Characterization of human neutrophil peptides (α-Defensins) in the tears of dry eye patients
Li-Hua
Lo
a,
Pei-Chang
Wu
*b,
Yi-Chen
Wu
b and
Jentaie
Shiea
*acd
aDepartment of Chemistry, National Sun Yat-Sen University, Kaohsiung, 80424, Taiwan. E-mail: jetea@mail.nsysu.edu.tw; Fax: +886-7-5253933; Tel: +886-7-5253933
bDepartment of Ophthalmology, Chang Gung Memorial Hospital, Kaohsiung Medical Center, Chang Gung University College of Medicine, Kaohsiung, Taiwan. E-mail: wooopc@gmail.com; Fax: +886-7-7317123-2801; Tel: +886-7-7317123-2801
cNational Sun Yat-Sen University–Kaohsiung Medical University Joint Research Center, Taiwan
dCancer Center, Kaohsiung Medical University Hospital, Taiwan
First published on 13th October 2010
Abstract
The potential peptide biomarkers of dry eye disease were rapidly and sensitively screened in tears by simple sample pretreatment and modern mass spectrometry. The tear sample was collected by simply wiping the corner of the eye with a small piece of Schirmer filter paper from the patients confirmed to have dry eye disease and from normal controls. The peptides adsorbed on the filter paper were extracted by vortexing it with sinapinic acid solution in a vial. A drop of the extracted solution was then deposited on the sample plate and examined using matrix-assisted laser desorption ionization/time-of-flight mass spectrometry (MALDI-TOF MS) without any other sample pretreatment. We found human neutrophil peptides (HNPs), so called α-defensins 1–3, which are potential biomarkers for inflammation, were significantly up-regulated in the tears collected from all the dry eye disease patients. The identity of the α-defensins in human tears was confirmed through (i) matching their molecular ions with those of standards, (ii) literature searches and (iii) electrophoretic purification and separation of the tears followed by in-gel tryptic digestion and MALDI-TOF analysis. The presence of large quantities of HNPs in the tears of dry eye patients suggests the occurrence of chronic inflammation in the ocular regions of dry eye patients.
Introduction
Tears are a kind of clear saline fluid secreted by the lacrimal gland to cover the ocular surface. It is generally diffused between the eye and the eyelid to moisten and facilitate their motion. Tears contain mucins, peptides, proteins, lipids, and salts. The presence of these biochemical compounds makes this biological medium an important one for smooth layer, lubrication, and protection against foreign bodies.1 Compared with serum and blood, it is relatively easy to characterize the biochemical compounds in the tears because the sample is relatively clean and can be collected easily and non-invasively.Dry eye disease (keratoconjunctivitis sicca), which is caused by a lack of tears on the ocular surface, is one of the most common diseases among aged individuals. It arises through either a decrease in tear secretion or an increase in tear evaporation. It has been estimated that approximately 20% of the aged adult population suffers from varying degrees of dry eye problems.2 Although the formative mechanisms of dry eye syndrome remain unclear, inflammatory mechanisms appear to underlie the condition.2
In clinical laboratories, dry eye syndrome is diagnosed using Schirmer's test—a filter paper strip is positioned under the eyelid and the tear tracking distance is measured after 5 min. If the distance is less than 5 mm, the patient is diagnosed with dry eye syndrome and natural tear eye drops will be prescribed to relieve the symptoms.3 The test is not suitable for some patients who are irritated by hanging the strip under the eyelid. Previous efforts to diagnose dry eye disease using biochemical assays for clinical purpose have not been very successful for lack of suitable biomarkers. However, several tear proteins such as proline-rich protein 3, the C-terminal fragment of PRP4, nasopharyngeal carcinoma-associated PRP4, α1-antitrypsin, and calgranulin A have been reported as candidate biomarkers for dry eye syndrome.4 Unfortunately, these proteins are usually present in trace amounts and require very complicated analytical procedures for their characterization in tears; as a result, none of these biomarkers are used at present for the clinical diagnoses of dry eye disease.
Defensins are a family of mammalian defensive peptides that have been known for decades to be present in many human biological fluids such as saliva, urine, human milk, serum and tear.5–9 These peptide molecules are 2–6 kDa in weight, cationic, contain three pairs of intramolecular disulfide bonds and are active microbicidal peptides against many Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses. On the basis of their sizes and patterns of disulfide bonds, mammalian defensins are classified into α, β and θ categories.10 α-Defensins, which have been identified in humans, monkeys, and several rodent species, are particularly abundant only in neutrophils—not in esoinophils or basophils. For this reason, α-defensins are also known as human neutrophil peptides (HNPs). The peptides comprise ca. 30–50% of the protein content in human azurophil granules and 5–7% of the total cellular protein content in the neutrophils; they are synthesized in the bone marrow in neutrophil precursor cells known as promyelocytes.11 Three α-defensins—HNP-1, −2 and −3—are usually found in neutrophils; these small (ca. 3.5 kDa) cationic antimicrobial peptides are known to be part of the innate immune system.12 Several studies have revealed that α-defensins are released in various biological fluids during inflammation and may be potential biomarkers of inflammation.13,14
Recent research has suggested that an inflammatory mechanism may underlie dry eye syndrome.2 A hallmark of dry eye syndrome is inflammation of the ocular surface and tear-producing glands.15 Since physiological and pathological eye conditions may associate with changes in the levels of tear peptides, characterization of HNPs in the tears of patients' having dry eye syndrome may be an alternative to Schirmer's strip test for diagnosing dry eye syndrome.
Previous methods developed to characterize the HNPs in different biological fluids are mainly immuno-based approaches such as Western blot analyses,16,17 enzyme-linked immunosorbent assays (ELISAs),18,19 radioimmunoassays (RIAs),20,21 and immunohistochemistry.22 Other methods to characterize HNPs include mRNA levels expression and flow cytometric analyses.17,23 Although the immuno-based techniques can provide highly sensitive results on diagnosing HNPs,21,24 they have limited dynamic range and the binding specificity is easily affected by the interferents in the sample solution. In addition, these approaches are usually time-consuming and require testing reagents that are expensive, carcinogenic, and/or unstable during storage.
Advances in specific peptide and protein identification through mass spectrometry (MS) have been applied previously to examine trace constituents in tears. Recently, liquid chromatography/mass spectrometry (LC/MS) has been used to characterize HNPs in tears.25,26 Although HNPs are successfully separated and detected, tedious sample pretreatment procedures are still required prior to analysis. The presence of co-eluting and isobaric interfering agents in the tears also result in these approaches lacking specificity.25,26 Surface-enhanced laser desorption ionization/time-of-flight mass spectrometry (SELDI-TOF MS) has been used for proteomic research into tear fluids,4 unfortunately, further applications of this approach to the screening of HNPs in tears have been prohibited by the tedious sample pretreatment procedures, the high cost of the affinity sample plate and the lack of reproducibility of the analytical results.
Matrix-assisted laser desorption ionization/time-of-flight (MALDI-TOF), which was introduced in the late 1980s,27–29 is a laser ionization MS technique that separates constituent peptide species on the basis of their mass-to-charge (m/z) ratios. The MALDI-TOF apparatus is an extremely sensitive instrument that allows sample molecules to be detected below femtomole level with a mass accuracy better than 10−4 (in the mass range up to ca. 30 kDa). Additionally, the experimental procedures required for a typical MALDI-TOF analysis are very simple, the reagents (i.e., organic matrix) for sample preparation are inexpensive, and, most importantly of all, the instruments are highly suited for automation so that they can be used to screen large numbers of samples. As a result, MALDI-TOF has been demonstrated to be a potential tool for clinical biomedical diagnoses of, for example, fecal occult blood (FOB) and proteinuria.30–32 Previously, MALDI-TOF was successfully used to characterize the HNPs in saliva, the relationship of saliva HNPs and age was studied.33 In this study, the approach using MALDI-TOF and simple sampling and extraction method was developed to rapidly characterize HNPs in tear samples collected from normal controls and from patients with dry eye disease. To confirm the identities of the HNPs in the tears, additional tests were performed including: (i) matching the molecular weights of the detected HNP ions with the standards, (ii) literature searching, and (iii) separating tear peptides and proteins by gel electrophoresis followed by in-gel tryptic digestion and MALDI-TOF analyses.
Experimental
Materials and sample preparation
This study was conducted at Chang Gung Memorial Hospital (CGMH)–Kaohsiung Medical Center and National Sun Yat-Sen University (NSYSU), Kaohsiung, Taiwan, respectively. This study conforms to Declaration of Helsinki and obtains patients oral form of consent. Patients diagnosed with dry eye disease and healthy controls were selected according to the results of Schirmer's tests in CGMH. A total of 30 participants were recruited in this study: 20 patients with dry eye disease and 10 healthy controls. The tear samples were collected by gently wiping the corner of the eyes with small pieces (5.0 × 5.0 mm2) of absorbent papers (including Kimwipes, Schirmer filter paper, and tissue paper). The absorbing materials were then saved in the Eppendorf tube, immediately delivered to the laboratory at NSYSU, and stored at −80 °C until required for examination.All solvents (HPLC grade) and chemicals were used without further purification. Various solvents—acetonitrile (ACN), ethanol (EtOH), doubly distilled water, and methanol (MeOH) (Merck, Darmstadt, Germany)—were used to examine the extraction efficiency of the HNPs from the pieces of absorbing paper. The MALDI matrices 4-hydroxy-α-cyanocinnamic acid (α-CHC), and sinapinie acid (SA) were purchased from Aldrich (Milwaukee, WI, USA); 2,5-dihydroxybenzoic acid (2,5-DHB) was purchased from KASEI (TCI CO., LTD., Tokyo, Japan). The protein and α-defensin standards were purchased from Aldrich or Peptides (Osaka, Japan). The peptide and protein solutions were prepared simply by dissolving the standards in doubly distilled water.
MALDI-TOF mass spectrometric analysis
For extraction, a solvent (20 μL) was added into the Eppendorf vial containing the absorbing paper and vortexed for 10 min. An aliquot (1 μL) of the extraction solution was removed by micropipette and mixed with 1 μL of the MALDI matrix solution on a 384-well MALDI sample plate. After drying, the sample spot was analyzed using a MALDI-TOF mass spectrometer (Bruker Daltonics, Autoflex, Leipzig, Germany) operated at linear mode by Flexcontrol 1.2 software package (v. 3). Inside the MALDI source, the sample spot was irradiated with a pulsed nitrogen laser (337 nm, 3 ns) for desorption and ionization. Approximately 240 laser shots were averaged for each sample to obtain representative mass spectra. Each positive-ion mass spectrum acquired in linear mode was collected at an acceleration voltage of 20 kV under the delayed extraction mode. External calibration of the MALDI mass spectra was performed using bovine cytochrome c, insulin, and ACTH (18–39) as standards. The SwissPort database, taxonomically restricted to Homo sapiens (human), was used to search for the peptide ions detected in the MALDI mass spectra. The following parameters were used for the MASCOT peptide mass fingerprint searches: missed cleavages for partial digest, up to 2; variable modifications, oxidation for methionine; peptide ion mass tolerance, 1.0 Da.Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis
SDS and other chemicals used for SDS-PAGE analysis were purchased from Sigma (Missouri, USA). Molecular weight markers (SeeBlue® Plus2 Pre-Stained Standard), 1.0 mm × 70.0 mm × 70.0 mm gels (NuPAGE® Novex® 12% Bis Tris MiniGels), and the corresponding NuPAGE® sample buffer were all obtained from Invitrogen (California, USA). Samples were prepared by adding the SDS sample buffer (NuPAGE® LOS Sample Buffer/NuPAGE® Reducing Agent, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 40 μL) to the tear sample solution. The solution was then immediately heated at 70 °C for 10 min in a water bath. The current used for running SDS gel electrophoresis was 40 mA for 40 min. Coomassie Blue was used as a stain to detect the proteins separated on the SDS-PAGE gel.
2; 40 μL) to the tear sample solution. The solution was then immediately heated at 70 °C for 10 min in a water bath. The current used for running SDS gel electrophoresis was 40 mA for 40 min. Coomassie Blue was used as a stain to detect the proteins separated on the SDS-PAGE gel.
For in-gel tryptic digestion, protein bands were cut from polyacrylamide gels and washed with water and buffer (25 mM NH4HCO3, 50% ACN). Reduction and alkylation of the protein molecules were performed by adding dithiothreitol (DTT) and iodoacetamide (IAA), respectively. An aliquot (20 μL) of a freshly prepared trypsin enzyme solution (20 ng μL−1 trypsin in 25 mM NH4HCO3) was added into the gel solution, which was then incubated at 37 °C overnight. The digested peptides were extracted by adding a solution (20 μL) of 100% ACN containing 0.1% trifluoroacetic acid into the solution and vortex mixing. After centrifugation, the supernatant was saved for peptide characterization through MALDI-TOF using α-CHC as the matrix and the SwissPort database.
Results and discussion
As generally known, the biochemical compounds in tears are mainly mucin, proteins, peptides, lipids and salts.1 To characterize HNPs in the tear with MALDI-TOF, the interferences from some of the chemicals in the tear must be considered. For example, the presence of large quantities of lipids and salts in tears may prevent co-crystallization of HNPs with the MALDI matrices and lower their ionization efficiency during MALDI-TOF analysis.34 To determine the best organic matrix for characterizing HNPs by MALDI-TOF, the tear samples and HNP standards were analyzed with three MALDI matrices-α-CHC, 2,5-DHB and SA.
Fig. 1a–c display the MALDI mass spectra of a tear sample collected from a normal control using different organic matrices. Several ion peaks were detected in the positive-ion MALDI mass spectra of this sample, but none for HNPs. Although the types of ions in the MALDI mass spectra seemed not to be influenced by the nature of the matrix, those obtained using SA as the matrix (Fig. 1c) exhibited the best results in terms of resolution and sensitivity. Fig. 1d–f display the positive-ion MALDI mass spectra of the HNP 1–3 standards (1.0 × 10−5 M each in pure water) using different MALDI matrices; no obvious differences in the ion signals for HNPs 1–3 are observed. Therefore, SA was used as the MALDI matrix for subsequent analyses of HNPs in tears. To determine the best analyte-to-matrix volume ratio (Vanalyte/Vmatrix) for MALDI-TOF analysis, three volumetric ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) were employed for the analyses of the HNP standards (data not shown). The optimal results were obtained when the ratio was 1
100) were employed for the analyses of the HNP standards (data not shown). The optimal results were obtained when the ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. This ratio was used for all subsequent MALDI-TOF analyses.
10. This ratio was used for all subsequent MALDI-TOF analyses.
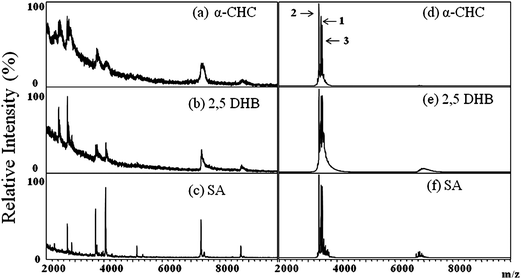 | ||
Fig. 1 Positive-ion MALDI mass spectra of tear samples collected from a normal control, recorded using (a) α-CHC, (b) 2,5-DHB, and (c) SA as MALDI matrices. Positive-ion MALDI mass spectra recorded from α-defensin 1–3 standards (HNPs 1–3) using (d) α-CHC, (e) 2,5-DHB, and (f) SA as matrices (Vanalyte/Vmatrix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Figure 1 (d) showed the numbers “1”, “2”, and “3” represent HNP1, HNP 2, and HNP 3, respectively. No signals of HNPs were found in tears from healthy donors. 10). Figure 1 (d) showed the numbers “1”, “2”, and “3” represent HNP1, HNP 2, and HNP 3, respectively. No signals of HNPs were found in tears from healthy donors. | ||
Of the several methods that can be used to collect tear samples, our experience suggests that dipping a small piece of soft absorbing paper at the corner of the eye for a few seconds is the easiest, fastest, and most efficient; it is acceptable by every tear donor in this study. Different absorbing papers (tissue paper, Kimwipes, and Schirmer filter paper) were examined for their abilities to absorb tears during sample collection and to release HNPs 1–3 molecules during extraction. The size of the absorbing paper was relatively small at 25 mm2 (5.0 mm × 5.0 mm). After absorbing the tears on the absorbing paper by gently touching of the edge of the eye with a piece of the paper for a few seconds, the absorbing paper was immersed and vortex-mixed in the solvent in an Eppendorf vial to extract the HNPs. An aliquot of the extractive solution was then mixed with SA matrix on the sample plate and subjected to MALDI-TOF analysis.
Fig. 2 displays the positive-ion MALDI mass spectra of the tears collected from a dry eye patient using different types of the absorbing paper. The ions detected in all three MALDI mass spectra were quite simple and nearly identical, with three ion signals (m/z 3371, 3442, and 3486) being the most prominent. For the similarity of the m/z values of the ions with the HNP standards, they were tentatively assigned as HNP 1–3. It seems even the sample was not pretreated with any chromatographic separation or affinity adsorption as they were in LC/MS and SELDI analysis, the HNPs in the tear were rapidly characterized with an extremely simple approach. In addition, compared to other reported methods used to collect the tear samples, absorbing the tear by touching the corner of the eye with a piece of absorbing paper is relatively quick, easy, and simple. Because Schirmer filter paper strips are disinfected and used clinically in the diagnosis of dry eye syndrome, it was then chosen to collect the tear samples throughout the study.
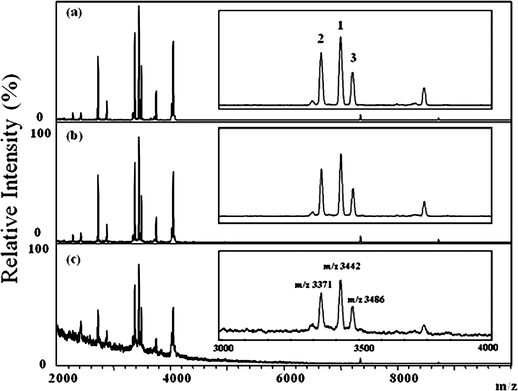 | ||
| Fig. 2 Positive-ion MALDI mass spectra of tear samples collected from dry eye patients using (a) Kimwipes, (b) Schirmer filter paper strips, and (c) tissue paper. Insets: Partial MALDI mass spectra (m/z 3000–4000) displaying the signals of the HNP ions. The numbers “1”, “2”, and “3” represent HNP 1, HNP 2, and HNP 3, respectively. The m/z of 3371, 3442 and 3486 labeled on Figure 2(c) represent HNP 2, HNP 1, and HNP 3, respectively. | ||
Next, five organic solvents (ACN, EtOH, pure water, MeOH, and saturated SA aqueous solution) were examined for their extraction efficiency toward the HNPs on the Schirmer filter paper. The absorbing paper was immersed and vortex-mixed in the solvent to extract the HNPs. An aliquot of the extractive solution was mixed with the SA matrix on the sample plate, after drying, the sample spot was subjected to MALDI-TOF analysis. As the saturated SA solution was used for extraction, the extractive solution was directly deposited on the sample plate and analyzed by MALDI-TOF after it was dry. Fig. 3 reveals that HNPs were detected as the peptides were extracted from the Shirmer filter paper with acetonitrils, water or saturated aqueous SA solution (Fig. 3a, b, and e). The saturated SA solution turned out to be the best extracting solvent because it provided the most intensive HNP ion signals (Fig. 3e). No peptide or protein ion signals were detected when less polar solvents such as methanol or ethanol were used for extraction (Fig. 3b and d).
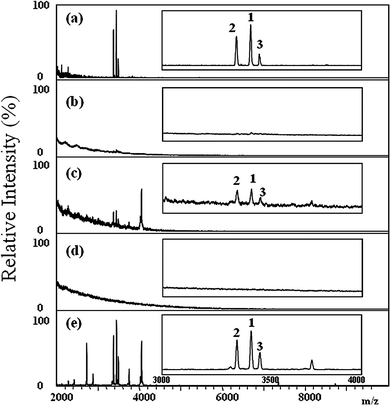 | ||
| Fig. 3 Positive-ion MALDI mass spectra of the extracts from Schirmer filter paper samples containing adsorbed tears from dry eye patient, recorded using (a) ACN, (b) EtOH, (c) pure water, (d) MeOH, and (e) saturated SA solution as extractants. Insets: Partial MALDI mass spectra (m/z 3000–4000) displaying the signals of the HNP ions. The numbers “1”, “2”, and “3” represent HNP1, HNP 2, and HNP 3, respectively. | ||
The identities of HNPs 1–3 detected in the tears of dry eye patients was confirmed first by comparing the mass spectra in the present study with those reported in the literature. The molecular ions of HNPs 1–3 in the MALDI mass spectra of the tears from dry eye patients appeared at m/z 3371, 3442, and 3486, respectively, matching well with those values reported in the literature using SELDI or liquid chromatography followed by MALDI-TOF analysis.35,36 The presence of the HNPs in the tears was further confirmed by “bottom up” approach. This was achieved by analyzing the tears collected from a dry eye patient tear through SDS-PAGE (separation and purification), in-gel tryptic digesting the spot, and characterizing the peptides through MALDI-TOF analysis. Fig. 4 displays the positive-ion MALDI mass spectrum of the peptides derived from the tryptic digestion of the HNPs spot on the SDS-PAGE gel. Through a library search using the MASCOT program, we confirmed that two of the peptide ions, at m/z 929.5 and 1060.5, were from HNPs. The inset to Fig. 4 displays the HNP band separated on the SDS-PAGE gel.
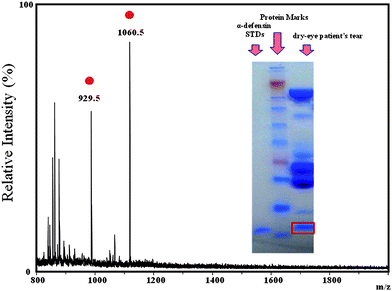 | ||
Fig. 4 Positive-ion MALDI mass spectrum of the peptides derived from the tryptic digestion of the HNP spot on the SDS-PAGE gel.  : Peptide ions derived from the HNPs. Inset: Photograph of the SDS-gel and its protein bands. : Peptide ions derived from the HNPs. Inset: Photograph of the SDS-gel and its protein bands. | ||
The sensitivity of using MALDI-TOF approach to characterize the tear HNPs absorbed on Shirmer paper was estimated by analyzing a series of the tear samples which were collected from a normal control and spiked with HNP 1–3 standards at concentrations ranging from 1.0 × 10−5 to 5.0 × 10−8 M. It was found that the HNP ion signals were detected only from those samples containing greater than 7.5 × 10−8 M of the HNP standards (Fig. 5); i.e., the limit of detection (LOD) of HNP ions in tears when using this MALDI-TOF approach is below 10−7 M.
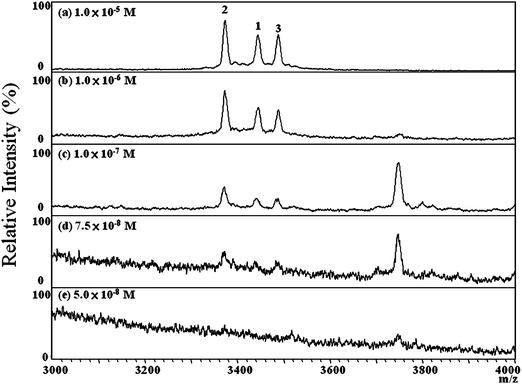 | ||
Fig. 5 Positive-ion MALDI mass spectra of tears from a normal control, spiked with HNP standards at concentrations ranging from 1.0 × 10−5 to 5.0 × 10−8 M, recorded using SA as the matrix (Vsample/Vmatrix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). The LOD of HNP was estimated to be 7.5 × 10−8 M. The numbers “1”, “2”, and “3” represent HNP1, HNP 2, and HNP 3, respectively. 10). The LOD of HNP was estimated to be 7.5 × 10−8 M. The numbers “1”, “2”, and “3” represent HNP1, HNP 2, and HNP 3, respectively. | ||
To evaluate the distribution of HNPs 1–3 in the tears of the normal controls and dry eye patients, 30 tear samples (10 from normal controls, 20 from dry eye patients) were collected on Schirmer filter paper and were analyzed by MALDI-TOF. Fig. 6 displays typical MALDI mass spectra of the tear samples collected from five normal controls. No ion signals for HNPs 1–3 in any of the tear samples collected from the normal control group were detected. Fig. 7 displays typical MALDI mass spectra of the tear samples collected from five dry eye patients; signals for HNPs 1–3 were clearly evident in all of these samples. The results shown in Fig. 6 and 7 suggest that HNPs 1–3 are potential biomarkers for the rapid diagnosis of dry eye disease.
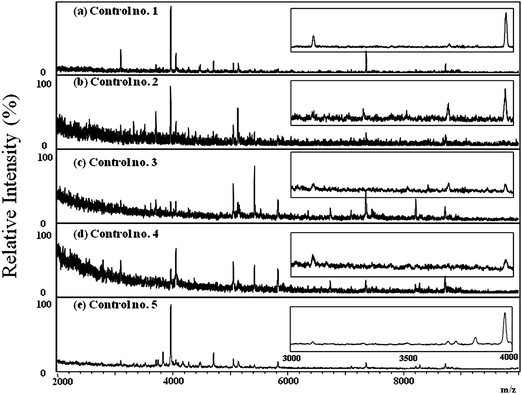 | ||
| Fig. 6 Positive-ion MALDI mass spectra of human tear samples collected from five healthy donors (a)-(e). The insets showed the partial MALDI mass spectra with m/z between 3,000 and 4,000. No ion signals of HNPs were detected in the tear samples collected from all healthy donors. | ||
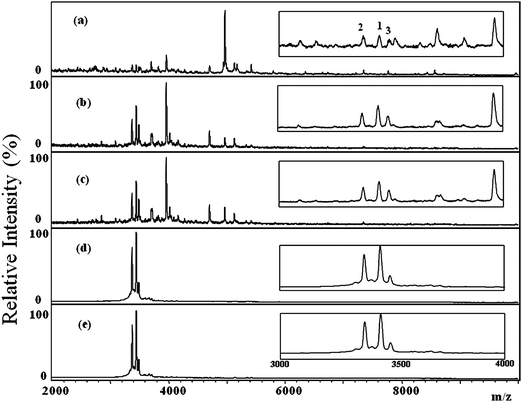 | ||
| Fig. 7 Positive-ion MALDI mass spectra of tear samples collected from five dry eye patients (a)-(e). The insets show the partial MALDI mass spectra with m/z between 3,000 and 4,000. The HNPs were detected in the tear samples collected from all patients. The numbers “1”, “2”, and “3” labeled on Figure 7(a) represent HNP1, HNP 2, and HNP 3, respectively. | ||
Previous studies have indicated that HNPs 1–3 (determined by immunoassay) may be useful biomarkers for certain eye diseases. For example, HNPs 1–3, upregulated in tear fluid from pterygium eyes, are associated with wounds and inflammation [37]; increased production of HNPs in human tears occurs after ocular surface surgery;35 and HNPs 1–3 appear in greater concentrations in the tears of allergic patients with corneal lesions,9 where they play a role in preventing secondary infections. In addition to their direct antimicrobial properties, HNPs appear to be capable of modulating other components of innate immunity.12,37 For example, the expression of TNF-α and IL-1β is stimulated by defensins, indicating that HNPs modulate inflammatory responses through the regulation of cytokine production.38 As is generally known, a common problem in patients with dry eye disease is that they usually have a low degree of inflammation in their eyes.15 Recent studies have revealed an increase in pro-inflammatory cytokine activity and inflammatory cell-surface markers at the ocular surface epithelia of subjects with Sjögren's or non-Sjögren's dry eye.39 The ocular surface for the dry eye patient is usually compromised and therefore, at high risk of microbial infections. Trabattoni et al. hypothesized that changes in the levels of HNPs 1–3 in biological fluids might result from repeated exposure to a pathogen—a potential index of immune activation.40 The presence of HNPs 1–3 in the tears of dry eye patients infers that dry eye syndrome induces inflammation to activate neutrophils and thereby, produce high levels of HNPs 1–3 in the tears.
Conclusions
In this pilot study, HNPs 1–3 were identified as novel potential biomarkers for the diagnosis of dry eye syndrome through MALDI-TOF analysis. The HNP ion signals were qualitatively characterized in all tear samples collected from patients with dry eye disease, but in none of those from the normal controls. The collection of tear samples was simple and noninvasive—simply by wiping the tear on the Shirmer filter paper. The approach for detecting HNPs 1–3 in the tear adsorbed on the Shirmer filter paper requires no further sample pretreatment other than mixing the filter paper with the MALDI matrix solution and drying under ambient conditions. The detection limit of HNPs in the tear was estimated to be below 10−7 M. This simple analytical procedure greatly reduces the analytical time, making it possible to screen large numbers of samples. Compared with other MS-based approaches (e.g., LC/MS and SELDI), the MALDI-TOF approach presented herein is cost-, time-, and labor-saving. One limitation of using this MALDI-TOF approach to diagnose dry eye disease is that eye injuries (e.g., after ocular surface surgery) can also lead to an increase in the production of HNPs in human tears. However, this limitation can be easily overcome by clinical diagnosis. The diagnosis of dry eye disease using HNPs as biomarkers must, therefore, accompany a physical examination to exclude the possibility of eye injury. In addition, even though HNPs are rapidly characterized with the current MALDI-TOF approach, quantification of HNPs in the tear with this approach is still limited because the sample volume used for each test is not constant.Acknowledgements
The authors would like to thank the National Science Council of Taiwan for financial support of this study.References
- J. J. Harding, in: Lens, In Biochemistry of the Eye. Chapman and Hall: London, 1997, pp. 94–135 Search PubMed.
- P. Timothy and S. E. Wilson, Allergan, 2003, 12, 14–19 Search PubMed.
- R. A. Dobie, C. W. Cummings, P. W. Flint, B. H. Haughey, K. T. Robbins and J. R. Thomas, in: Philadelphia, Pa: Mosby Elsevier, Otolaryngology: Head & Neck Surgery, 2005 Search PubMed.
- F. H. Grus and V. N. Podust, Invest. Ophthalmol. Visual Sci., 2005, 46, 863–876 CrossRef.
- R. Tao, R. J. Jurevic, K. K. Coulton, M. T. Tsutsui, M. C. Roberts, J. R. Kimball, W. Norma, J. Berndt and B. A. Dale, Antimicrob. Agents Chemother., 2005, 49, 3883–3888 CrossRef CAS.
- N. P. Munro, D. A. Cairns, P. Clarke, M. Rogers, A. J. Stanley, J. H. Barrett, P. Harnden, D. Thompson, I. Eardley, R. E. Banks and M. A. Knowles, Int. J. Cancer, 2006, 119, 2642–2650 CrossRef CAS.
- L. Kuhn, D. Trabattoni, C. Kankasa, K. Semrau, P. Kasonde, F. Lissoni, M. Sinkala, M. Ghosh, C. Vwalika, G. M. Aldrovandi, D. M. Thea and M. Clerici, J. Acquir. Immune Defic. Syndr., 2005, 39, 138–142 Search PubMed.
- Z. M. Sthoeger, S. Bezalel, N. Chapnik, I. Asher and O. Froy, Immunology, 2009, 127, 116–122 CrossRef CAS.
- R. Y. Hida, Y. Ohashi, Y. Takano, M. Dogru, E. Goto, H. Fujishima, I. Saito, K. Saito, Y. Fukase and K. Tsubota, Curr. Eye Res., 2005, 30, 737–730 Search PubMed.
- T. Ganz, Science, 1999, 286, 420–421 CrossRef CAS.
- K. Shiomi, M. Nakazato, T. Ihi, K. Kanagawa, H. Matsuo and S. Matsukura, Biochem. Biophys. Res. Commun., 1993, 195, 1336–1344 CrossRef CAS.
- R. E. W. Hancock and G. Diamond, Trends Microbiol., 2000, 8, 402–410 CrossRef CAS.
- D. Yang, O. Chertov and J. J. Oppenheim, Cell. Mol. Life Sci., 2001, 58, 978–989 CrossRef CAS.
- F. Niyonsaba, H. Ushio and I. Nagaoka, J. Immunol., 2005, 175, 1776–1784 CAS.
- M. E. Stern, R. W. Beuerman and R. I. Fox, Cornea, 1998, 17, 584–589 CrossRef CAS.
- P. Y. Ong, T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo and D. Y. M. Leung, N. Engl. J. Med., 2002, 347, 1151–1160 CrossRef CAS.
- J. J. Song, S. W. Chae, J. S. Woo, H. M. Lee, H. H. Jung and S. J. Hwang, Ann. Otol. Rhinol. Laryngol., 2007, 116, 235–240.
- C. Melle, G. Ernst, B. Schimmel, A. Bleul, H. Thieme, R. Kaufmann, H. Mothes, U. Settmacher, U. Claussen, K. J. Halbhuber and F. von Eggeling, Gastroenterology, 2005, 129, 66–73 CrossRef CAS.
- A. V. Panyutich, E. A. Panyutich, V. A. Krapivin, E. A. Baturevich and T. Ganz, J. Lab Clin. Med., 1993, 122, 202–207 CAS.
- T. Hiratsuka, M. Nakazato, Y. Date, J. Ashitani, T. Minematsu, N. Chino and S. Matsukura, Biochem. Biophys. Res. Commun., 1998, 249, 943–947 CrossRef CAS.
- T. Ihi, M. Nakazato, H. Mukae and S. Matsukura, Clin. Infect. Dis., 1997, 25, 1134–1140 CrossRef CAS.
- Q. Lu, L. J. Jin, R. P. Darveau and L. P. Samaranayake, J. Periodontal Res., 2004, 39, 221–227 CrossRef CAS.
- M. E. Klut, B. A. Whalen and J. C. Hogg, Eur. J. Haematol., 2000, 64, 114–120 CrossRef CAS.
- A. V. Panyutich, N. N. Voitenok, R. I. Lehrer and T. Ganz, J. Immunol. Methods, 1991, 141, 149–155 CrossRef CAS.
- E. Pisano, T. Cabras, C. Montaldo, V. Piras, R. Inzitari, C. Olmi, M. Castagnola and I. Messana, Eur. J. Oral Sci., 2005, 113, 462–468 CrossRef CAS.
- N. Mizukawa, K. Sugiyama, T. Ueno, K. Mishima, S. Takagi and T. Sugahara, Oral Surg., Oral Med., Oral Pathol., Oral Radiol. Endodontol., 1999, 87, 539–543 Search PubMed.
- M. Karas, D. Bachman and F. Hillenkamp, Anal. Chem., 1985, 57, 2935–2939 CrossRef CAS.
- M. Karas and F. Hillenkamp, Anal. Chem., 1988, 60, 2299–2301 CrossRef CAS.
- K. Tanaka, H. Waki, Y. Ido, S. Akita, Y. Yoshida and T. Yoshida, Rapid Commun. Mass Spectrom., 1988, 2, 151–153 CAS.
- S. Y. Lin, S. H. Shih, D. C. Wu, Y. C. Lee, C. I. Wu, L. H. Lo and J. Shiea, Rapid Commun. Mass Spectrom., 2007, 21, 3311–3316 CrossRef CAS.
- C. I. Wu, C. C. Tsai, C. C. Lu, P. C. Wu, D. C. Wu, S. Y. Lin and J. Shiea, Clin. Chim. Acta, 2007, 384, 86–92 CrossRef CAS.
- J. Shiea, Y. T. Cho, Y. H. Lin, C. W. Chang, L. H. Lo, Y. C. Lee, H. L. Ke, W. J. Wu and D. C. Wu, Rapid Commun. Mass Spectrom., 2008, 22, 3754–3760 CrossRef CAS.
- M. H. Yang, L. H. Lo, Y. H. Chen, J. Shiea, P. C. Wu, Y. C. Tyan and Y. J. Jong, Rapid Commun. Mass Spectrom., 2009, 23, 3220–3226 CrossRef CAS.
- B. K. Anna, B. K. Anna, T. Dylag, D. Anna, P. Suder, M. Noga, J. Jarzebinska and J. Silberring, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 849, 1–31 CrossRef CAS.
- L. Zhou, L. Q. Huang, R. W. Beuerman, M. E. Grigg, S. F. Y. Li, F. T. Chew, L. Ang, M. E. Stern and D. Tan, J. Proteome Res., 2004, 3, 410–416 CrossRef CAS.
- L. Zhou, R. W. Beuerman, L. P. K. Ang, C. M. Chan, S. F. Y. Li, F. T. Chew and D. T. H. Tan, Invest. Ophthalmol. Visual Sci., 2009, 50, 2077–2086 CrossRef.
- T. Ganz, Nat. Rev. Immunol., 2003, 3, 710–720 CrossRef CAS.
- Y. V. Chaly, E. M. Paleolog, T. S. Kolesnikova, I. I. Tikhonov, E. V. Petratchenko and N. N. Voitenok, Eur. Cytokine Netw., 2000, 11, 257–266 Search PubMed.
- N. Tomosugi, K. Kitagawa, N. Takahashi, S. Sugai and I. Ishikawa, J. Proteome Res., 2005, 4, 820–825 CrossRef CAS.
- D. Trabattoni, S. L. Caputo, G. Maffeis, F. Vichi, M. Biasin, P. Pierotti, F. Fasano, M. Saresella, M. Franchini, P. Ferrante, F. Mazzotta and M. Clerici, JAIDS, J. Acquired Immune Defic. Syndr., 2004, 35, 455–463 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
