DOI:
10.1039/C0AY00316F
(Paper)
Anal. Methods, 2010,
2, 1156-1159
Received
2nd April 2010
, Accepted 17th May 2010
First published on
24th June 2010
Introduction
The development of a fast and sensitive colorimetric sensor or reagent for detection and identification of ions1–7 and small molecules8–12 has emerged as an actively investigated area of research in recent years. Aromatic amines are one of the most important compounds and are used widely, not only in the synthesis of drugs13–17 and pesticides18–23 as key structure units, but also in the field of polymer materials chemistry.24–28 However, a number of aromatic amines have recently been found to be carcinogens29–33 and have been polluting the human environment34–36 including food and water. Therefore, to have a fast, simple and sensitive assay for the detection of aromatic amines with a low-cost and facile sensor or reagent is important and necessary. Although some reagents including fluorescamine,37 3-methyl-2-benzothiazolone hydrazone,38 2,6-diaminopyridine,39 and 2,6-dichloroquinone-4-chloroimide,40 and some detection methods including gas chromatography,41,42 liquid chromatography43,44 and mass spectrometry45,46 were described for the detection and determination of aromatic amines, some disadvantages such as high cost, unavailability, toxicity, and time-consuming are encountered with these reagents or methods. In this paper, we employ cinnamaldehyde (Scheme 1) as a reagent for the detection of aromatic amines, and the method has several merits as follows: 1) The reagent is cheap, commercially available and nontoxic, 2) the detection is simple, fast and colorimetric, and 3) the detection can be performed in real-time and online.
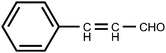 |
| | Scheme 1 Structure of reagent. | |
Experimental
Reagents and equipment
Both cinnamaldehyde and aromatic amines were purchased from chemical companies (Alfa Aesar, Acros Organics) without further purification. Solvent (dichloromethane, HPLC Grade), TLC plate (SiO2, 10–40 μm, pH = 6.2–6.8) and filter paper were purchased from Chinese Chemical Company. 1H NMR spectra were recorded at 400 MHz with tetramethylsilane (TMS) as an internal reference and CDCl3 as solvent. Mass spectra were recorded with Trio-20000 GC-MS spectrometer. UV absorption spectra were measured on an absorption spectrophotometer (Hitachi U-3010).
Procedure of spot test on TLC plate for selectivity
A drop (0.002 ml) of aromatic amine solution (0.1 M, dichloromethane) was added onto the TLC plate by capillary. After evaporation of solvent, a drop (0.002 ml, 50 μg) of cinnamaldehyde solution (0.1 M, dichloromethane) was added to the same place on the TLC plate (diameter: ϕ = 0.3 mm).
Procedure of spot test on TLC plate for sensitivity
To cinnamaldehyde (50 μg, 0.002 ml) on the TLC plate (diameter: ϕ = 0.3 mm) was added aromatic amines solution (0.1 M, dichloromethane) until a yellow color appeared.
Preparation of the yellow product for mechanism
To a solution of cinnamaldehyde (1.32 g, 0.01 mol) in dichloromethane (5 ml) was added a solution of p-toluidine (1.23 g, 0.01 mol) in dichloromethane (5 ml), the mixture was stirred at room temperature until no starting material was detected by TLC plate. After evaporation of the solvent, the crude product was purified by recrystallization from ethanol and a yellow solid was obtained. 1H NMR (CDCl3): δ = 8.33 (d, 1H, J = 8.4 Hz), 7.58 (d, 2H, J = 7.4 Hz), 7.45 − 7.36 (m, 4H), 7.23 − 7.20 (m, 5H), 2.4 (s, 3H). MS: m/z [M+]: 221.
Results and discussion
Selectivity of detection
The detection for aromatic amines is preformed on a TLC plate according to the procedure described in the Experimental Section, and results are presented in Table 1. It was found a yellow spot appeared immediately when cinnamaldehyde reagent was treated with aromatic primary amines on TLC plate. It is worth noting that only aromatic primary amines with electron-donating groups give positive results. No yellow color was detected using aromatic primary amines with electron-acceptor groups in the same condition. A negative result was also obtained when cinnamaldehyde reagent was treated with aromatic secondary amines, aromatic tertiary amines, and aliphatic amines. Besides, control experiment showed that no positive results were obtained when aromatic primary amines were replaced by other compounds such as phenol and thiophenol.
The detection of amines with cinnamaldehyde reagent in solution has also been explored. No color change was observed when colorless solution of cinnamaldehyde mixed with colorless solution of p-toluidine in dilute solution (1 × 10−2 M). Further investigation found that no new absorption band was detected in UV-Vis spectroscopy. As presented in Fig. 1, the absorption bands at 290 nm (ε = 5.1 × 103) and 236 nm (ε = 2.8 × 104) corresponded to p-toluidine, and the absorption bands at 220 nm (ε = 1.5 × 104) and 285 nm (ε = 2.8 × 104) attributed to cinnamaldehyde. Mixture of p-toluidine and cinnamaldehyde in solution produced the absorption bands around 220 nm, 236 nm and 285 nm, which were just the sum of the absorption bands above. The results suggested that no reaction occurred between cinnamaldehyde and p-toluidine in dilute solution. This is probably due to the fact that the collision between cinnamaldehyde molecules and p-toluidine molecules is small because of dilute solution, which resulted in a slow reaction between them. The suggestion above was confirmed by the following experiments: increase of concentration of mixture solution (1.0 M) or prolongation of the reaction time of dilute solution (more than 72 h). In both cases, it was found that a yellow color solution was observed and a new absorption at 330 nm was detected (Fig. 1 inset).
Sensitivity of detection
The sensitivity of aromatic primary amine detection is preformed according to the procedure described in the Experiment section, and the results are listed in Table 2. It was found that phenylenediamines are most active in the detection test and the detectable limit was less than 1.0 μg with 50 μg of cinnamaldehyde reagent on TLC plate. For other detected amines, detectable limits were between 2.0 μg and 6.0 μg with 50 μg of cinnamaldehyde reagent on TLC plate except aniline, whose detectable limit was 19.6 μg. The results indicated that the amines could be detected in the nanomolar range.
Table 2 Minimum amount of detected amines with 50 μg cinnamaldehyde reagent
It is worth noting that the detectable limits of amines can be regulated with a change in the amount of cinnamaldehyde reagent. It was found that the detectable limit of p-phenylenediamine was decreased from 0.1 μg to 0.04 μg when the amount of cinnamaldehyde reagent was increased from 50 μg to 130 μg and the lower detectable limit for p-phenylenediamine is 0.02 μg with cinnamaldehyde reagent (more than 150 μg) on TLC plate. The lower detectable limits for various primary aromatic amines on TLC plate with cinnamaldehyde reagent were listed in Table 3. It is necessary to emphasize that modification of the general procedure might be necessary to obtain optimum results for the detection of different aromatic primary amines.
Table 3 Lower detectable limit of amines on TLC plate
Competition experiments
Competition experiments are conducted in which the reagent is exposed to a mixed solution of amines. It was found that a yellow color spot appeared immediately when the solution of cinnamaldehyde was added to the TLC plate that was composed of p-phenylenediamine and some aliphatic amines including diethylamine, trimethylamine, and piperidine. A yellow spot was also obtained when the solution of cinnamaldehyde was added to the TLC plate composed of p-phenylenediamine and some aromatic amines including 4-nitroaniline, N,N-dimethylaniline and diphenylamine, and similar results were obtained when using other aromatic primary amines with electron donator groups. All results suggest that the detection for aromatic primary amines with electron doner on the TLC plate is anti-interference. Further investigation also showed that weak acid, base and inorganic salts had not significantly influenced the detection limit, and it implies that the detection may be performed in real-time and online.
Reaction mechanism
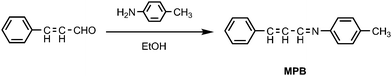
The reaction mechanism of cinnamaldehyde with amines is investigated. First, the product of the reaction of cinnamaldehyde and p-toluidine in solution was isolated and identified by 1H NMR and MS spectra, respectively. It was found that the typical signal around at 9.7 ppm arising from the proton of aldehyde disappeared and a new signal around at 8.3 ppm, corresponding to the proton of imine, appeared in the 1H NMR spectra. Furthermore, the mass spectra analysis of product showed the relative abundance of the molecular ion (m/z = 221) was 100%. All results indicated that the reaction of cinnamaldehyde with p-toluidine produced 4-methyl-N-(3-phenylallylidene) benzenamine (MPB), which is illustrated in Scheme 2. Second, the TLC test showed that a yellow spot appeared at the TLC plate with Rf 0.52 (elute: ethyl acetate/petroleum = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v) when solution of cinnamaldehyde mixed with the solution of p-toluidine on TLC plate, which was exactly the same as the result of MPB on the TLC plate. Third, comparing the absorption spectra of mixture (resulted from cinnamaldehyde with p-toluidine on TLC plate) with that of MPB in solution found that the profile of mixture was similar to that of MPB and there were two absorption bands appearing at 330 nm and 295 nm, respectively (Fig. 2). All indicated that treatment of cinnamaldehyde with p-toluidine on the TLC plate produced MPB. It is worth noting that the reaction, unlike in the solution, of cinnamaldehyde with amines on TLC plate could not be completed as the reaction occurred on the interface, therefore, the detection could not be performed quantitatively.
6, v/v) when solution of cinnamaldehyde mixed with the solution of p-toluidine on TLC plate, which was exactly the same as the result of MPB on the TLC plate. Third, comparing the absorption spectra of mixture (resulted from cinnamaldehyde with p-toluidine on TLC plate) with that of MPB in solution found that the profile of mixture was similar to that of MPB and there were two absorption bands appearing at 330 nm and 295 nm, respectively (Fig. 2). All indicated that treatment of cinnamaldehyde with p-toluidine on the TLC plate produced MPB. It is worth noting that the reaction, unlike in the solution, of cinnamaldehyde with amines on TLC plate could not be completed as the reaction occurred on the interface, therefore, the detection could not be performed quantitatively.
The mechanism is also applied to explain why amines with electron-donating groups give positive results and amines with electron-acceptor groups give negative results. With electron-donating substitutent groups, the electron cloud density of N atom of amines is increased, which results in activation of amine attack on the aldehyde group of cinnamaldehyde, and promotion of the condensation reaction between amines and cinnamaldehyde. While with electron-acceptor groups, the electron cloud density of N atom of amines is decreased, which results in weak or no reaction between amines and cinnamaldehyde, and a negative detection test is obtained in this case.
The study described above highlights the fact that the detection of primary aromatic amines can be performed on a TLC plate using cinnamaldehyde as reagent. This method is simple and fast. Moreover, the reagent is cheap, non-toxic, and commercial, which is are main advantages over other reagents. In addition, preliminary experiments shows that the reagent can also be used as a spray regent for the detection of primary aromatic amines on a TLC plate and further experiments are under way.
Conclusion
A new colorimetric detection method for aromatic primary amines has been developed. The method is advantageous, as the reagent is inexpensive, commercially available and non-toxic, the detection is sensitive and selective, and the procedure is simple and fast.
Acknowledgements
This work was supported by National Basic Research Program of China (2010CB934103) and the National Nature Science Foundation of China.
Notes and references
- R. Shunmugam, G. J. Gabriel, C. E. Smith, K. A. Amer and G. N. Tew, Chem.–Eur. J., 2008, 14, 3904 CrossRef CAS.
- J. Lee, J. W. Lee and Y. Chang, J. Comb. Chem., 2007, 9, 926 CrossRef CAS.
- E. Quinlan, S. E. Mattews and T. Gunnlaugsson, J. Org. Chem., 2007, 72, 7497 CrossRef.
- F. Han, Y. Bao, Z. Yang, T. M. Fyles, J. Zhao, X. Peng, J. Fan, Y. Wu and S. Sun, Chem.–Eur. J., 2007, 13, 2880 CrossRef CAS.
- E. Coronado, J. R. Galan-Mascaros, C. Marti-Gastaldo, E. Palomares, J. R. Durrant, R. Vilar, M. Gratzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2005, 127, 12351 CrossRef CAS.
- P. A. Jr PA, M. A. Palacios, K. Jursikova and M. Marquez, Org. Lett., 2005, 7, 5027 CrossRef.
- E. J. Cho, B. J. Ryu, Y. J. Lee and K. C. Nam, Org. Lett., 2005, 7, 2607 CrossRef CAS.
- C. Zhang and K. S. Suslick, J. Am. Chem. Soc., 2005, 127, 11548 CrossRef CAS.
- R. Rathore, S. H. Abdelwahed and I. A. Guzei, J. Am. Chem. Soc., 2004, 126, 13582 CrossRef CAS.
- S. C. McCleskey, M. J. Griffin, S. E. Schneider, J. T. McDevitt and E. V. Anslyn, J. Am. Chem. Soc., 2003, 125, 1114 CrossRef CAS.
- M. N. Stojanovic and D. W. Landry, J. Am. Chem. Soc., 2002, 124, 9678 CrossRef CAS.
- Y. K. Jung, T. W. Kim, J. Kim, J. M. Kim and H. G. Park, Adv. Funct. Mater., 2008, 18, 701 CrossRef CAS.
- M. D. Hall, H. R. Mellor, R. Callaghan and T. W. Hambley, J. Med. Chem., 2007, 50, 3403 CrossRef CAS.
- P. W. Elsinghorst, C. M. Gonzalez Tanarro and M. Gutschow, J. Med. Chem., 2006, 49, 7540 CrossRef CAS.
- P. Singh, A. M. Mhaka, S. B. Christensen, J. J. Gray, S. R. Denmeade and J. T. Isaacs, J. Med. Chem., 2005, 48, 3005 CrossRef CAS.
- S. Talukdar, R. J. Chen, C. T. Chen, L. C. Lo and J. M. Fang, J. Comb. Chem., 2001, 3, 341 CrossRef CAS.
- I. Roufos, S. Hays and R. D. Schwarz, J. Med. Chem., 1996, 39, 1514 CrossRef CAS.
- J. Hu, W. Wang, Z. Zhu, H. Chang, F. Pan and B. Lin, Environ. Sci. Technol., 2007, 41, 4806 CrossRef CAS.
- S. E. Duirk and Y. W. Collette, Environ. Sci. Technol., 2006, 40, 546 CrossRef CAS.
- P. Westerhoff, Y. Yoon, S. Snyder and E. Wert, Environ. Sci. Technol., 2005, 39, 6649 CrossRef CAS.
- D. A. Lambropoulou and T. A. Albanis, J. Agric. Food Chem., 2002, 50, 3359 CrossRef CAS.
- Y. Zhu, K. Yanagihara, F. Guo and Q. X. Li, J. Agric. Food Chem., 2000, 48, 4097 CrossRef CAS.
- R. Benigni, A. Giuliani, R. Franke and A. Gruska, Chem. Rev., 2000, 100, 3697 CrossRef CAS.
- H. Kanazawa, M. Higuchi and K. Yamamoto, Macromolecules, 2006, 39, 138 CrossRef CAS.
- G. S. Liou, S. H. Hsiao, W. C. Chen and H. J. Yen, Macromolecules, 2006, 39, 6036 CrossRef CAS.
- D. H. Wang, P. Mirau, B. Li, C. Y. Li, J. B. Baek and L. S. Tan, Chem. Mater., 2008, 20, 1502 CrossRef CAS.
- S. L. Burkett, N. Ko, N. D. Stern, J. A. Caissie and D. Sengupta, Chem. Mater., 2006, 18, 5137 CrossRef CAS.
- M. A. Abd El-Ghaffar, N. R. El-Halawany and H. A. Essawy, J. Appl. Polym. Sci., 2008, 108, 3225 CrossRef CAS.
- K. M. Gorlewska-Roberts, C. H. Teitel, J. O. Lay, D. W. Roberts and F. F. Kadlubar, Chem. Res. Toxicol., 2004, 17, 1659 CrossRef CAS.
- P. L. Skipper, L. J. Trudel, T. W. Kensler, J. D. Groopman, P. A. Egner, R. G. Liberman, G. N. Wogan and S. R. Tannembaum, Chem. Res. Toxicol., 2006, 19, 1086 CrossRef CAS.
- D. Nikolic and R. B. van Breemen, Chem. Res. Toxicol., 2001, 14, 351 CrossRef CAS.
- P. Pais, C. P. Salmon, M. G. Knize and J. S. Felton, J. Agric. Food Chem., 1999, 47, 1098 CrossRef CAS.
- M. Novak, L. Xu and R. A. Wolf, J. Am. Chem. Soc., 1998, 120, 1643 CrossRef CAS.
- C. Funk, P. Weber, J. Thilker, J. H. Grabber, H. Steinhart and M. Bunzel, J. Agric. Food Chem., 2006, 54, 1860 CrossRef CAS.
- H. Li and L. S. Lee, Environ. Sci. Technol., 1999, 33, 1864 CrossRef CAS.
- M. Fukushima, K. Tatsumi and K. Morimoto, Environ. Sci. Technol., 2000, 34, 2006 CrossRef CAS.
- E. Rinde and W. Troll, Anal. Chem., 1976, 48, 542 CrossRef CAS.
- E. Sawicki, T. W. Stanley, T. R. Hauser, W. Elbert and J. L. Noe, Anal. Chem., 1961, 33, 722 CrossRef CAS.
- L. J. Dombrowsk and E. L. Pratt, Anal. Chem., 1971, 43, 1042 CrossRef.
- J. H. Ross, Anal. Chem., 1968, 40, 2138 CrossRef CAS.
- B. E. Bowen, Anal. Chem., 1976, 48, 1584 CrossRef CAS.
- K. W. Sigvardson, J. M. Kennish and J. W. Birks, Anal. Chem., 1984, 56, 1096 CrossRef CAS.
- C. Funk, P. Weber, J. Thilker, J. H. Grabber, H. Steinhart and M. Bunzel, J. Agric. Food Chem., 2006, 54, 1860 CrossRef CAS.
- L. S. Debruin, J. B. Pawliszyn and P. D. Josephy, Chem. Res. Toxicol., 1999, 12, 78 CrossRef CAS.
- M. Rampfl, M. Stefan, F. Mayer, K. Sedlbauer, K. Breuer and R. Niessner, Environ. Sci. Technol., 2008, 42, 5217 CrossRef CAS.
- S. Yang, J. Han, Y. Huan, Y. Cui, X. Zhang, H. Chen and H. Gu, Anal. Chem., 2009, 81, 6070 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v) when solution of cinnamaldehyde mixed with the solution of p-toluidine on TLC plate, which was exactly the same as the result of MPB on the TLC plate. Third, comparing the absorption spectra of mixture (resulted from cinnamaldehyde with p-toluidine on TLC plate) with that of MPB in solution found that the profile of mixture was similar to that of MPB and there were two absorption bands appearing at 330 nm and 295 nm, respectively (Fig. 2). All indicated that treatment of cinnamaldehyde with p-toluidine on the TLC plate produced MPB. It is worth noting that the reaction, unlike in the solution, of cinnamaldehyde with amines on TLC plate could not be completed as the reaction occurred on the interface, therefore, the detection could not be performed quantitatively.
6, v/v) when solution of cinnamaldehyde mixed with the solution of p-toluidine on TLC plate, which was exactly the same as the result of MPB on the TLC plate. Third, comparing the absorption spectra of mixture (resulted from cinnamaldehyde with p-toluidine on TLC plate) with that of MPB in solution found that the profile of mixture was similar to that of MPB and there were two absorption bands appearing at 330 nm and 295 nm, respectively (Fig. 2). All indicated that treatment of cinnamaldehyde with p-toluidine on the TLC plate produced MPB. It is worth noting that the reaction, unlike in the solution, of cinnamaldehyde with amines on TLC plate could not be completed as the reaction occurred on the interface, therefore, the detection could not be performed quantitatively.