Preconcentration of ultra-trace arsenic with nanometre-sized TiO2 colloid and determination by AFS with slurry sampling
Shahua
Qian
*,
Zhen
Huang
,
Jiaqi
Fu
,
Jie
Kuang
and
Chaozhen
Hu
School of Resources and Environmental Science, Wuhan University, Luoyu Road NO.129, Wuhan City, Wuhan, Hubei 430079, China. E-mail: qiansh@whu.edu.cn; Fax: +86 27-68778893; Tel: +86 27-68778551
First published on 12th July 2010
Abstract
This study describes a united method for the analysis of ultra-trace arsenic in environmental water samples by preconcentration of TiO2 colloid and determination of Atomic Fluorescence Spectrometry (AFS). When the pH values of solution are between 6.0 and 7.0, the adsorption for arsenic is above 98.0% with the dosage of nanometre-sized TiO2 colloid (0.2%) being 6.0 mL. Without desorbing the concentrated arsenic, the precipitated TiO2 is directly inverted to colloid after centrifugation. Arsenic is then determined by AFS with slurry sampling. This united method is simple, fast and sensitive. The detection limit (3σ) and the relative standard deviation (R.S.D) of this method are 10.6 ng L−1 and 7.04% (n = 6), respectively. When the method is applied to the determination of ultra-trace arsenic in environmental standard samples, the results are in good agreement with the standard values. And when it comes to environmental samples, satisfactory results are obtained.
1. Introduction
Arsenic is an ubiquitous element, and its abundance ranks 20th, 14th and 12th in the earth’s crust, seawater and human body, respectively.1 Arsenic can exist in four oxidation states: As(−III), As(0), As(III), and As(V). Environmental forms include arsenites, arsenates, methylarsenic acid, dimethylarsinic acid, arsine, etc. The predominant form of inorganic arsenic in aqueous, aerobic environments is arsenate, whereas arsenite is more prevalent in anoxic environments.2 Arsenic was one of the first chemicals recognized as a cause of cancer.3 The WHO guideline value for As in drinking water was provisionally reduced in 1993 from 50 to 10 μg L−1. The new recommended value was based on the increasing awareness of the toxicity of arsenic, particularly its carcinogenicity, and on the ability to measure it quantitatively.4 Arsenic is known for its physiological and toxicological effects.5 For some waters that have not been polluted or subsurface water, the background value of arsenic can be very low.6,7 It is difficult to determine directly. Therefore, the preconcentration and separation as well as the determination of arsenic are of great importance and necessity.Nanoparticles, the building blocks of nanotechnology, have been broadly defined as having at least one dimension at 100 nm or less.8 Nanomaterials are made of nanoparticles. The sizes, surface structures and interparticle interactions of nanomaterials determine their unique properties and the improved performances, and make their potential application in many areas.9 The atomic number, the area, energy, and chemical combination energy of its surface all rapidly increase as the particle radius decreases.10 The atoms on the surface of the nanoparticles are unsaturated and can easily bind with other atoms. The high chemical activity they possess makes their combination easier. Consequently, compared with general adsorbents, nanomaterials have a much higher adsorption capacity for many metal ions and are an ideal kind of adsorbent. Nanometer-size TiO2 powder shows great potential and advantages as solid-phase extractant for the separation and preconcentration of trace amounts of many metals. Some research on the adsorption of nanometre-size TiO2 powder for heavy metals has been done for years, and methods of concentration and separation for these elements by using nanometre-size TiO2 powder have been established. Relevant research has shown that nanometre-size TiO2 powder is effective in the adsorption for trace metals.11–17
Nanometer-size TiO2 colloid was first applied to the analyses of trace elements by our seminar in 2006, and the methods have been continuously improved.18 Compared with nanometre-size TiO2 powder, its colloid is more advantageous as adsorbent for the concentration and separation of trace elements. Smaller particle radius, larger specific surface and better dispersal without agglomeration are the advantages that nanometre-size TiO2 colloid has over its powder. Compared with the conventional adsorption materials, TiO2 colloid has better adsorption efficiency. With slurry sampling, the complicated processes of filtration and desorption were omitted, so that the time of analysis was shortened and the possibility of contamination was reduced.
A simple, fast, sensitive united-method for the determination of ultra-trace arsenic is developed in this study. Nanometer-size TiO2 colloid, which is applied as adsorbent, shows its full advantages. The concentration of arsenic is determined by atomic fluorescence spectrometry (AFS). This method is applied to the determination of arsenic in environmental water samples with great success.
2. Experimental
2.1 Apparatus and reagents
The PF6-2 non-dispersive atomic fluorescence spectrometer (Beijing Purkinje General Instruments Co., Ltd., Beijing, China), equipped with the HAF-2 arsenic hollow cathode lamp, was used for arsenic determination. The pH values were measured with the Mettler-Toledo 320-S pH meter (Mettler-Toledo, Greifensee, Switzerland). The LD5-2A centrifuge (Beijing Medical Centrifuge Factory, Beijing, China) was applied to separate the suspensions, and the AY-120 electronic balance (Shimadzu, Tokyo, Japan) was used in this experiment. The instrumental conditions are displayed in Table 1.| Negative high voltage/V | Flow rate of Ar/mL min−1 | Lamp current/mA | Atomization T/°C | |
|---|---|---|---|---|
| 265 | 500 | Main | Auxiliary | 170 |
| 30 | 30 | |||
Colloid nanometre-size TiO2 (2.0% w/w) was supplied by Infrared Academy of Wuhan University and was diluted 10 times to 0.2% w/w for use. Thiourea-ascorbic acid mixed solution (5% w/w) was used as reductant. Solution (1.5%) of KBH4 and hydrochloric acid (2% v/v) were involved in the procedure of determination. Ar (99.99%) was applied as carrier gas. As2O3 (G.R.) was used to prepare standard stock solution (1.000 g L−1) of As(III) and Na2HAsO4·7H2O (G.R.) was used when preparing standard stock solution (1.000 g L−1) of As(V). A series of working standard solutions were prepared by diluting standard stock solution. HNO3 and NaOH aqueous solutions were used to adjust pH. Water samples were from Donghu Lake (Wuhan, Hubei Province, China), Yangtze River and tap water (in the laboratory). All solutions were prepared with ultra pure water.
2.2 Methods
3. Results and discussion
3.1 Effect of pH
The particles of Nanometer-size TiO2 colloid were well dispersed when the pH value was lower than 4.0 (the isoelectric point of TiO2), and they could not be effectively separated. In order to separate the Nanometer-size TiO2 colloid with adsorption of As from solution, the pH value should be higher than 4.0 theoretically. And experiments showed that satisfactory centrifugal effect could be attained with a pH value higher than 5.0 and a centrifugal speed of 4000 rpm.The adsorption rates of different pH values were determined with the amount of Nanometer-size TiO2 colloid being 6.00 mL. The results were shown in Fig. 1.
 | ||
| Fig. 1 Effect of pH on adsorption. | ||
It can be seen that the adsorption rate could reach 98.0% or higher when the pH value is between 6.0 and 7.0. The pH was shown to have a significant influence on the adsorption of metal ions. Different results were reported for the adsorption of As(III) and As(V) on the metal oxide surface.19–21 Generally, the adsorption amount of As(III) and As(V) was smaller in alkaline solution than that of acidic solution. At acidic pH the surface has a net positive charge that would attract arsenite and arsenate negative ions. At basic pH the surfaces has a net negative charge that would cause an electrostatic repulsion between the surface and arsenite and arsenate negative ions. However, Dutta et al. found the adsorption of As(III) onto TiO2 particles increased with increases in pH, which was opposite to the adsorption behavior found for As(V).20 Actually, for the adsorption of As(V), the adsorption between pH 5.0 and 6.0, was also negligible. When use ferrihydrite as the adsorbent, there was a trend of increasing adsorption with increasing pH for As(III), with maximum adsorption at pH 8.2–10. As for As(V), a different trend was observed, with a broad adsorption maximum at pH 3.8–7.4.21 Therefore, we think it was difficult to explain the mechanism at this moment and further work has already been carried out in our lab.
3.2 Effect of the amount of nanometre-size TiO2 colloid
The adsorption rates of different volumes of nanometre-size TiO2 colloid were determined. The adsorption rate reached 98.0% when the volume of nanometre-size TiO2 was 6.00 mL. Therefore, 6.00 mL of nanometre-size TiO2 colloid (0.2%) was adopted in this experiment. The results were showed in Fig. 2.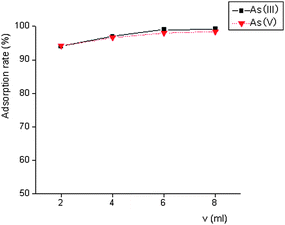 | ||
| Fig. 2 Effect of nanometre-size TiO2 colloid dosage on adsorption. | ||
3.3 Adsorption capacity
A series of concentration of standard solution of As(III) were prepared with addition of 6.00 mL nanometre-size TiO2 colloid (0.2%) respectively. Several adsorption rates were calculated and a curve of adsorption amounts of As(III) with initial concentration was attained. The adsorption capacity of nanometre-size TiO2 colloid (0.2%) for As(III) was 111.9 μg mL−1. The results were showed in Fig. 3. | ||
| Fig. 3 Adsorption capacity of nanometre-size TiO2 colloid on As(III). | ||
3.4 Effect of coexistent ions
A certain amount of different ions were added to 100.00 mL solution containing 0.10 μg L−1 (the content of arsenic after being concentrated for 20 times was 2.00) arsenic. The solution then contained K+ (4.2 mg), Na+ (110.4 mg), Ca2+ (4.4 mg), Mg2+ (13.2 mg), Sr2+ (81.0 μg), Zn2+ (24.0 μg), Fe3+ (60.0 μg), Al3+ (38.0 μg), Mn2+ (20.0 μg), Cl− (198.8 mg), SO42− (26.6 mg), NO3− (1242 μg), PO43− (260 μg), Br− (673 μg) and F− (13.0 μg). The fluorescence was determined under the procedure of enrichment experiment. The recovery of the samples was above 97.5%. It showed that there was almost no influence on the determination of arsenic.In the control group, 2.00 μg L−1 of arsenic with coexistent ions was directly determined without concentration. The recovery of the samples was about 30%. The coexistent ions showed significant effect on the detection and the obtained result was not accurately.
By comparing the data of these two groups, this method of concentration and separation can accurately determine the content of As in the composition of complex samples. This indicates that the method has strong anti-ion interference ability.
3.5 Detection limit and relative standard deviation
Under the optimum experimental conditions, 100.00 mL of standard solution (0.05 μg L−1) of As(III) was enriched. Its fluorescence then were determined by AFS (n = 6). The detection limit (3σ) and the relative standard deviation (R.S.D) of this method were 10.6 ng L−1 and 7.04%, respectively.3.6 Analysis of standard reference material
To study the accuracy of this method, an environmental water sample certified reference material (GSBZ 50004-88) with an arsenic content of 5.03 ± 0.34 μg L−1, was analyzed. The obtained arsenic content value 4.89 ± 0.11 μg L−1, based on the average of four replicates, was in good agreement with the certified value. The standard curve was obtained with the samples determined in suspended state with slurry sampling, with an R2 of 0.9990.3.7 Environmental water samples analysis and standard adding recovery experiments
The above-mentioned method was adopted to determine arsenic in several water samples, including Donghu Lake, Yangtze River and tap water. The results were listed in Table 2.| sample | As added/μg L−1 | As found/μg L−1 | Recovery (%) |
|---|---|---|---|
| Donghu Lake | 0 | 0.167 | — |
| 0.20 | 0.362 | 97.5 | |
| Yangtze River | 0 | 0.063 | — |
| 0.10 | 0.159 | 96.0 | |
| Tap water | 0 | 0.0268 | — |
| 0.04 | 0.0645 | 95.3 |
4. Conclusion
This method adopted nanometre-size TiO2 colloid as adsorbent for separation and concentration, and used AFS with slurry sampling for determination of arsenic in water samples. Short-time separation and enrichment, low detection limit, free of interference, simple operation and fast analysis are the biggest advantages of this method. Compared with the traditional method which needs a desorption process, the method introduced in this paper appears to be more advantaged. Consequently, it can be applied to determine ultra-trace amount of arsenic in environmental water samples with great success.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No. 20877059) and National Key Technology R&D Program in the 11th Five year Plan of China (Grant NO.2006BAJ08B07).References
- B. K. Mandal and K. T. Suzuki, Talanta, 2002, 58, 201–235 CrossRef CAS.
- R. S. Oremland and J. F. Stolz, Science, 2003, 300, 939–944 CrossRef CAS.
- A. H. Smith, P. A. Lopipero, M. N. Bates and C. M. Steinmaus, Science, 2002, 296, 2145–2146 CrossRef CAS.
- P. L. Smedley and D. G. Kinniburgh, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- E. Muñoz and S. Palmero, Talanta, 2005, 65, 613–620 CrossRef CAS.
- X. Chen, J. Z. Li and Z. F. He, Shanghai Geol., 2006, 1, 24–27, Search PubMed in Chinese.
- X. G. Pang and J. C. Zhan, Shandong Land Resour., 2005, 5, 1–5, Search PubMed in Chinese.
- S. T. Stern and S. E. McNeil, Toxicol. Sci., 2007, 101, 4–21 CrossRef.
- P. Liang, J. Cao, R. Liu and Y. Liu, Microchim. Acta, 2007, 159, 35–40 CrossRef CAS.
- M. Uehara, B. Barbara, B. Dieny and P. C. E. Stamp, Phys. Lett. A, 1986, 114, 23–26 CrossRef.
- E. Vassileva and K. Hadjiivanov, Fresenius J. Anal. Chem., 1997, 357, 881–885 CrossRef CAS.
- P. Liang, Y. C. Qin, B. Hu, C. X. Li, T. Y. Peng and Z. C. Jiang, Fresenius J. Anal. Chem., 2000, 368, 638–640 CrossRef CAS; P. Liang, Y. C. Qin, B. Hu, T. Y. Peng and Z. C. Jiang, Anal. Chim. Acta, 2001, 440, 207–213 CrossRef CAS.
- S. H. Qian, Y. Luo, S. B. Mo and X. Weng, Wuhan Univ. J. Nat. Sci., 2006, 11, 437–440 CrossRef CAS.
- F. Y. Zheng, S. H. Qian, S. X. Li, X. Q. Huang and L. X. Lin, Anal. Sci., 2006, 22, 1319–1322 CrossRef CAS.
- R. Liu and P. Liang, J. Hazard. Mater., 2008, 152, 166–171 CrossRef CAS.
- P. Matúš, I. Hagarová, M. Bujdoš, P. Diviš and J. Kubová, J. Inorg. Biochem., 2009, 103, 1473–1479 CrossRef CAS.
- L. Zhang, Y. N. Wang, X. J. Guo, Z. Yuan and Z. Y. Zhao, Hydrometallurgy, 2009, 95, 92–95 CrossRef CAS.
- S. H. Qian, X. Q. Li, M. Xiao, H. B. Deng and L. J. Xiang, Chinese. Chem. Lett., 2006, 17, 933 Search PubMed; S. H. Qian, S. J. Zhang, Z. Huang, M. Xiao and F. Huang, Microchim. Acta, 2009, 166, 251–254 CrossRef CAS.
- H. Jézéquel and K. H. Chu, Environ. Chem. Lett., 2005, 3, 132–135 Search PubMed.
- P. K. Dutta, A. K. Raya, V. K. Sharma and F. J. Millero, J. Colloid Interface Sci., 2004, 278, 270–275 CrossRef CAS.
- K. P. Raven, A. Jain and R. H. Loeppert, Environ. Sci. Technol., 1998, 32, 344–349 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
