Acrylamide risk in food products: The shortbread case study
O.
Marconi
*a,
E.
Bravi
a,
G.
Perretti
a,
R.
Martini
b,
L.
Montanari
a and
P.
Fantozzi
a
aDepartment of Economic and Food Science, University of Perugia,
via S. Costanzo, 06126, Perugia, Italy. Fax: +39.075.585.7939; Tel: +39.075.585.7923
bColussi, Petrignano di Assisi, Perugia, Italy
First published on 5th October 2010
Abstract
Among advanced hypotheses concerning acrylamide formation in cooked food, the Maillard browning reactions of sugars and amino acids have received much attention as the most likely pathway. Bakery products such as biscuits represent a class of food in which the effect of ingredients and processing promote acrylamide formation. The International Agency for Research on Cancer (IARC) classified acrylamide as “potential carcinogen to humans”; therefore, there is an urgent requirement for the development and validation of sensitive, robust and inexpensive analytical methods to quantify acrylamide in different food matrices to the μg kg −1 levels. The aims of this research were the set up of an analytical method to determine acrylamide concentration in shortbread biscuits, and the study of the technological parameters influencing the acrylamide formation during shortbread processing. Five technological tests were conducted to produce five different experimental types of shortbread. The experimental shortbreads were obtained using different concentrations of baking agents (sodium bicarbonate and ammonium bicarbonate) and applying different cooking temperatures and different times. A HPLC-DAD reliable and sensitive method to determine acrylamide in shortbread was achieved. The chromatographic separation was achieved with isocratic analysis in a 15-min run. The coefficient of determination of the acrylamide standard calibration curve is 0.997; the limit of detection was 8 μg Kg−1, and the limit of quantification was 28 μg Kg−1. The recovery test was conducted adding three different concentrations of acrylamide standard solution to the blank sample. The acrylamide recovery ranged from 90.5 ± 0.3% to 99.1 ± 1.8%. The results showed that the ammonium bicarbonate concentration in shortbread, and the high temperature, influenced the acrylamide formation during the processing highlighting that the final acrylamide concentration is influenced by the processing parameters.
Introduction
Acrylamide (2-propenamide) is a low molecular weight hydrophilic compound known mostly for its use as a monomer in the production of polyacrylamide, which in turn is used in plastics and as an electrophoresis medium.1 The International Agency for Research on Cancer (IARC) has therefore classified acrylamide as “potential carcinogen to humans”,2 as a result of this classification, the maximum work place concentration list defined it as a Category III A2 substance. In early 2002, the Swedish National Food Administrator (SNFA) and the University of Stockholm announced that certain foods that are processed or cooked at high temperatures contain relatively high levels of acrylamide. Various fried and oven-cooked foods based on meats and flour respectively, corn crisps, muesli, as well as some of the breakfast cereals and crisp breads, were in the concentration interval up to 100 μg kg−1. French fries and other fried, deep fried or oven-baked potato products, together with some crisp breads, biscuits, crackers and breakfast cereals, were in the interval 100–1000 μg kg−1. About half of the potato crisp samples were also in this group while the levels above 1000 μg kg−1 were detected in the remaining half.3,4 Acrylamide formation takes place when browning carbohydrate rich foods like potato or cereal based products by frying, baking or roasting, but acrylamide can also be found in, e.g., coffee or chocolate.5In general, thermal processes during the production of foodstuffs are complex, and the initial result on acrylamide level do not seem to indicate a common pattern, except that carbohydrate-rich foods seems to generate relatively more acrylamide.3 The low water content seems important for the reactions, and acrylamide is nearly not detected in boiled foods containing starch. Deep frying or roasting seems to be propitious to the formation of acrylamide.6 Recent studies suggested that the acrylamide in food is largely derived from heat-induced reaction between the amino group of free amino acid asparagine and the carbonyl group of reducing sugar such as glucose during baking and frying.7–9 Two main mechanisms of acrylamide formation in foods have been proposed. However, acrylamide is also generated by direct decarboxylation from Schiff bases prior to the Amadori rearrangement, as well as from carnosine, Strecker aldehydes or from pyrolysis of gluten.10–12 Finally, acrolein and ammonia have also been identified as precursors of acrylamide from thermal degradation of triglycerides in lipid-rich foods.13 Acrylamide chemistry, biochemistry, occurrence, metabolism and toxicology have been reviewed elsewhere.14
An urgent requirement is for the development and validation of sensitive, robust and inexpensive analytical methods to quantify acrylamide in different food matrices to the μg kg −1 levels.1 A number of chromatographic methods for determination and detection of acrylamide in heat-treated foods has been developed. Gas chromatographic (GC) and high-performance liquid chromatographic (HPLC) techniques are used for both determination and detection of acrylamide in water, biological fluids and non cooked foods.3,5,15–17 The GC-MS methods require derivatization of the acrylamide before the analysis (e.g., bromination), LC-MS instead analyzes the compound directly. Moreover, many researchers believe that the liquid chromatography (LC) methods were not found to be appropriate for the analysis of acrylamide in processed food at low levels, and many authors believe that LC must be coupled to mass spectrometry (MS) for better identification of acrylamide processed foods. Although MS is a selective system for detection, the mass of acrylamide itself or its ions are not specific due to the presence of co-extractive compounds that yield the same magnitude of m/z with acrylamide in the sample matrix.1,15 The sample clean-up plays a fundamental role in acrylamide analysis. High-performance liquid chromatographic (HPLC) techniques are easy and low cost with respect to MS techniques. As reported in the literature,15,18 the HPLC method coupled with Diode Array Detector (DAD) can be suitable for the analysis of acrylamide in potato chips, crisps and deep-fried flour based food so it could be applied to other processed foods such as biscuits. Gökmen et al.15 found that a conventional HPLC instrument coupled to DAD can also be used accurately and precisely as an alternative to tandem LC-MS methods for the determination of acrylamide in potato-based foods after extraction of acrylamide with methanol, purification with Carrez I and II solution, evaporation and solvent change to water. In different foods, acrylamide formation has been show to correlate with pre-processing levels of asparagine, fructose, glucose or the product of asparagine and reducing sugars. However, the observed wide variations in levels of acrylamide in different food categories as well as in different brands of the same food category appear to result not only from the amounts of the precursors present but also from variations in processing conditions (e.g., temperature, time, nature of food matrix).20 Bakery products such as biscuits represent a class of food in which the effect of ingredients and processing promote the acrylamide formation.21 The highest contents were often found in products prepared with the baking agent ammonium bicarbonate such as gingerbread products.22 Some studies about model experiments showed that the ammonium bicarbonate strongly promotes the acrylamide formation in sweet bakery.23,24 Shortbread represents a biscuits class containing acrylamide because of the ingredients and the thermal process. Shortbread ingredients that influence the acrylamide formation are the baking agents, reducing sugars and the asparagine which derived from cereals, milk or eggs.19 The cereals cultivars containing low concentrations of reducing sugar and asparagine represent an important way to reduce the acrylamide content of shortbread. Nevertheless it is shown that the breakdown of the stark grain during the milling causes the increase of acrylamide in the final product. The damaged grains are more susceptible to the enzymatic hydrolysis which led to the reducing sugars formation. The bran cereal displacement to obtain white flour represents another way to prevent the acrylamide formation in bakery products. In fact the bran contains a high concentration of acrylamide precursors as asparagine.25 Graft et al., 20067 conducted a study about influence of the baking agents at different concentrations on acrylamide formation during the production of a semi-finished biscuit on an industrial scale. Recently, Sadd et al., 200826 evaluated the effect of dough age, yeast, fermentation times, the addition of amino acids or metal ions and the use of sodium instead of ammonium raising agents for reducing acrylamide in pilot scale bakery products.
The aims of the present investigation were the set up of an analytical method to determine acrylamide concentration in the level of 30–650 μg kg−14 in shortbread biscuits and to study the technological parameters influencing the acrylamide formation during shortbread processing. The paper presents a reliable, sensitive, fast and low-cost analytical method for the determination of acrylamide in shortbread biscuits. The method utilized LC with UV detection that can be easily adopted by non-specialized analytical laboratories. The second purpose of this study was to check the influence of the cooking temperature, time, and the baking agents—like ammonium bicarbonate and sodium bicarbonate—on the acrylamide content of shortbread biscuits produced in a pilot plant.
Experimental
Materials
HPLC grade methyl alcohol and water were purchased from Carlo Erba (Milano, Italy). Carrez I (15 g of potassium ferrocyanide in 100 ml of water) and Carrez II (30 g zinc sulfate in 100 ml of water) and sodium sulfate anhydrous. SPE (Solid Phase Extraction) Oasis HLB. Number 2 Wathman paper filters.Eight different shortbread samples. Samples 1, 2, 3A, 3B, and 3C, obtained with different technological trials, were prepared in a pilot plant. Samples 1CS, 2CS and 3CS were commercial shortbreads purchased in local market.
Preparation of biscuits
The samples 1, 2, 3A, 3B, and 3C considered in this study were prepared using five different recipes (1, 2, 3A, 3B, and 3C) that planned the variation of the following parameters: the amount of baking agents (ammonium and sodium bicarbonate) and baking time and temperature (Table 1). All the recipes consisted of the following ingredients: flour 90–110 (1860 g), water (186 g), powdered sugar (sucrose) (612 g), vegetable fat (360 g), skimmed milk (72 g), a starch (wheat) preparation (12 g), monohydrate dextrose (21 g), eggs (48 g), mineral salt (8 g), orange (690b) (0.5 g) and vanilla (01218) aroma (0.9 g), and baking agents. For the preparation of samples 1, 2, and 3A the baking parameters were fixed to 240 °C for 7 min, whereas the amount of baking agents varied. In particular 3.0 g of ammonium bicarbonate and 3.0 g of sodium bicarbonate were added in recipe 1, 4.5 g of each baking agent were added in recipe 2, and in recipe 3A 11.9 g each were added.| RECIPE | (NH4)HCO3 g | NaHCO3 g | COOKING PARAMETERS |
|---|---|---|---|
| 1 | 3.0 | 3.0 | T = 240 °C t = 7′ |
| 2 | 4.5 | 4.5 | T = 240 °C t = 7′ |
| 3A | 11.9 | 11.9 | T = 240 °C t = 7′ |
| 3B | 11.9 | 11.9 | T = 300 °C t = 3′ |
| 3C | 11.9 | 11.9 | T = 180 °C t = 12′ |
Finally for the recipes 3B and 3C they had same ingredients of 3A but different baking parameters, 300 °C for 3 min and 180 °C for 12 min respectively.
The baking process was carried out in a static oven (TMP, C/2B Mod.).
Equipment
The samples and standards were weighed using an A&D Instrument Ltd (Oxon, UK) HR-200 analytical balance with a sensitivity of ±0.1 mg. Samples were grinded using a Sunbeam mod. 4153-50 grinder and dried in a Binder ED 115 airy stove. Sample solutions were homogenate with an Ultraturrax T25 Jankle&Kunkel Ika-Labortecknick and centrifuged with a Sorvall RC-5B refrigerated, superspeed centrifuge (Du Pont Instruments) and with a centrifuge 5415D Eppendorf. The following equipment was used for the HPLC analysis: a Jasco Pu-2089 plus pump, a Rheodyne 7025 injector, equipped with a 50 μL injection loop, a model Agilent 1100 DAD and a model Agilent 1100 thermostat for HPLC columns. The system was managed by Agilent Chem-Station for LC 3D System (Agilent Technology, Santa Clara, CA).Standard and sample preparation
For the calibration curve plotting the standard solution concentrations ranged between 0.05–1.00 μg ml−1, which is the region that contains the measures.Two grams of ground shortbread sample and 20 ml of methyl alcohol were mixed in a 50 ml Falcon tube. The obtained solution was homogenated with Ultraturrax at 10000 rpm for 2 min and centrifuged at 12000 rpm for 5 min. The supernatant was recovered, transferred in a Falcon tube and Carrez I (200 μl) and II (200 μl) were added. The mixture was centrifuge at 12000 rpm for 5 min. After centrifugation 5 ml of supernatant were collected, filtered and anhydrified with sodium sulfate anhydrous. The filtered sample was evaporated to dryness, dissolved in 2 ml of water in Eppendorf tubes and centrifuge at 14000 rpm for 2 min. The supernatant was then purified in SPE Oasis HLB cartridges. The cartridge was conditioned with 2 ml of methanol and equilibrated with 2 ml of water. Then the supernatant was loaded, the first 1.5 ml drops were discarded and the remaining solution injected into the HPLC-DAD system.
Chromatographic conditions
The chromatographic separation was achieved at room temperature with C18 reverse-phase column (Inertsil ODS 3, 4.6 mm ID × 150 mm, 5 μm, Varian Inc., Lake Forest, CA). The acrylamide was determined at 200, 226, and 240 nm wavelengths.All procedures were carried out isocratically using a mixture of 0.01M sulfuric acid of water–methanol 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5. The flow rate was 0.7 mL min−1.
2.5. The flow rate was 0.7 mL min−1.
Results and discussion
Method development
The method was developed using methanol solvent for the extraction, Carrez reagent for sample cleanup by protein precipitation, and SPE-cartridges for extract cleanup before HPLC analysis (Fig. 1).5,15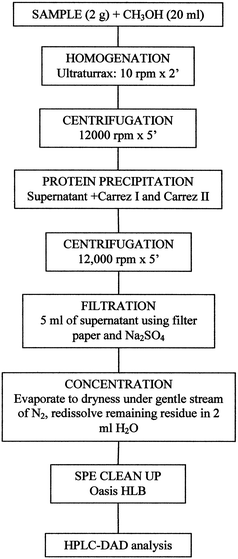 | ||
| Fig. 1 Sample preparation procedure. | ||
Sample preparation
Many researchers have focused on the extraction efficiency of acrylamide in different sample matrices using water, methanol or other solvent.3,27,28 Because acrylamide is highly soluble in methanol (1.55 g ml−1) and it is an extracting solvent much compatible with the food matrices containing high amounts of fat as shortbread, the sample preparation was started by extracting the ground shortbread with methanol. The sample preparation was conducted as reported by Gokmen and Senyuva, (2006).29 The extraction was performed at room temperature using a ratio sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent 1
solvent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Heating or ultrasonicating during the extraction step may as well be avoided because this may have generated large amounts of slight particles that can saturate the solid phase extraction (SPE) cartridges used in further clean-up steps.3 According to literature the extracting step of acrylamide needs to have a defatting or deproteinating step according to sample matrices. In the shortbread case the proteins were precipitated by Carrez reagents that also prevent the loss of acrylamide during the evaporation under stream of nitrogen.3,5,15 In order to remove coextractives the extract was cleaned up using Oasis HLB SPE. HLB cartridge consisting of an uncharged sorbent made from a balanced ratio of the hydrophilic N-vinylpyrrolidone and the lipophilic divinylbenzene. The cartridges were conditioned with methanol and water and, after discarding the first 30 drops, the remaining effluents were collected and analysed.
10. Heating or ultrasonicating during the extraction step may as well be avoided because this may have generated large amounts of slight particles that can saturate the solid phase extraction (SPE) cartridges used in further clean-up steps.3 According to literature the extracting step of acrylamide needs to have a defatting or deproteinating step according to sample matrices. In the shortbread case the proteins were precipitated by Carrez reagents that also prevent the loss of acrylamide during the evaporation under stream of nitrogen.3,5,15 In order to remove coextractives the extract was cleaned up using Oasis HLB SPE. HLB cartridge consisting of an uncharged sorbent made from a balanced ratio of the hydrophilic N-vinylpyrrolidone and the lipophilic divinylbenzene. The cartridges were conditioned with methanol and water and, after discarding the first 30 drops, the remaining effluents were collected and analysed.
HPLC-DAD analysis
A satisfactory separation of acrylamide contained in the extract was achieved by HPLC-DAD with an isocratic analysis in 15 min using a C18 column. The mobile phase was a solution of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (97.5
methanol (97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) 0.01 M sulfuric acid mixture. As reported in the literature a 0.01 M sulfuric acid solution gave the highest peaks and shorter retention times.1Fig. 2 shows the chromatogram and UV spectrum of a 1 μg ml−1 standard solution of acrylamide. The acrylamide retention time was 4.481 min and it showed the maximum absorption spectrum at 200 nm. The signals at 226 and 240 nm are useful to confirm the acrylamide assigned peak. The retention time of acrylamide was stable. To monitor the retention time and the performances of the column every day before starting the HPLC analysis, a standard solution of acrylamide was injected. The acrylamide in shortbread extract was identified by comparison of its retention time with that of standard and it was quantified by using its calibration curve and by evaluating the UV absorbance spectrum. Fig. 3 shows a typical elution profile of acrylamide in shortbreads and reported a small segment of the chromatogram in which the acrylamide peak is highlighted. The performance of the method was evaluated with regard to the calibration, the linearity, the repeatability, the limit of detection (LOD), the limit of quantification (LOQ) and the recovery. Calibration curves were determined for five different concentrations of standard solution injected in triplicate. The calibration graphs, at 200 nm which is the wavelength with the maximum absorption, were obtained by plotting concentrations against peak area. Table 2 reports the slope (a), the intercept (b) and the coefficient of determination (r2) of the calibration plots. Moreover, considering the standard deviation (SD) for a (SDa) and b (SDb) parameters, the confidence interval for intercept (b) and slope (a) was calculated considering P ≤ 0.05 and degree of freedom (n–2) was n = 15. The slope and its confidence interval is reported in Table 2 for acrylamide as a = a ± t0.05, 13 SDa, while the intercept and its confidence interval is b = b ± t0.05, 13 SDb. The intercept was substantially near the zero and the confidence interval included origin axes. The coefficient of determination was r2 = 0.997. The LOD was determined as three times the signal to noise ratio, while the LOQ was ten times the signal to noise ratio.1,5,18 The LOD and the LOQ were 8 μg Kg−1 and 28 μg Kg−1respectively at a detection wavelength of 200 nm. The accuracy of the method was verified by analyzing spiked shortbreads. The recovery of acrylamide was determined by analyzing each of the spiked samples three times (n = 3) for levels ranging from 75 to 250 μg kg −1. The mean percentage recoveries exceeded 90% for all spiking levels for shortbread (Table 3).
2.5) 0.01 M sulfuric acid mixture. As reported in the literature a 0.01 M sulfuric acid solution gave the highest peaks and shorter retention times.1Fig. 2 shows the chromatogram and UV spectrum of a 1 μg ml−1 standard solution of acrylamide. The acrylamide retention time was 4.481 min and it showed the maximum absorption spectrum at 200 nm. The signals at 226 and 240 nm are useful to confirm the acrylamide assigned peak. The retention time of acrylamide was stable. To monitor the retention time and the performances of the column every day before starting the HPLC analysis, a standard solution of acrylamide was injected. The acrylamide in shortbread extract was identified by comparison of its retention time with that of standard and it was quantified by using its calibration curve and by evaluating the UV absorbance spectrum. Fig. 3 shows a typical elution profile of acrylamide in shortbreads and reported a small segment of the chromatogram in which the acrylamide peak is highlighted. The performance of the method was evaluated with regard to the calibration, the linearity, the repeatability, the limit of detection (LOD), the limit of quantification (LOQ) and the recovery. Calibration curves were determined for five different concentrations of standard solution injected in triplicate. The calibration graphs, at 200 nm which is the wavelength with the maximum absorption, were obtained by plotting concentrations against peak area. Table 2 reports the slope (a), the intercept (b) and the coefficient of determination (r2) of the calibration plots. Moreover, considering the standard deviation (SD) for a (SDa) and b (SDb) parameters, the confidence interval for intercept (b) and slope (a) was calculated considering P ≤ 0.05 and degree of freedom (n–2) was n = 15. The slope and its confidence interval is reported in Table 2 for acrylamide as a = a ± t0.05, 13 SDa, while the intercept and its confidence interval is b = b ± t0.05, 13 SDb. The intercept was substantially near the zero and the confidence interval included origin axes. The coefficient of determination was r2 = 0.997. The LOD was determined as three times the signal to noise ratio, while the LOQ was ten times the signal to noise ratio.1,5,18 The LOD and the LOQ were 8 μg Kg−1 and 28 μg Kg−1respectively at a detection wavelength of 200 nm. The accuracy of the method was verified by analyzing spiked shortbreads. The recovery of acrylamide was determined by analyzing each of the spiked samples three times (n = 3) for levels ranging from 75 to 250 μg kg −1. The mean percentage recoveries exceeded 90% for all spiking levels for shortbread (Table 3).
| Concentration range | a | b | r2 | LODb | LOQb | |
|---|---|---|---|---|---|---|
| (μg ml−1) | a ± t0.05, 13 | b ± t0.05, 13 | (μg Kg−1) | (μg Kg−1) | ||
| a p < 0.05 a = a± t0.05, 13 × sda, sda = standard error of slope, b = b ± t0.05, 13 × sdb, sdb = standard error of intercept, y = ax + b. b n = 10. | ||||||
| Acrylamide | 0.05–1.00 | 915.23 ± 47.33 | 18.48 ± 15.19 | 0.997 | 8 | 28 |
| Background level in shortbread (μg Kg−1) | Spike level (μg Kg−1) | Detected (μg Kg−1) | Recovery (%) |
|---|---|---|---|
| a n = 3. | |||
| 257 | 75 | 315 ± 5 | 94.8 ± 1.4 |
| 100 | 323 ± 1 | 90.5 ± 0.3 | |
| 250 | 502 ± 9 | 99.1 ± 1.8 |
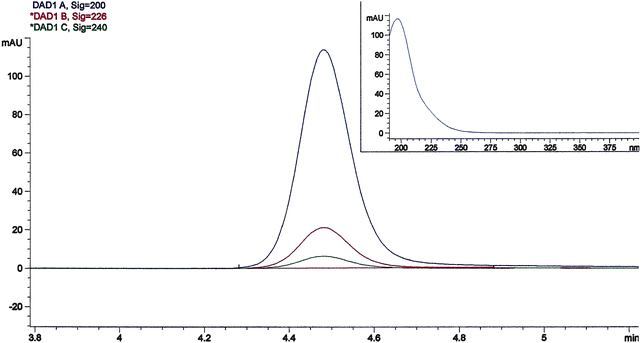 | ||
| Fig. 2 Chromatogram and UV spectrum of a 1 μg ml−1 standard solution of acrylamide. | ||
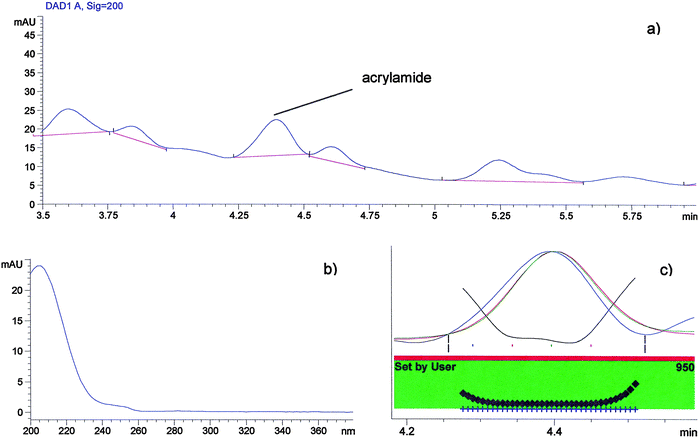 | ||
| Fig. 3 (a) A typical elution profile of acrylamide in shortbread; (b) acrylamide spectra; (c) acrylamide peak purity test. | ||
Acrylamide shortbread analysis
Table 4 reports the concentration of acrylamide in three commercial shortbread (CS) samples purchased at a local market. The standard deviation value ranges from 10 to 22 μg kg −1 and the total concentration of acrylamide detected ranged from 278 to 326 μg kg −1. The relative standard deviation ranged from 4 to 7% and these data highlight the good repeatability of the proposed method. The different concentrations of acrylamide of the three samples are the consequence of the different ingredients of the shortbreads. These data are in the range of acrylamide concentration for this type of food reported in the literature14 and showed that the concentration of acrylamide in shortbread was lower than other foods, such as fried potato which presented a concentration of acrylamide ranging from 200 to 12000 μg kg −1. The average long-term dietary intake in developed countries was 0.3–0.8 μg kg −1 body weight per day, so the contribution of acrylamide due to shortbread biscuits is low.30 The second step of this work is the study of the technological parameters influencing the acrylamide formation during shortbread processing. Five different technological tests were conducted using a pilot plant consisting of a vertical kneader, a pilot rotary press, a static oven, five different recipes characterized by different concentrations of ammonium bicarbonate, and sodium bicarbonate and different cooking times and temperatures.Table 5 reports the acrylamide concentrations of the five experimental shortbreads. The samples 1, 2 and 3A were obtained using different amounts of baking agents (3.0, 4.5 and 11.9 g respectively) but were cooked at the standard parameters of 240 °C for 7 min. The acrylamide concentration of sample 1 was lower than sample 2 and 3A. The sample 1 recipe contained a lower amount of ammonium bicarbonate, and these data confirmed that ammonium bicarbonate had a positive correlation with the acrylamide formation. Literature data reported that the recipes without ammonium bicarbonate lead to gingerbread without acrylamide.22 The replacement with sodium bicarbonate as the only baking agent could be an approach to decrease the acrylamide formation in shortbread but the product had an alkaline taste. The samples 3A, 3B and 3C were obtained utilizing the same amount of ammonium bicarbonate (11.9 g) but with different cooking temperatures and times. The sample 3A, cooked at 240 °C for 7 min, showed a concentration of acrylamide higher than 3B and 3C. The sample 3B has the minor acrylamide concentration. It was cooked at 300 °C for 3 min and we hypothesize that the high temperatures promote the acrylamide degradation as suggested by literature.25,31 Sample 3C was obtained applying a cooking temperature of 180 °C for 12 min and it presented a lower acrylamide concentration than samples 3A. Gokmen et al.32 reported that after the initial lower rate period, acrylamide concentration of cookies reaches a plateau of about 250 μg kg −1 within a baking time of 15 min at 180 °C, using sucrose in the recipe. They obtained cookies with an acrylamide concentration less than 150 μg kg −1 by baking them at 160 °C for 25 min which will convert the surface to a fully brownish color. Sample 3C presented a concentration of acrylamide of 127 μg kg−1 and the typical surface color of shortbread. It is well known that the acrylamide formation is influenced by the ingredients and their composition. The obtained results also highlighted the role of the cooking time and temperature. In fact, the evolution of acrylamide concentration with respect to the thermal treatment time in model system follows a “bell course” (gaussian course). Moreover, the acrylamide concentration of a food system subject to an intense thermal treatment for a determined time should be the result of its formation and degradation.33 The acrylamide content of heating processed food first increases in the time at a constant cooking temperature. Then, after prolonged heating, a decrease in the acrylamide content was observed because degradation of acrylamide becomes predominant.34
Conclusions
This work describes a quantitative analytical method for the determination of acrylamide in shortbread biscuits. It requires relatively low cost instrumentation to perform when compared to tandem MS detection based methods already published, and can be easily adopted by many laboratories worldwide. The sample preparation is simple and rapid. The Diode Array Detector set at 200, 226, and 240 nm enables quantification as low as 28 μg kg−1 of acrylamide in shortbreads.The obtained results highlight the strong influence of ammonium bicarbonate in acrylamide formation. The acrylamide concentration in the finished product was influenced by the process parameters. The high cooking temperatures obtained a decrease of acrylamide concentration. The experimental shortbreads showed a lower acrylamide concentration in comparison with commercial shortbreads, and it should be the consequence of the simplified recipe. In fact, the acrylamide formation depends on the food matrix.
References
- E. K. Paleologos and M. G. Kontominas, J. Chromatogr., A, 2005, 1077, 128 CrossRef CAS.
- International Agency for Reaserch on Cancer (IARC), Some Industrial Chemical, IARC Monographs on the on the Evaluation for Carcinogenic risk for Chemicals to Humans, vol. 60, IARC, Lyon, 1994, p. 435 Search PubMed.
- Y. Zhang, G. Zhang and Y. Zhang, J. Chromatogr., A, 2005, 1075, 1 CrossRef CAS.
- Swedish National Food Administration web site, http:/www.slv.se, consulted on the 29 June 2010.
- J. N. Nielsen, K. Granby, R. V. Hedegaard and L. H. Skibsted, Anal. Chim. Acta, 2006, 557, 211 CrossRef CAS.
- B. E. Erickoson, Anal. Chem, 2004, 76, 247.
- M. Graf, T. M. Amrein, S. Graf, R. Szaalay, F. Escher and R. Amadò, LWT–Food Sci. Technol., 2006, 39, 724 CrossRef CAS.
- D. S. Mottram, B. L. Wedzicha and A. T. Dodson, Nature, 2002, 419, 448 CrossRef CAS.
- R. H. Stadler, I. Blank, N. Varga, F. Robert, J. Hau, P. A. Guy, M.-C. Robert and S. Riediker, Nature, 2002, 419, 449 CrossRef.
- V. A. Yaylayan, A. Wronowski and L. C. Peres, J. Agric. Food Chem., 2003, 51, 1753 CrossRef CAS.
- V. A. Yaylayan, L. C. Peres, A. Wronowski and J. O'Brien, J. Agric. Food Chem., 2004, 52, 5559 CrossRef CAS.
- A. Yasuhara, Y. Tanaka, M. Hengel and T. Shibamoto, J. Agric. Food Chem., 2003, 51, 3999 CrossRef CAS.
- A. Claus, G. M. Weisz, D. R. Kammerer, R. Carle and A. Schieber, Mol. Nutr. Food Res., 2005, 49, 918 CrossRef CAS.
- M. Friedman, J. Agric. Food Chem., 2003, 51, 4504 CrossRef CAS.
- V. Gokmen, H. Z. Senyuva, J. Acar and K. Sarioglu, J. Chromatogr., A, 2005, 1088, 193 CrossRef.
- L. Castle, J. AOAC Internat, 2005, 88, 274 Search PubMed.
- K. Hoenicke, R. Gatermann, W. Harder and L. Hartig, Anal. Chim. Acta, 2004, 520, 207 CrossRef CAS.
- H. Wang, A. W. M. Lee, S. M. Shuang and M. M. F. Choi, Microchem. J., 2008, 89, 90 CrossRef CAS.
- M. Croft, P. Tong, D. Fuentes and T. Hambridge, Food Addit. Contam., Part A, 2004, 21, 721 Search PubMed.
- G. Weiss, Science, 2002, 297, 27 CAS.
- M. Anese, B. Quarta, M. Foschia and R. Bortolomeazzi, Mol. Nutr. Food Res., 2009, 53, 1526 CrossRef CAS.
- T. M. Amrein, B. Schonbachler, F. Escher and R. Amadò, J. Agric. Food Chem., 2004, 52, 4282 CrossRef CAS.
- M. Biedermann and K. Grob, Mitt. Lebensm Hyg., 2003, 94, 406 Search PubMed.
- R. Weisshaar, Deutsche Lebensm.-Rund., 2004, 100, 92 Search PubMed.
- M. Anese and S. Sovrano, Ind. Aliment., 2006, 459, 625 Search PubMed.
- P. A. Sadd, C. G. Hamlet and L. Liang, J. Agric. Food Chem., 2008, 56, 6154 CrossRef CAS.
- F. Tateo and M. Bononi, Ital. J. Food Sci., 2003, 15, 149 CAS.
- A. F. Lagante and M. A. Felter, J. Agric. Food Chem., 2004, 52, 3744 CrossRef CAS.
- V. Gokmen and H. Z. Şenyuva, J. Chromatogr., A, 2006, 1120, 194 CrossRef.
- A. M. Tritscher, Toxicol. Lett., 2004, 149, 177 CrossRef CAS.
- A. Becalski, B. P. Y. Lau, D. Lewis, S. W. Seaman, S. Hayward, M. Sahagian, M. Ramesch and Y. Leclerc, J. Agric. Food Chem., 2004, 52, 3801 CrossRef CAS.
- V. Gokmen, O. C. Acar, H. Koksel and J. Acar, Food Chem., 2007, 104, 1136 CrossRef.
- D. Andrzejewsky, J. A. G. Roach, M. L. Gay and S. M. Musser, J. Agric. Food Chem., 2004, 52, 3744 CrossRef CAS.
- P. Rydberg, S. Erikkson, E. Tareke, K. Karlsson, L. Ehrenberg and M. Törqvnist, J. Agric. Food Chem., 2003, 51, 7012 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
