same solvent (should be freshly prepared).12–14
Procedure. To a test tube 1 mL CuCl2 solution (1.0 × 10−2 M), 1 mL neocuproine alcoholic solution (7.5 × 10−3 M) and 1 mL NH4Ac buffer solution were added and mixed. Then, (x) mL antioxidant followed by (1.1 − x) mL water were added (total volume, 4.1 mL) and mixed well. The absorbance against a reagent blank was measured at 450 nm after 30 min.12–14
Preparation of solutions. The FRAP solutions were prepared as follows: a suitable mass of FeCl3·6H2O was weighed so that the final concentration of Fe(III) in solution would be 2.0 × 10 −2 M; 1 mL of 1 M HCl solution was added, dissolved in some water and diluted to 50 mL with H2O. A suitable mass of TPTZ was weighed such that its final concentration would be 1.0 × 10 −2 M, dissolved in absolute EtOH, and diluted to 50 mL. In order to prepare 0.3 M CH3COOH/CH3COONa buffer solution at pH 3.6, 3.1 g CH3COONa·3H2O was weighed and 16 mL glacial acetic acid was added, diluted with water to 1 L. The FRAP reagent was prepared as follows: the pH 3.6 acetic acid buffer, 1.0 × 10−2 M TPTZ solution, and 2.0 × 10−2 M FeCl3·6H2O solution were mixed in this order at a volume ratio of 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The FRAP reagent was prepared and used freshly.8–10
1. The FRAP reagent was prepared and used freshly.8–10
Procedure. To 3 mL of the FRAP reagent, x mL antioxidant solution, (0.1 − x) mL of EtOH, and 0.3 mL H2O were added such that the final volume was 3.4 mL. The absorbance at 595 nm (A595) was read against a reagent blank at the end of 6 min.8–10
Preparation of solutions. Folin-Ciocalteu's phenol reagent was diluted at a volume ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with 96% EtOH prior to use. Lowry A solution was prepared from sodium carbonate such that the strength of Na2CO3 in 0.1 M NaOH solution was 2% (w/v). Lowry B solution was prepared from copper(II) sulfate such that the strength of CuSO4 in 1% sodium potassium tartrate (NaKC4H4O6) solution was 0.5% (w/v). Lowry C solution was prepared by freshly mixing 50 mL Lowry A with 1 mL Lowry B.7
3 with 96% EtOH prior to use. Lowry A solution was prepared from sodium carbonate such that the strength of Na2CO3 in 0.1 M NaOH solution was 2% (w/v). Lowry B solution was prepared from copper(II) sulfate such that the strength of CuSO4 in 1% sodium potassium tartrate (NaKC4H4O6) solution was 0.5% (w/v). Lowry C solution was prepared by freshly mixing 50 mL Lowry A with 1 mL Lowry B.7
Procedure. To (x) mL phenolic sample solution was added (2 − x) mL H2O. An aliquot of 2.5 mL Lowry C solution was added, and the mixture was let to stand for 10 min. At the end of this period, 0.25 mL Folin reagent was added, and 30 more min was allowed for stabilization of the blue colour formed. The absorbance against a reagent blank was read at 750 nm.7
Preparation of herbal tea and tea bag infusions. One gram of each of the commercial tea bags and herbal teas were dipped separately into 250 mL of freshly boiled water in a beaker, occasionally shaken for 2 min, and allowed to stand in the same solution for an additional 3 min, enabling a total steeping time of 5 min. The herbal tea solution (infusion) was allowed to cool to room temperature, and filtered through a Whatman black-band filter paper for removing particulates. Tea infusions were appropriately diluted with water such that their initial absorbance using the mentioned antioxidant assays was around 0.2 absorbance units, and standard additions of antioxidants were made to these solutions as required. The dilution ratios of nettle and green tea were 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, respectively, and the aliquot volume taken from each diluted infusion was 0.1 mL for applying the ‘method of standard additions’.
20, respectively, and the aliquot volume taken from each diluted infusion was 0.1 mL for applying the ‘method of standard additions’.
Preparation of herbal plant extracts. 1 g of each medicinal plant (in dried form) was weighed. These plant samples were extracted in stoppered flasks with 80% (v/v) methanol; three successive batch extractions were carried out using an ultrasonic bath. The first extraction was made with 20 mL MeOH for 60 min, the second with 20 mL MeOH for 45 min, and the third with 10 mL MeOH for 15 min. The three extracts were filtered, filtrates combined in a single graduated flask, and diluted to 50 mL with 80% MeOH. The dilution ratio of the final extracts and the appropriate volumes taken for TAC measurement from each extract with respect to the antioxidant assay selected were as follows: Ferric-ferrozine method 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dilution, 0.1 mL; CUPRAC method 1
8 dilution, 0.1 mL; CUPRAC method 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dilution, 0.1 mL; FRAP method 1
8 dilution, 0.1 mL; FRAP method 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dilution, 0.05–0.1 mL; and Folin-Ciocalteau method 1
8 dilution, 0.05–0.1 mL; and Folin-Ciocalteau method 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution, 0.2 mL.
4 dilution, 0.2 mL.
Calculation of trolox equivalent antioxidant capacity (TEAC) of antioxidant compounds and of total antioxidant capacity (TAC) of mixtures and complex samples
The TEAC value of a pure antioxidant compound is defined as the equivalent trolox concentration (mM) giving the same response as a 1 mM solution of the tested antioxidant compound in a given antioxidant assay. Practically this definition reduces to the ratio of the ‘apparent molar absorptivity’ of the tested antioxidant compound to that of trolox measured under identical conditions of the given assay. Since TEAC is calculated from the indicated ratio, it is unitless. The apparent molar absorptivity values of trolox in the aforementioned methods are shown in Table 1. Although trolox is not the authentic light-absorbing species itself in these assays, it is converted into a coloured product after having a redox reaction with the oxidizing chromogenic reagent, and thus its apparent molar absorptivity can be calculated.| Antioxidant capacity methods | The apparent molar absorptivity of trolox/L mol−1cm−1 |
|---|---|
| Ferric-ferrozine method | ε TR = 6.01 × 104 |
| CUPRAC method | ε TR = 1.67 × 104 |
| FRAP method | ε TR = 4.62 × 104 |
| Folin-Ciocalteau method | ε TR = 4.65 × 103 |
The TAC value of an unknown sample (in the units of gram-trolox equivalent antioxidant content per g-weight of sample) was calculated as follows: If a herbal infusion or extract (initial volume = Vcup) prepared from (m) g dry matter was diluted (r) times prior to analysis, and a sample volume of (Vs) was taken for analysis from the diluted extract, and colour development (after addition of reagents) was made in a final volume (Vf) to yield an absorbance (Af), then the total antioxidant capacity (TAC) of the herb (mmol TR/g dry matter, or simply mmol TR/g) was found using the equation: TAC (mmol TR/g) =(Af/εTR) × (Vf/Vs) × r(Vcup/m).33
The calculation of expected and found antioxidant capacity (in the units of mM TR-equivalent) of synthetic mixtures was performed as follows: Possible binary, ternary and quaternary mixtures of antioxidant compounds were synthetically prepared, and the suitably diluted solutions were analyzed for TAC using the proposed ferric-ferrozine method. Since 1-cm optical cells were used in absorbance measurements, the experimental TAC was found by dividing the measured absorbance to the apparent molar absorptivity of trolox (εTR) measured under identical conditions of the given assay, and multiplying the quotient by 1000. The theoretical TAC of the synthetic mixture was calculated using eqn (1) where 1,2,…, i denote the corresponding constituents of the mixture
| (TAC)expected = (TEAC)1 (concn)1 + (TEAC)2 (concn)2 + … + (TEAC)n (concn)n | (1) |
Results and discussion
Hierarchic order of antioxidants, structure–activity relationships, and correlations with other electron transfer-based assays
The proposed ferric–ferrozine antioxidant assay had a high sensitivity for antioxidants; the molar absorptivities achieved for trolox (TR), quercetin (QR), caffeic acid (CF), ferulic acid (FR), rutin (RT), ascorbic acid (AA), catechin (CT), gallic acid (GA), rosmarinic acid (RA), ellagic acid (EA) being 6.01 × 104, 2.19 × 105, 7.42 × 104, 5.52 × 104, 9.02 × 104, 6.07 × 104, 7.95 x104, 1.58 × 105, 1.59 × 105, 1.47 × 105 L mol−1cm−1, respectively (Table 2). The hierarchic order of TEAC coefficients of the proposed method reflecting relative antioxidant power were: QR > RA ≥ GA ≥ EA > RT > CT > CF > AA = TR > FR > CYS. This order quite resembled that of CUPRAC, i.e., QR ≥ RA > CF > CT > GA > RT > FR > AA = TR > CYS,28,34 meaning in common that QR and RA had the highest antioxidant power while AA, TR, and CYS had the lowest, and CF, CT, GA, RT, and FR lying in between. On the other hand, though generally conforming to the above order, the ABTS/persulfate assay6 assigns an abnormally high TEAC value to GA, and interchanges the order of CF and FR. The proposed method assigns a CUPRAC-harmonious TEAC value of 0.5 to cysteine (Table 2), corresponding to its reversible 1−e oxidation to the disulfide, while the low TEAC value of GSH combined with its poor linearity (Table 2) reflects the inherent thiol responsiveness problems of ferric ion-based assays like FRAP.28 The TEAC values found with the proposed assay (Table 2) were correlated to those existing in literature for the reference methods of FRAP and ABTS/persulfate (separate table not shown), and the resulting correlation equations (for the first seven antioxidant compounds listed in Table 2, excluding glutathione and cysteine, which lower the correlation coefficient) were:| TEACFRAP = 0.701 TEACferric-ferrozine + 0.222 (r = 0.982) |
| TEACABTS = 1.12 TEACferric-ferrozine + 0.444 (r = 0.932) |
| Antioxidants | Slope × 10−4 | Intercept × 102 | LCR × 105 | Correlation coefficient | TEAC |
|---|---|---|---|---|---|
| Trolox (TR) | 6.01 | 1.34 | 0.3–0.6 | 0.9992 | 1.00 |
| Ascorbic acid (AA) | 6.07 | 1.21 | 0.3–0.6 | 0.9994 | 1.01 |
| Caffeic acid (CF) | 7.42 | 4.56 | 0.3–0.6 | 0.9998 | 1.23 |
| Ferulic acid (FR) | 5.52 | −2.37 | 0.3–0.6 | 0.9994 | 0.92 |
| Gallic acid (GA) | 15.8 | −6.47 | 0.2–0.7 | 0.9996 | 2.63 |
| Quercetin (QR) | 21.9 | −7.01 | 0.1–0.5 | 0.9992 | 3.64 |
| Rutin (RT) | 9.02 | −0.93 | 0.2–1.3 | 0.9999 | 1.50 |
| Catechin (CT) | 7.95 | −1.66 | 0.2–1.3 | 0.9999 | 1.32 |
| Rosmarinic acid (RA) | 15.9 | −2.44 | 0.1–0.7 | 0.9998 | 2.65 |
| Ellagic acid (EA) | 14.7 | −0.88 | 0.1–0.8 | 0.9983 | 2.45 |
| Glutathione (GSH) | 1.43 | −17.7 | 1.5–7.7 | 0.9990 | 0.24 |
| Cysteine (CYS) | 3.03 | −5.23 | 1.1–8.8 | 0.9997 | 0.50 |
Since FRAP is a ferric reducing assay of similar (ET: electron transfer) mechanism to that of the ferric-ferrozine assay, it showed a higher correlation compared to the ABTS assay which is considered to be a mixed ET- and HAT-based method. A further advantage to ABTS may be the TEAC coefficient of cysteine which was measured by the proposed method as 0.5, corresponding to its physiological role as reversible 1−e oxidation to cystine. The ABTS/persulfate antioxidant assay method was shown to treat GSH as a reductant capable of giving ≥ 2 electrons (TEAC of ABTS/persulfate method for GSH varied between 1.3 and 1.5).13 However, reversible oxidation reactions of protein thiols as a part of antioxidant action involving two- or more electrons are less likely in vivo.35
The hierarchy of antioxidant power involves some structure–activity relationships, discussed in detail by Apak et al.28 According to kinetic studies of aryloxy (Ar–O˙) radical formation and decomposition reactions, the antioxidant activity (AOA) of a flavonoid is closely related to its chemical structure. Three structural requirements are important for high AOA of a flavonoid.36
(i) the ortho-dihydroxy (catechol) structure in the B-ring, imparting a greater stability to the formed aryloxy radicals as a result of flavonoid oxidation, possibly through H-bonding and electron-delocalization.37 Another function of the catechol moiety in the B-ring is the possible chelation of transition metal ions that may otherwise cause ROS formation via Fenton-type reactions;36
(ii) the 2,3-double bond, in conjugation with the 4-oxo function, enhancing electron-transfer and radical scavenging actions through electron-delocalization;36
(iii) the presence of both 3- and 5-OH groups, enabling the formation of stable quinonic structures upon flavonoid oxidation.38 Substitution of the 3-OH results in increase in tortion angle and loss of coplanarity, and subsequently reduced AOA.39 A typical flavonoid which meets the above three criteria is quercetin, showing the highest antioxidant capacity in both the proposed method and CUPRAC.
Aside from these structural requirements, the number of hydroxyl substituents on the flavonoid molecule, the position of these hydroxyls, the presence of glycosides (–OR) or aglycons (–OH), and the overall degree of conjugation are important in determining AOA28,40 applying for the medium power antioxidants RT and CT. For hydroxycinnamic acids, the number of phenolic –OH groups is important, and thus, caffeic acid having two –OH substituents should have a higher AOA than one –OH bearing ferulic acid.41 Likewise, rosmarinic acid having four phenolic –OH groups in a perfectly conjugated molecule should exhibit the highest AOA among hydroxycinnamic acids28 which is the case for both the proposed method and CUPRAC (Fig. 1).
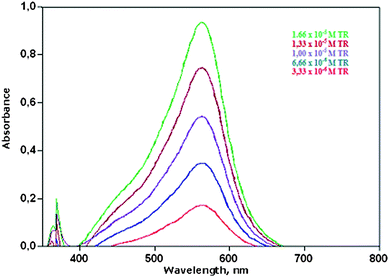 | ||
| Fig. 1 UV-visible absorption spectra of reduced iron-ferrozine (Fe(II)-FZ) complex as a result of redox reaction with varying concentrations of trolox. | ||
The expected and found total antioxidant capacities (TAC, as mM trolox-equivalent) of binary, ternary and quaternary synthetic mixtures of antioxidants using the proposed ferric-ferrozine assay are shown in Table 3, verifying the additivity of TAC values of constituents forming the mixtures. The proposed method was also applied to green tea, nettle tea, ceylon tea, linden, chamomile, mint and sage herbal tea as real sample solutions of complex nature. The calibration curves of QR in pure aqueous solution and in two selected herbal tea (green tea and nettle) infusions were parallel to each other (Fig. 2), and the same was observed for TR (figure not shown). The calibration lines of trolox (TR) in different media, namely alone, in nettle infusion having an initial absorbance of A562nm = 0.161, and in green tea infusion having an initial absorbance of A562nm = 0.240): TR alone; A = 1.34 × 10−2 + 6.01 × 104CTR, TR in nettle infusion; A = 1.65 × 10−1 + 6.04 × 104CTR, and TR in green tea infusion; A = 2.48 × 10−1 + 6.03 × 104CTR. The obtained calibration lines of the tested antioxidants in pure solution and in the real sample extracts showed good parallelism. A similar parallelism was observed for the calibration curves of CF in QR solution, and of FR in TR solution (figures not shown). These findings verified that in the proposed ferric-ferrozine assay of TAC, either antioxidant compounds among themselves or constituents of real matrix solutions with pure antioxidants did not show chemical interactions so as to cause a chemical deviation from Beer's law disrupting the additivity of optical absorbances. This means the TAC of real mixtures measured with the proposed method is approximately equal to the sum of TAC values of constituents forming the mixture, an essential property in reliable comparison of TAC of food matrices.
| Composition of mixture | Capacity expected | Capacity found | Composition of mixture | Capacity expected | Capacity found | ||
|---|---|---|---|---|---|---|---|
| RT | (3 × 10−4 M 30 μL) | 7.40 × 10−3 | 8.94 × 10−3 | QR | (3 × 10−4 M 20 μL) | 8.95 × 10−3 | 8.35 × 10−3 |
| CA | (3 × 10−4 M 50 μL) | CF | (3 × 10−4 M 50 μL) | ||||
| GA | (3 × 10−4 M 30 μL) | 9.66 × 10−3 | 1.08 × 10−2 | QR | (3 × 10−4 M 20 μL) | 8.12 × 10−3 | 7.96 × 10−3 |
| CA | (3 × 10−4 M 50 μL) | EA | (3 × 10−4 M 20 μL) | ||||
| RA | (3 × 10−4 M 20 μL) | 7.24 × 10−3 | 9.39 × 10−3 | TR | (3 × 10−4 M 50 μL) | 7.92 × 10−3 | 8.36 × 10−3 |
| FR | (3 × 10−4 M 75 μL) | FR | (3 × 10−4 M 75 μL) | ||||
| CF | (3 × 10−4 M 50 μL) | 1.27 × 10−2 | 1.20 × 10−2 | CF | (3 × 10−4 M 50 μL) | 1.20 × 10−2 | 9.87 × 10−3 |
| TR | (3 × 10−4 M 50 μL) | FR | (3 × 10−4 M 75 μL) | ||||
| GA | (3 × 10−4 M 30 μL) | TR | (3 × 10−4 M 50 μL) | ||||
| RA | (3 × 10−4 M 20 μL) | 1.05 × 10−2 | 1.03 × 10−2 | AA | (3 × 10−4 M 50 μL) | 1.27 × 10−2 | 1.29 × 10−2 |
| FR | (3 × 10−4 M 75 μL) | CF | (3 × 10−4 M 50 μL) | ||||
| TR | (3 × 10−4 M 50 μL) | GA | (3 × 10−4 M 30 μL) | ||||
| RA | (3 × 10−4 M 20 μL) | 1.06 × 10−2 | 1.19 × 10−2 | RA | (3 × 10−4 M 20 μL) | 1.00 × 10−2 | 9.27 × 10−3 |
| FR | (3 × 10−4 M 75 μL) | AA | (3 × 10−4 M 50 μL) | ||||
| TR | (3 × 10−4 M 50 μL) | CF | (3 × 10−4 M 50 μL) | ||||
| TR | (3 × 10−4 M 50 μL) | 1.08 × 10−2 | 1.15 × 10−2 | TR | (3 × 10−4 M 50 μL) | 1.09 × 10−2 | 1.00 × 10−2 |
| QR | (3 × 10−4 M 20 μL) | RT | (3 × 10−4 M 30 μL) | ||||
| RA | (3 × 10−4 M 20 μL) | FR | (3 × 10−4 M 75 μL) | ||||
| GA | (3 × 10−4 M 30 μL) | 1.25 × 10−2 | 1.39 × 10−2 | AA | (3 × 10−4 M 50 μL) | 1.20 × 10−2 | 1.18 × 10−2 |
| RA | (3 × 10−4 M 20 μL) | CF | (3 × 10−4 M 50 μL) | ||||
| FR | (3 × 10−4 M 75 μL) | FR | (3 × 10−4 M 75 μL) | ||||
| QR | (3 × 10−4 M 20 μL) | 1.34 × 10−2 | 1.58 × 10−2 | RT | (3 × 10−4 M 30 μL) | 1.15 × 10−2 | 1.26 × 10−2 |
| CA | (3 × 10−4 M 50 μL) | CA | (3 × 10−4 M 50 μL) | ||||
| CF | (3 × 10−4 M 50 μL) | CF | (3 × 10−4 M 50 μL) | ||||
| GA | (3 × 10−4 M 30 μL) | 1.27 × 10−2 | 1.57 × 10−2 | GA | (3 × 10−4 M 30 μL) | 1.24 × 10−2 | 1.61 × 10−2 |
| CA | (3 × 10−4 M 50 μL) | RT | (3 × 10−4 M 30 μL) | ||||
| RT | (3 × 10−4 M 30 μL) | CF | (3 × 10−4 M 50 μL) | ||||
| QR | (3 × 10−4 M 20 μL) | 1.45 × 10−2 | 1.47 × 10−2 | TR | (3 × 10−4 M 50 μL) | 1.42 × 10−2 | 1.46 × 10−2 |
| GA | (3 × 10−4 M 30 μL) | RT | (3 × 10−4 M 30 μL) | ||||
| CA | (3 × 10−4 M 50 μL) | RT | (3 × 10−4 M 30 μL) | ||||
| AA | (3 × 10−4 M 50 μL) | ||||||
| RA | (3 × 10−4 M 20 μL) | 1.37 × 10−2 | 1.30 × 10−2 | ||||
| AA | (3 × 10−4 M 50 μL) | ||||||
| CA | (3 × 10−4 M 50 μL) | ||||||
| TR | (3 × 10−4 M 50 μL) | ||||||
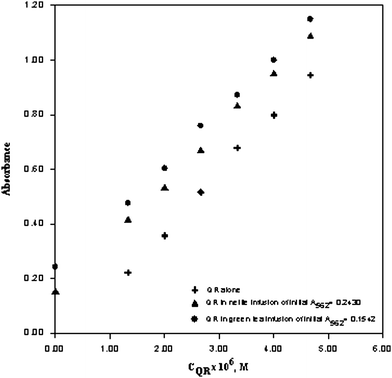 | ||
| Fig. 2 Calibration line of quercetin (QR) in pure aqueous solution, in green tea infusion, and in nettle infusion with respect to the ferric-ferrozine method. QR alone A = −7.01 × 10−2 + 2.19 × 105CQR, QR in nettle infusion A = 1.46 × 10−1 + 2.02 × 105CQR, QR in green tea infusion A = 2.88 × 10−1 + 2.02 × 105CQR. | ||
The TAC values of these herbal tea extracts were measured in the units of mmol TR/g (Table 4). The TAC values of these real extracts, as assayed by the proposed ferric-ferrozine and reference antioxidant assay methods, showed the following binary correlations:
| TACCUPRAC = 1.19 TACFe(III)-ferrozine + 0.063 (r = 0.966) |
| TACFRAP = 0.386 TACFe(III)-ferrozine + 0.108 (r = 0.888) |
| TACFolin = 0.560 TACFe(III)-ferrozine + 0.275 (r = 0.862) |
| Extracted medicinal plants | Ferric-Ferrozine | CUPRAC | FRAP | Folin-Ciocalteu |
|---|---|---|---|---|
| Nettle tea, (Urtica diocia/urens) | 1.46 × 10−1 | 3.26 × 10−1 | 1.17 × 10−1 | 2.76 × 10−1 |
| Mint, (Mentha piperita) | 1.33 × 10−1 | 1.17 × 10−1 | 2.54 × 10−1 | 4.64 × 10−1 |
| Chamomile, (Matricaria chamomilla L.) | 6.69 × 10−2 | 1.17 × 10−1 | 5.52 × 10−2 | 1.85 × 10−1 |
| Sage herbal tea, (Salvia officinalis) | 3.12 × 10−1 | 6.0 × 10−1 | 2.74 × 10−1 | 5.38 × 10−1 |
| Linden, (Tilia) | 1.34 × 10−2 | 2.76 × 10−2 | 1.13 × 10−1 | 3.35 × 10−1 |
| Green tea, (Camellia sinensis) | 6.32 × 10−1 | 7.52 × 10−1 | 3.72 × 10−1 | 5.34 × 10−1 |
| Ceylon tea, (Camellia sinensis) | 7.80 × 10−1 | 9.76 × 10−1 | 3.73 × 10−1 | 7.59 × 10−1 |
These TAC relationships reveal that the best correlation and one-to-one correspondence to the proposed assay is supplied by the CUPRAC assay also based on the same principle of reducing power measurement. Both the proposed ferric ferrozine and the widely used CUPRAC assays are electron-transfer (ET)-based assays, producing coloured species when the corresponding reagents are reduced by antioxidants. In literature, it has been observed that the results of ET-based assays show a much better agreement among themselves than with those of HAT–based assays, due to the similarity in mechanism. In regard to kinetics, the CUPRAC reagent is fast enough to oxidize thiol-type antioxidants, whereas according to the protocol developed by Benzie and Strain,8 the FRAP method does not measure thiol-type antioxidants like glutathione42 and slowly responds to certain hydroxycinnamic acids.28 The reason for this may be the half-filled d-orbitals of high spin Fe(III) attributing it a chemical inertness, while the electronic structure of Cu(II) enables fast kinetics. Another reason for this difference between the kinetic behaviours of Fe(III) and Cu(II) toward thiols may be the softer character (with respect to the “Hard and Soft Acids and Bases”, HSAB Theory) of Cu(II) enabling the coordination of the latter to the soft –SH groups as the electron donor. However, in spite of these kinetic differences, the proposed assay gave a close agreement with CUPRAC, showing that the higher stabilization of ferrous ion in the presence of ferrozine probably provides faster kinetics for Fe(III) oxidation of a number of antioxidants than the conventional FRAP assay.
Reaction kinetics and solvent dependency
The oxidation reactions of cysteine (1.0 × 10−4 mmol), glutathione (3.5 × 10−4 mmol), caffeic acid (3.0 × 10−5 mmol), and ferulic asid (7.5 × 10−5 mmol) with the ferric ferrozine reagent were followed for a time period of 30 min (absorbance data recorded at the end of 1, 2, 4, 8, 12, 15, 20, 25 and 30 min, as the mean of N = 3 measurements). In general, the reaction kinetics for the proposed assay was definitely faster than for the FRAP assay. The reaction kinetics (shown in Fig. 3) demonstrate that while the oxidations of caffeic acid and cysteine to stable products were almost complete within 10 min, the corresponding reaction in 30 min was almost complete for ferulic acid and incomplete for glutathione (Fig. 3). While the exact redox potential of the ferric ferrozine reagent is not known in literature, it can be estimated that this potential should be higher than the standard ferric-ferrous potential of 0.77 V due to preferential stabilization of the ferrous state with ferrozine binding. Since the standard potentials of most antioxidants lie in the range of 0.2–0.8 V, it can be estimated that ferric ferrozine should be able to oxidize these antioxidants. As a general rule, it may be envisaged that as the redox potentials of the oxidant and reductant approach each other, complete oxidation of the antioxidant with the oxidizing reagent should take more time. The formal potentials (E°′), estimated by the equation: E°′ = (Ep,a + Ep/2)/2, where Ep,a and Ep/2 are the anodic peak potential and half-peak potential of the oxidation wave, respectively, were measured against a Ag/AgCl electrode, and found to be 0.546 V for caffeic acid and 0.740 V for ferulic acid.43 The standard redox potential of glutathione (GSH) was measured as E°GSSG,GSH = 0.85 V44 much higher than that of cysteine, i.e., E°CSSC,CYS = 0.22 V at pH = 7.45 Thus, the oxidations of both ferulic acid and glutathione with ferric ferrozine were slower, in accordance with their high redox potentials, though these reactions were definitely faster than encountered in FRAP.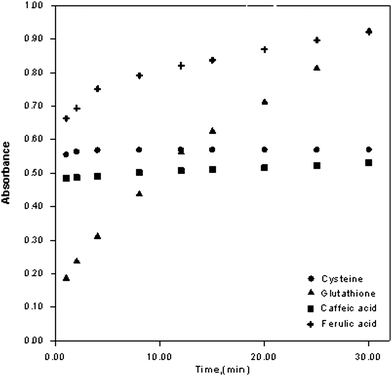 | ||
| Fig. 3 The change of absorbance of cysteine, glutathione, caffeic acid, and ferulic acid as a function of time (oxidant: ferric ferrozine reagent). | ||
When the apparent molar absorptivities of quercetin and ferulic acid as a result of ferric ferrozine oxidation in media of different ethanol contents (being progressively varied from 50% to 100% EtOH) were examined, the relative change was within 7% for ferulic acid and 9% for quercetin, demonstrating that the composition of alcohol-aqueous mixtures do not have a significant effect on the formation of the ferrous ferrozine chromophore (table not shown). So, the proposed methodology can easily assay antioxidants from plant extracts containing different levels of alcohol, without a special consideration for the composition of extracting solvent mixture.
Interferences
The following potential interferents common in plant food and related foodstuffs did not affect the determination of 1 × 10−2 mM trolox at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 fold concentration levels (i.e., caused less than 5% relative error): 0.5 mM levels of dextrose, fructose, tartaric acid, mannitol, glycine, serine, lysine, valine, proline, and alanine. Also, a 5-fold concentration level of citric acid (at 5.0 × 10−2 mM) and 5.56 × 10−1 mg mL−1 starch did not affect the determination. This proved that the proposed ferric-ferrozine assay has a favourable redox potential enabling selective oxidation of true antioxidant compounds without affecting other similar substances not genuinely belonging to the antioxidant class.
50 fold concentration levels (i.e., caused less than 5% relative error): 0.5 mM levels of dextrose, fructose, tartaric acid, mannitol, glycine, serine, lysine, valine, proline, and alanine. Also, a 5-fold concentration level of citric acid (at 5.0 × 10−2 mM) and 5.56 × 10−1 mg mL−1 starch did not affect the determination. This proved that the proposed ferric-ferrozine assay has a favourable redox potential enabling selective oxidation of true antioxidant compounds without affecting other similar substances not genuinely belonging to the antioxidant class.
Advantages over the FRAP assay
The most widely used antioxidant assay based on Fe(III) reduction, the FRAP method,8,9 has been criticised for not measuring thiol-type antioxidants like glutathione42 and slowly responding to certain hydroxycinnamic acids.28 The trolox-equivalent antioxidant capacity (TEAC) coefficients for cysteine and glutathione measured by FRAP were very low (0.060 and 0.042, respectively), with calibration lines not passing through the origin.46 In this work, the TEAC value of the proposed ferric ferrozine assay for cysteine was 0.5, in accordance with its physiological role regarding reversible 1−e oxidation of this compound to cystine (e.g., 2 CSH − 2 e− ↔ CSSC) and in total agreement with the results of the CUPRAC assay.13 Moreover, though being less responsive to glutathione (GSH), the TEAC value of the proposed method for this compound (i.e., 0.24) was better than that of FRAP. Thus, the proposed method is superior to the widely used FRAP in regard to thiols response.A second advantage is that this new reagent (FZ) for a modified ferric reducing assay provides a very high apparent molar absorptivity for antioxidants (e.g., ε = 6.0 × 104 Lmol−1cm−1 for trolox and ascorbic acid), and therefore enhanced sensitivity in total antioxidant capacity (TAC) measurement. Since most laboratories dealing with plant antioxidants already use the FZ reagent for ‘iron-binding capacity’ assays, this reagent may also be used for an additional aim of TAC measurement in such laboratories. The proposed method is easy, flexible, and of low-cost. The extremely high sensitivity provided by the proposed assay (due to preferential stabilization of divalent iron over trivalent iron by ferrozine) may also be advantage for on-line HPLC applications using a chromogenic derivatizing agent in the post-column mode,47 because in post-column applications, only authentically antioxidant compounds give a signal, often with weak sensitivity, and very sensitive indirect methods of antioxidant characterization need to be used in such assays.
A third advantage over FRAP may be the relatively stronger chelation of ferrous ion—produced as a result of reaction with antioxidants—using ferrozine, hindering Fenton-type reactions.48 The FRAP reagent contains excessive Fe(III)—higher than the stochiometric ratio required for complex formation—compared to the ferric ferrozine reagent. As a result, some free Fe(II) accompanying Fe(II)-TPTZ chelate may form after the reaction with antioxidants. The FRAP method was previously criticised for producing Fe(II) which may cause redox cycling of antioxidants during the assay as a result of Fe(II)-mediated Fenton-type reactions.29
A fourth advantage is that the near-neutral pH of this work (pH 5.5) is significantly higher than that of the widely used FRAP test having a working pH of 3.6. As the solubility product of ferric hydroxide is very low, i.e., Ksp [Fe(OH)3] = 6.0 × 10−38, ferric ion-based assays are usually performed at low pH to prevent the hydrolysis of Fe(III), which would otherwise lower the Fe(III)/Fe(II) potential and consequently obstruct the oxidation of antioxidants by the ferric-based reagent. The acidic medium of FRAP is rather unrealistic (pH 3.6) in regard to simulation of antioxidant action under physiological conditions, because at significantly more acidic conditions than the physiological pH, the reducing capacity may be suppressed due to protonation on antioxidant compounds. Another widely used antioxidant test is the Folin phenolics assay, which provides a redox reaction at pH 10, again not in accordance with physiological requirements. Therefore, a pH of 5.5 should not be considered out of biological relevance. Such a high (near-neutral) pH in conjunction with a ferric-based reagent in the proposed assay was only possible by virtue of extra stabilization of the ferrous state by ferrozine complexation.
The only possible disadvantage compared to FRAP may be the higher redox potential of the ferric ferrozine reagent due to extreme stabilization of the lower oxidation state of iron. However, higher potential compounds such as the food preservative citric acid (which is not classified as a true antioxidant) has been shown in this work not to be oxidized by the ferric ferrozine reagent at 5-fold concentrations (i.e., 5.0 × 10−2 mM).
Conclusions
A ferric-ferrozine method of antioxidant capacity measurement has been developed for the simple, low-cost and versatile assay of food antioxidants. In the presence of ferrozine ligand, ferric ion easily oxidizes antioxidants and is itself reduced to Fe(II)-FZ, yielding a very high molar absorptivity (for Fe(II) at the order of 2.8 × 104 Lmol−1cm−1) and thus enhanced sensitivity for most antioxidants. The order of antioxidant power for common antioxidants measured with the proposed method is in accordance with theoretical considerations expected from structure–activity relationships. The necessary equipment (a colorimeter or a spectrophotometer) is available to most common laboratories, and the method is non-laborious. Ferrozine is already purchased by food laboratories for iron-binding capacity assays, and may also be advantageously used for TAC measurement. The stability with respect to pH of Fe(II)-ferrozine chelate covers a wide range (i.e., pH 4–9). The method is not susceptible to serious interference from common food ions; heavy metal cations capable of complexing with ferrozine do not affect the results when the reagent is used in excess, while the only anionic interferences are oxalate (at ≥ 500 mg L−1), cyanide and nitrite, the latter being decomposed upon acid heating.17,21 As the conditional stability constant (Log β3) of the Fe(II)-FZ complex is very high, at the order of 15.5 (e.g., as compared to that of ferrous-tripyridyltriazine complex of the widely used FRAP method, where Log β2 of Fe(II)-TPTZ is 11.4)49, FZ preferentially stabilizes the ferrous state compared to ferric ion, resulting in increased redox potential of the Fe(III)/Fe(II) couple, and consequently, the ferric-ferrozine reagent is even capable of oxidizing antioxidants having a weak reducing ability. The assay has a wide linear concentration range and low blank values, and is not adversely affected either from dissolved oxygen or common food ingredients such as simple sugars and citrate. The ferric-ferrozine assay results for food TAC well compare with those of CUPRAC, and show good additivity. The assay is expected to be useful to conventional food laboratories not necessitating sophisticated equipment and highly skilled operators.References
- B. Halliwell and J. M. C. GutteridgeFree radicals in biology and medicine. Oxford, University Press, 1989 Search PubMed.
- B. Halliwell, R. Aeschbach, L. Löliger and O. I. Aruoma, Food Chem. Toxicol., 1995, 33, 601–617 CrossRef CAS.
- A. Yıldırım, A. Mavi and A. A. Kara, J. Agric. Food Chem., 2000, 48, 5030–5034 CrossRef.
- B. Ou, D. Huang, M. Hampsch-Woodill, J. A. Flanagan and E. K. Deemer, J. Agric. Food Chem., 2002, 50, 3122–3128 CrossRef CAS.
- N. J. Miller, C. A. Rice-Evans, M. J. Davies, V. Copinathan and A. Milner, Clin. Science, 1993, 84, 407–412 Search PubMed.
- R. Re, N. Pellegrini, A. Oroteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237 CrossRef CAS.
- O. Folin and V. Ciolcalteu, J. Biol. Chem., 1927, 73, 627–650 CAS.
- I. F.F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS.
- I. F. F. Benzie and Y. T. Szeto, J. Agric. Food Chem., 1999, 47, 633–636 CrossRef CAS.
- R. Pulido, L. F. Bravo and J. Saura-Calixto, J. Agric. Food Chem., 2000, 48, 3396–3402 CrossRef CAS.
- K. I. Berker, K. Güçlü, I. Tor, B. Demirata and R. Apak, Food Analytical Methods, 2010, 3, 154–168 CrossRef.
- R. Apak, K. Güçlü, M. Özyürek and S. E. Karademir, J. Agric. Food Chem., 2004, 52, 7970–7981 CrossRef CAS.
- R. Apak, K. Güçlü, M. Özyürek, S. E. Karademir and M. Altun, Free Radical Res., 2005, 39, 949–961 CrossRef CAS.
- M. Özyürek, S. E. Çelik, K. I. Berker, K. Güçlü, İ. Tor and R. Apak, React. Funct. Polym., 2007, 67, 1478–1486 CrossRef.
- D. Ozyurt, B. Demirata and R. Apak, Talanta, 2007, 71, 1155–1165 CrossRef CAS.
- C. Sanchez-Moreno, J. A. Larrauri and F. Saura-Calixto, J. Sci. Food Agric., 1998, 76, 270–276 CrossRef CAS.
- L. L. Stookey, Anal. Chem., 1970, 42, 779–781 CrossRef CAS.
- M. I. Pascual-Reguera, I. Ortega-Carmona and A. Molina-Diaz, Talanta, 1997, 44, 1793–1801 CrossRef CAS.
- A. Molina-Diaz, I. Ortega-Carmona and M. I. Pascual-Reguera, Talanta, 1998, 47, 531–536 CrossRef CAS.
- L. D. Giokas, E. K. Paleologos and M. I. Karayannis, Anal. Bioanal. Chem., 2002, 373, 237–243 CrossRef CAS.
- P. Carter, Anal. Biochem., 1971, 40, 450–458 CAS.
- H. Y. Yee and A. Zin, Clin. Chem., 1971, 17, 950–953 CAS.
- I. Gülçin, M. Oktay, E. Kireççi and O. Küfrevioğlu, Food Chem., 2003, 83, 371–382 CrossRef CAS.
- I. Gülçin, Amino Acids, 2007, 32, 431–438 CrossRef CAS.
- T. Ak and I. Gülçin, Chem.-Biol. Interact., 2008, 174, 27–37 CrossRef CAS.
- T. C. P. Dinis, V. M. C. Madeira and L. M. Almeida, Arch. Biochem. Biophys., 1994, 315, 161–169 CrossRef CAS.
- K. I. Berker, K. Güçlü, I. Tor and R. Apak, Talanta, 2007, 72, 1157–1165 CrossRef CAS.
- R. Apak, K. Güçlü, B. Demirata, M. Özyürek, S. E. Çelik, B. Bektasoglu, K. I. Berker and D. Ozyurt, Molecules, 2007, 12, 1496–1547 Search PubMed.
- R. L. Prior and G. Cao, Free Radical Biol. Med., 1999, 27, 1173–1181 CrossRef CAS.
- C. R. Gibbs, Anal. Chem., 1976, 48, 1197–1201 CrossRef CAS.
- B. Halliwell and J. M. C. Gutteridge, Methods Enzymol., 1990, 186, 1–85 CAS.
- Y. Y. Lim, T. T. Lim and J. J. Tee, Food Chem., 2007, 103, 1003–1008 CrossRef CAS.
- R. Apak, K. Güçlü, M. Özyürek, S. E. Karademir and E. Erçağ, Int. J. Food Sci. Nutr., 2006, 57, 292–304 CrossRef CAS.
- L. Yıldız, K. S. Başkan, E. Tütem and R. Apak, Talanta, 2008, 77, 304–313 CrossRef CAS.
- D. A. Dickinson and J. J. Forman, Biochem. Pharmacol., 2002, 64, 1019–1026 CrossRef CAS.
- P.-G. Pietta, J. Nat. Prod., 2000, 63, 1035–1042 CrossRef.
- S. A. B. E. Van Acker, D.-J. van den Berg, M. N. J. L. Tromp, D. H. Griffioen, W. P. van Bennekom, W. J. F. van der Vijgh and A. Bast, Free Radical Biol. Med., 1996, 20, 331–342 CrossRef.
- O. Firuzi, A. Lacanna, R. Petrucci, G. Marrosu and L. Saso, Biochim. Biophys. Acta, Gen. Subj., 2005, 1721, 174–184 Search PubMed.
- N. P. Seeram and M. G. Nair, J. Agric. Food Chem., 2002, 50, 5308–5312 CrossRef CAS.
- E. Tripoli, M. L. Guardia, S. Giammanco, D. D. Majo and M. Giammanco, Food Chem., 2007, 104, 466–479 CrossRef CAS.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Trends Plant Sci., 1997, 2, 152–159 CrossRef.
- B. Ou, M. Hampsch-Woodill and R. L. Prior, J. Agric. Food Chem., 2001, 49, 4619–4626 CrossRef CAS.
- P. A. Kilmartin, Antioxid. Redox Signaling, 2001, 3, 941–955 Search PubMed.
- G. Galati, M. Y. Moridani, T. S. Chan and J. O. Brien, Free Radical Biol. Med., 2001, 30, 370–382 CrossRef CAS.
- P. C. Jocelyn, Eur. J. Biochem., 1967, 2, 327–331 CAS.
- S. Demirci Çekiç, K. Sözgen Başkan, E. Tütem and R. Apak, Talanta, 2009, 79, 344–351 CrossRef.
- S. E. Çelik, M. Özyürek, K. Güçlü and R. Apak, Anal. Chim. Acta, 2010, 674, 79–88 CrossRef CAS.
- M. Guo, C. Perez, Y. Wei, E. Rapoza, G. Su, F. Bou-Abdallah and N. D. Chasteen, Dalton Trans., 2007, 4951–4961 RSC.
- K. L. Cheng, K. Ueno and T. Imamura, CRC Handbookof Organic Analytical Reagents, CRC Press, Boca Raton, Florida, 1982, p. 316 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
