Nickel oxide screen printed electrodes for the sensing of hydroxide ions in aqueous solutions
Philip M.
Hallam
,
Dimitrios K.
Kampouris
,
Rashid O.
Kadara
,
Norman
Jenkinson
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Biology, Chemistry and Health Science, Division of Chemistry and Materials, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, Lancs, UK. E-mail: c.banks@mmu.ac.uk; Fax: +44 (0)1612476831; Tel: +44 (0)1612471196
First published on 10th June 2010
Abstract
Nickel oxide bulk modified screen printed electrodes are developed for the first time and explored towards the electroanalytical sensing of hydroxide ions and shown to be analytically useful. The nickel oxide screen printed sensor allows the detection of hydroxide ions over the low micro-molar to milli-molar range with a detection limit of 23 µM. The sensor is simplified over existing analytical methodologies and given its disposable and economical nature holds promise for the sensing of hydroxide ions and consequently the measurement of pH in aqueous solutions.
1. Introduction
Reliable sensors for the monitoring of hydroxide ion concentrations which can operate in harsh environments are urgently sought.1–3 Hydroxide is used worldwide in many industrial processes and rapid and reliable methodologies for the sensing of hydroxide ions for quality control purposes and monitoring during industrial processing are required.4 Problems arise quite simply due to the corrosive nature of the concentrated alkali and glass pH electrodes become insensitive and unstable at high concentration levels.1,2,5 Sensors based on fluorescence and absorbance have been reported but have been criticised due to tedious fabrication processes.6Daniele et al. have extensively explored the electrochemical oxidation of hydroxide ions in aqueous solution using gold microelectrodes.7–11 The electroanalytical signal resulting from the electrochemical oxidation of hydroxide ions is well documented to be proportional to the hydroxide ion over a wide concentration range8 providing a novel methodology for the measurement of pH as an alternative to potentiometry, particularly at high alkaline concentrations beyond the limit of applicability of the glass electrode.11 Current state-of-the-art for hydroxide ion sensing has been reported by Compton and co-workers who reported the low micro-molar level detection of hydroxide ions using gold ultra-microelectrode arrays.12
The electrochemical oxidation of hydroxide ions always occurs at high oxidation potentials typically in the region of 1.3 to 1.6 V (vs. SCE).5 Recently Mentus et al. have explored the role of cathodic pre-treatment on the electrochemical oxidation of hydroxide ions using gold electrodes where it was demonstrated that the electrochemical oxidation potential can be reduced to lower oxidation potentials through forming different types of gold oxide.5 Generally the electrochemical oxidation of hydroxide ions has been undertaken on gold and platinum electrodes. The modification of gold electrodes with metal ion phthalocyanines and porphyrins has reported modest decreases in the electrochemical oxidation potentials of hydroxide ions.4 It was observed by Daniele et al.9 that the electrochemical oxidation of hydroxide ions occurred at lower oxidation potentials than that at gold and platinum electrodes through the use of a nickel electrode. However, it was noted that the background oxide formation potentially makes measurements difficult.9
Herein we revisit the electrochemical oxidation of hydroxide ions using nickel oxide screen printed electrodes which have micron-sized nickel oxide incorporated into the bulk of screen printed working electrodes. These nickel oxide screen printed sensors have been developed for the first time and are explored towards the electroanalytical sensing of hydroxide ions.
2. Experimental section
All chemicals used were of analytical grade and were used as received without any further purification and were obtained from Sigma-Aldrich. Micron-sized nickel oxide (NiO 97.3%, 10 microns) was used as received. All solutions were prepared with deionised water of resistivity not less than 18.2 MΩ cm−1.Voltammetric measurements were carried out using a µ-Autolab III (ECO-Chemie, The Netherlands) potentiostat. All measurements were conducted using a screen-printed three electrode configuration with a geometric working electrode area of 3 mm diameter. Connectors for the efficient connection of the screen printed electrochemical sensors were purchased from Kanichi Research Services Ltd.13
Screen-printed carbon electrodes were fabricated in-house with appropriate stencil designs using a microDEK 1760RS screen-printing machine (DEK, Weymouth, UK). A carbon–graphite ink formulation previously utilised14 was first screen printed onto a polyester flexible film (Autostat, 250 µm thickness). This layer was cured in a fan oven at 60 degrees for 30 minutes. Next a silver/silver chloride reference electrode was included by screen printing Ag/AgCl paste (Gwent Electronic Materials Ltd, UK) onto the plastic substrate. Last a dielectric paste ink (Gwent Electronic Materials Ltd, UK) was printed to cover the connection and define the 3 mm diameter graphite working electrode. After curing at 60 degrees for 30 minutes the screen printed electrode is ready to use.
Nickel oxide screen printed electrodes were fabricated as described above with commercially purchased nickel oxide efficiently mixed into the ink formulation prior to screen printing. Note that in doing so, the rheology of the ink changes such that the careful addition of solvents is needed along with modification of the standard printing parameters. Increasing amounts of nickel oxide were incorporated into the screen printed electrodes over the range of 0–10% (MP/MI), where MP is the mass of particulate and MI is the mass of ink formulation used in the printing process.
Scanning electron microscope (SEM) images and surface element analysis were obtained with a JEOL JSM-5600LV model having an energy-dispersive X-ray microanalysis package.
3. Results and discussion
Nickel oxide (NiO) screen printed electrochemical sensing platforms were fabricated as described in the Experimental section where increasing amounts of nickel oxide were incorporated into the screen printed electrodes over the range of 0–10% (MP/MI), where MP is the mass of particulate and MI is the mass of ink formulation used in the printing process. SEM images of the nickel oxide sensor are shown in Fig. 1 where a relatively rough surface is observed but is not distinctly different to that expected for a non-modified screen printed electrode.14 EDAX was performed with typical values revealing that the examined surface is 92.59% atomic carbon, 4.49% atomic oxygen, 2.72% atomic chloride and 0.2% atomic nickel. The micron sized nickel oxide likely resides in the carbon layer as random dispersed micron sized particles, or larger, which are clearly exposed to the electrolyte solution. | ||
| Fig. 1 SEM images of a standard, non-modified screen printed sensor (left image) compared with a 2% (MP/MI) nickel oxide modified screen printed sensor (right image). | ||
The response of a 2% (MP/MI) nickel oxide screen printed sensor towards the electroanalytical sensing of hydroxide was initially explored. Fig. 2 shows typical cyclic voltammetric responses resulting from 250 µM additions of hydroxide into a 0.01 M Na2SO4 solution. Inspection of the cyclic voltammetric response in the absence of hydroxide (dotted line, Fig. 2) reveals a small reduction wave at −0.11 V (vs. SCE) which is likely due, based on inspection of the nickel Pourbaix diagram, to the electrochemical reduction of the nickel oxide,15 likely to Ni2+. The nickel then likely reacts with the added hydroxide analyte forming Ni(OH)2 in accordance with the Pourbaix diagram where the electrochemical oxidation wave at +0.65 V is likely due to two processes: Ni(OH)2 → NiOOH + H+ + e− and Ni(OH)−3 → NiOOH + H2O + e−. On the reverse scan a pre-wave and large reduction wave are observed. Analysis of the peak potential (Ep) as a function of pH for both waves, where the pH of the solution is changed through the addition of hydroxide ions, reveals a small non-Nerstian response for the pre-wave and a linear response for the large reduction wave at +0.05 V (vs. SCE) with a gradient of 51 mV per pH indicating a one proton and one electron process. Thus we tentatively assign the pre-wave and the large reduction wave to the electrochemical reduction of NiOOH + H2O + e− → Ni(OH)3− and NiOOH → Ni(OH)2 + H+ + e− respectively. Following the additions of hydroxide, a large voltammetric wave is observed at +0.65 V (vs. SCE) which is likely due to the following process:5,12
| 4OH− − 4e− ⇌ O2 + 2H2O |
 | ||
| Fig. 2 Cyclic voltammetric profiles resulting from the addition of 250 µM of NaOH into a 0.01 M Na2SO4 aqueous solution using a 2% (MP/MI) nickel oxide screen printed sensor. Scan rate: 50 mV s−1 (vs. SCE). Also shown is the analysis of the peak height as a function of hydroxide concentration. | ||
Returning to the analytical performance of the nickel oxide screen printed sensor, the electrochemical oxidation of hydroxide, which is observed at +0.65 V (vs. SCE), occurs at a lower overpotential compared to previous literature reports using nickel microelectrodes which was observed to exhibit an electrochemical oxidation wave at ∼1.0 V (vs. SCE),9 and additionally that observed at gold and platinum microelectrodes9 indicating some possible ‘electro-catalytic’ activity of the nickel oxide domains. Analysis of the oxidation wave observed at +0.65 V (vs. SCE) as a function of hydroxide additions is shown in Fig. 2 where a linear response is observed from 250 µM to 2.75 mM (IH/A = 8.0 × 10−3 A/M + 9.3 × 10−7 A; R2 = 0.996; N = 11) with a second linear range observed from 3 mM to 10 mM (IH/A = 2.9 × 10−3 A/M + 1.6 × 10−5 A; R2 = 0.992; N = 29). Based on the first linear range the limit of detection (based on three sigma) was found to correspond to 137 µM.
The response of differing % (MP/MI) nickel oxide screen printed sensor towards the electroanalytical sensing of hydroxide was initially explored where it was found that as the % (MP/MI) nickel oxide content in the screen printed sensor was increased, the background current also enlarged until at the 10% (MP/MI) nickel oxide screen printed sensor, the large background engulfs the electroanalytical oxidation signal which arises from the electrochemical oxidation of hydroxide. This phenomenon has been observed before which is due to the reduced number of conductive pathways throughout the electrode.16,17
Next the above experiment was replicated under identical conditions with the 2% (MP/MI) nickel oxide screen printed sensor towards smaller additions of NaOH. Fig. 3 displays the analysis of cyclic voltammetric responses resulting from successive additions of NaOH (25 µM) into a solution containing 0.01 M Na2SO4. Analysis of the anodic wave (Fig. 3A) reveals two linear ranges: the first from 25 µM to 275 µM (IH/A = 31 × 10−3 A/M + 8.2 × 10−7 A; R2 = 0.985; N = 11) and the second from 300 µM to 500 µM (IH/A = 16 × 10−3 A/M + 4.1 × 10−6 A; R2 = 0.923; N = 9). Based on the first linear region, a limit of detection (based on three sigma) was found to correspond to 21(±3) µM. The analytical performance of the nickel oxide screen printed sensor is competitively comparable to gold ultra-microelectrodes.12 Given the disposable nature and low cost, the nickel oxide screen printed sensor is highly desirable for the analytical sensing of hydroxide. As shown in Fig. 3B, the cathodic wave can also be used as an indirect analytical measurement of hydroxide where a linear response takes place at concentrations greater than 125 µM to 500 µM (IH/A = 38 × 10−3 A/M − 4.3 × 10−6 A; R2 = 0.992; N = 16). The reproducibility of the screen printed batch was explored using a fixed hydroxide concentration of 7 mM with electrode randomly selected from the batch. The percentage relative standard deviation (%RSD) found to correspond to 3.84% (n = 7) indicating sufficient reproducibility throughout the batch indicating that an electrode selected randomly should act quantitatively similar to the rest.
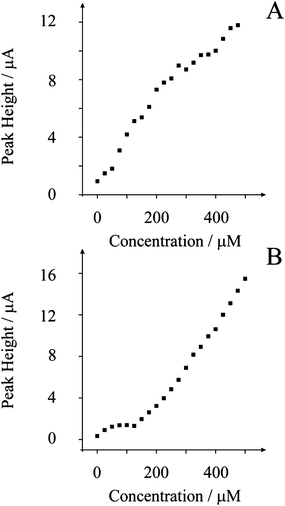 | ||
| Fig. 3 Analysis of cyclic voltammetric profiles resulting from 25 µM additions of NaOH into a 0.01 M Na2SO4 aqueous solution using a 2% (MP/MI) nickel oxide screen printed sensor. Scan rate: 50 mV s−1 (vs. SCE). Plot A is the analysis of the anodic wave while plot B is the cathodic wave. | ||
Next, the application of chronoamperometry as an electrochemical measurement was explored as it can be simpler to analyse. Fig. 4A illustrates the chronoamperometric responses of a 2% (MP/MI) nickel oxide screen printed sensor from additions of hydroxide into a 0.01 M Na2SO4 solution. Analysis of the limiting current (IL) as a function of hydroxide concentration (current taken at 20 second time-moment) is also shown (Fig. 4B) which reveals two linear regions: the first over the range 50 to 250 µM (IL/A = 1 × 10−3 A/M − 2.4 × 10−8 A; R2 = 0.98; N = 9) and the second over the range 275 to 500 µM (IL/A= 0.8 × 10−3 A/M − 6.5 × 10−9A; R2 = 0.987; N = 10). Based on the first linear region, a limit of detection (based on three sigma) was found to correspond to 27 µM even though the sensitivity is considerably less than observed above but still suggests that this approach is analytically viable.
 | ||
| Fig. 4 Chronoamperometric responses obtained using a 2% (MP/MI) nickel oxide screen printed sensor towards the sensing of hydroxide in 0.01 M Na2SO4. Parameters: +0.74 V (vs. SCE). | ||
It has been shown that the measurement of hydroxide concentrations can allow the pH of the solution under investigation to be determined.10Fig. 5 depicts an overview of the entire analytical response using the voltammetric peak height as a function of hydroxide concentration. Analysis of this plot in terms of voltammetric peak height and a function of the solution pH, which changes as increasing amounts of hydroxide are added to the 0.01 M Na2SO4 solution, is shown which correspond from hydroxide additions of 2 to 50 mM. Clearly a direct correlation between the peak height of the voltammetric profiles obtained using the nickel oxide screen printed electrodes and the concentration/pH is observed, this in turn means that there is the possibility of using this electrode as a single use, disposable pH sensor which is either beyond the limit of applicability of the glass electrode5 or be utilised in extreme environments where the glass electrode is non-operational and can be easily incorporated into existing devices. Interestingly a titration type curve is observed at pH 12.2 corresponding to a hydroxide concentration of 15 mM. At this point the peak height versus hydroxide concentration plot deviates from linearity and inspection of the nickel Pourbaix diagram reveals that as the pH has been changed the dominate nickel species becomes Ni(OH)3−. Further additions of hydroxide using the nickel oxide screen printed sensor were explored but were found to produce no response indicating the upper pH limit which is in accordance with previous studies using gold microelectrodes.11
 | ||
| Fig. 5 Analysis of cyclic voltammetric responses resulting from additions of hydroxide into a 0.01 M Na2SO4 aqueous solution using a 2% (MP/MI) nickel oxide screen printed sensor. Also shown is the response of the peak height as a function of solution pH. | ||
4. Conclusions
We have reported the first literature report of a screen printed electrode bulk modified with nickel oxide. The nickel oxide screen printed sensor has been explored towards the analytical quantification of hydroxide ions which has found to be analytically useful over the low micro-molar to milli-molar range. The disposable sensor is competitively comparable to current state-of-the-art which employs ultra-microelectrode arrays but is greatly simplified and cost effective and holds promise for hydroxide sensing and holds potential for the sensing of pH in aqueous solutions which is inaccessible for the standard glass pH electrode and may be incorporated into existing sensing devices as appropriate.References
- R. J. Berman, G. D. Christian and L. W. Burgess, Anal. Chem., 1990, 62, 2066 CrossRef CAS.
- L. R. Allain and Z. Xue, Anal. Chem., 2000, 72, 1078 CrossRef CAS.
- T. A. Canada and Z. Xue, Anal. Chem., 2002, 74, 6073 CrossRef CAS.
- K. De Wael and A. Adriaens, Talanta, 2008, 74, 1562 CrossRef CAS.
- A. Abu-Rabi, D. Jasin and S. Mentus, J. Electroanal. Chem., 2007, 600, 364 CrossRef CAS.
- H. Xu and O. A. Sadik, Analyst, 2000, 125, 1783 RSC.
- I. Ciani and S. Daniele, J. Electroanal. Chem., 2004, 564, 133 CrossRef CAS.
- S. Daniele, C. Bragato, M. E. Abdelsalam and G. Denualt, Anal. Chem., 2002, 74, 3290 CrossRef CAS.
- M. E. Abdelsalam, G. Denualt, M. A. Baldo, C. Bragato and S. Daniele, Electroanalysis, 2001, 13, 289 CrossRef CAS.
- S. Daniele, M. A. Baldo, C. Bragato, G. Denualt and M. E. Abdelsalam, Anal. Chem., 1999, 71, 811 CrossRef CAS.
- M. E. Abdelsalam, G. Denualt, M. A. Baldo and S. Daniele, J. Electroanal. Chem., 1998, 449, 5 CrossRef CAS.
- O. Ordeig, C. E. Banks, T. J. Davies, F. Javier del Campo, F. X. Munoz and R. G. Compton, Anal. Sci., 2006, 22, 679 CrossRef CAS.
- http://kanichi-research.com/ .
- R. O. Kadara, N. Jenkinson and C. E. Banks, Electrochem. Commun., 2009, 11, 1377 CrossRef CAS.
- D. Giovanelli, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Analyst, 2003, 128, 173 RSC , and articles cited therein.
- N. A. Choudhry, D. K. Kampouris, R. O. Kadara, N. Jenkinson and C. E. Banks, Anal. Methods, 2009, 1, 183 RSC.
- R. O. Kadara, N. Jenkinson and C. E. Banks, Electroanalysis, 2009, 21, 2410 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
