Chemiluminescence based technique for the detection of methyl parathion in water and fruit beverages
Raghuraj Singh
Chouhan
,
Aaydha Chidambara
Vinayaka
and
Munna Singh
Thakur
*
Fermentation Technology and Bioengineering Department, Central Food Technological Research Institute (A constituent laboratory of Council of Scientific and Industrial Research, New Delhi), Mysore, 570020, India. E-mail: msthakur@cftri.res.in; msthakur@yahoo.com; Fax: +91-821-2517233; Tel: +91-821-2515792
First published on 13th May 2010
Abstract
Organophosphorus (OP) pesticides, being used extensively in agricultural practices, are highly toxic even at trace levels and their detection with high sensitivity is a challenging task. The present study addresses the development of a chemiluminescence (CL) based flow injection technique for the sensitive detection of methyl parathion (MP). Different fruit samples were spiked with MP and extracted according to the AOAC method. A mean recovery of 84.3%–103.1% was obtained for MP spiked fruit juice samples with 0.18%–9.29% reproducibility (RSD). The assay specificity was attributed to the use of highly specific immunological reactions. Competitive binding was monitored between free MP and MP-Horseradish Peroxidase (MP-HRP) with immobilized anti-MP IgY antibodies in an immunoreactor column. The unbound MP-HRP conjugate eluted out of the column as a non retained component and was reacted with urea-H2O2 (U–H2O2) and luminol. The photons generated during the biochemical interaction were determined by a photomultiplier tube (PMT) based detector, and were found to be directly proportional to the MP concentration. In the present investigation, IgY has proved advantageous over the IgG class of immunoglobulins in terms of yield, stability, cost effectiveness and enhancement of assay sensitivity. Using the proposed CL method, MP was detected in samples and showed linearity in the range of 0.001–500 ng mL−1 with a limit of quantification (LOQ) of 0.005 ng mL−1 and with a limit of detection (LOD) of 0.001 ng mL−1. The current findings show that this method can provide valuable information for the estimation of the pesticide traces in water and fruit beverages.
Introduction
Methyl parathion (MP) is used in mango, grape and apple plantations to control pests and insects,1–3 there is every possibility that it passes in to the processed beverages. The exposure to high levels of MP can potentially generate consequent toxicological risks such as itching, skin irritation, stomach cramps, epigastria, abdominal pain, chest tightness, productive cough, dyspnea and basal crepitation of both lungs. It can also cause certain hematological diseases such as significant decrease in hemoglobin count, decreased size of red blood cells (RBCs), and impairment in heme biosynthesis.3 MP has also contributed greatly to increase the agricultural productivity and it has also created a negative impact on human health, environment and ecological balance.4 These pesticides are hazardous in very low concentrations and caused immense damage to the ecosystem. The high persistence level of pesticides in the environment leads to their accumulation in soil, water and food,5 thereby causing severe health hazards. The permissible pesticide residue levels in food, water and environment have been made more stringent in the recent years by regulatory bodies all over the world.Conventional methods reported for the detection of MP such as HPLC, GC, spectrophotometry and ELISA6–9 are not sensitive at picogram levels, laborious, required skilled analysts and expensive instrumentation. Wherein, biosensors are alternatives that can be adopted for broad practical applications due to their specificity, rapidity, ease for mass fabrication and field applicability. Several biosensors techniques for organophosphorus pesticides (OP), based on enzyme inhibition or antibody-antigen reaction have been reported.10–14 An amperometric enzyme electrode was reported for the rapid determination of OP pesticides using ascorbic acid oxidase immobilized on the surface of the electrode.15 During the last two decades, a variety of assays for pesticides have been reported based on electrochemical,16 and optical detection,17 which however, have significant limitations. Moreover, immunosensor techniques reported for the detection of pesticides in combination with fluorescence, surface plasmon resonance (SPR), amperometric and thermometric detection18,19 have a narrow detection range. However the use of hen egg yolk immunoglobulin (IgY) has recently been considered more advantageous for immunoassays, ELISA, Western blot, immuno-staining, and immunoprecipitation applications, because of the ease with which these antibodies can be produced as well as the relative low cost and high yield. Yields in excess of 100 mg IgY/yolk have been reported.20–22 Alternatively, CL based immunoassay methods are known to be highly sensitive23 and also it depends upon the light signals generated by the biochemical reaction between horseradish peroxidase (HRP), luminol and U–H2O2. Hence this method has become an attractive analytical tool in clinical diagnosis and related applications.24,25 CL techniques exhibit high detection sensitivity and do not require an excitation source as reaction itself produces light and also do not require monochromators (often not even a filter).
The present study focus on the development of a highly sensitive CL method for the detection of MP in water and fruit beverages using a PMT based detector system.
Experimental
Reagents
HRP, luminol, urea-H2O2 (urea used as a stabilizer), bovine serum albumin (BSA), sodium cyanoborohydride, Freund's complete adjuvant (FCA), and Freund's incomplete adjuvant (FICA) were procured from Sigma chemicals, USA. Methyl parathion (analytical grade, 98% pure) was procured from Fluka, Germany. Sepharose CL-4B used for the immobilization of anti-MP IgY antibodies were supplied by Amersham Pharmacia Biotech, Sweden. All other reagents used in the present study were of analytical grade and procured from different Indian companies and used without further purification. The antibodies (anti-MP IgY) employed in the present investigation were raised in chicken. Rabbit anti-chicken IgY secondary antibody labeled with Alkaline Phosphatase (ALP) was procured from Bangalore Genie Ltd, Bangalore, India. Amicon Bioseparation centrifugal filter devices used for the purification of antibody and bioconjugates having molecular cutoff (MWCO) 30,000 Da, was procured from Millipore Corporation, Bedford, MA 01730, USA. Immuno plates used in the present study were supplied by Nunc, Denmark.A stock solution of 10 mM luminol was prepared by dissolving 17.5 mg of luminol solid in 1.5 mL of 0.1 M NaOH and the volume was made up to 10 mL in 0.2 M Tris buffer 10 mM urea-H2O2 stock solution prepared in distilled water. MP-HRP dilutions were made in PBS-BSA (0.1%). Stock solution of MP was prepared by dissolving 1 mg of MP in 1 mL of methanol. Fruit beverages were purchased from local market.
Apparatus
The flow injection apparatus used in this study for the determination of MP is shown in Fig. 1. Peristaltic pumps (Alitea, Sweden) were used to deliver all solutions. Silicon tubing (0.2 mm i.d.) was used to connect all components in the flow system. Luminol, U–H2O2 and MP-HRP were mixed in the reaction chamber. The CL signals generated during the reaction were monitored using PMT based luminometer (Luminoskan, TLPLUS, Finland), and the signals obtained were further converted into readable format by Hexa-terminal software. ELISA based analysis were carried out in VERSA max tunable micro-plate reader, (Molecular devices, USA). | ||
| Fig. 1 Schematic diagram of the flow system for determination of MP. | ||
Sample preparation for analysis
Apple, mango and grape beverages were brought from a local market; no pesticide residues were detected by HPLC analysis. For the spiking study, known concentrations of MP were added to water, apple, grape and mango fruit beverages. The preparations were mixed thoroughly and incubated at room temperature for 12 h. The spiked MP was extracted from these preparations using dichloromethane (DCM) as per standard AOAC method.26 The extraction step was repeated for 4–6 times and the combined DCM extracts passed over sodium sulfate to remove moisture and solvent was evaporated under reduced pressure. The resultant residue was then re-dissolved in acetonitrile for further analysis.Synthesis of hapten and production of anti-MP IgY antibodies
Both MP-BSA and MP-HRP conjugates were synthesized as reported in our earlier publication with slight modification in the methodology.23 Briefly, O-(4-aminophenyl)-O,O-dimethyl thiophosphate were dissolved in dry dioxane in the presence of succinic anhydride and refluxed for 4 h at 80 °C under inert atmosphere. Prepared MP-hapten (20 mg) was dissolved in 200 μL of dimethyl formamide (DMF). 100 μL of this preparation was mixed with 5 mg of BSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) dissolved in 200 μL of phosphate buffer saline (PBS, 50 mM, and pH 7.4) and another 100 μl of MP hapten was mixed with 20 mg of HRP (1
0.5) dissolved in 200 μL of phosphate buffer saline (PBS, 50 mM, and pH 7.4) and another 100 μl of MP hapten was mixed with 20 mg of HRP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) dissolved in 200 μL of PBS respectively and the total volume was made up to 1 mL with PBS. The preparations were incubated for 2 h at room temperature (RT) under mild agitation in the presence of N-hydroxysuccinimide (NHS) and 1 ethyl 3(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC).
2) dissolved in 200 μL of PBS respectively and the total volume was made up to 1 mL with PBS. The preparations were incubated for 2 h at room temperature (RT) under mild agitation in the presence of N-hydroxysuccinimide (NHS) and 1 ethyl 3(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC).
Isolation and purification of anti-MP IgY antibodies
White Leghorn hens were immunized subcutaneously with MP-BSA. The initial dose of the hapten (1 mg/Kg body weight) was administrated using FCA. After a period of 21 days, booster doses were given with flow injection analysis at the concentration of 0.5 mg kg−1 body weight at time intervals of 2, 8 and 13 weeks. The eggs were collected and processed by water dilution precipitation method to isolate anti-MP IgY antibodies according to the standard protocol.22 The titer development was monitored after each booster immunization and isolated anti-MP IgY antibodies were further purified by passing through sepharose-BSA affinity column to remove any anti-BSA antibodies that would have been produced as a result of immune response to the BSA portion of the hapten.Immobilization of anti-MP IgY antibodies
Purified anti-MP IgY antibodies were immobilized on sepharose CL-4B matrix by the periodate activation method.27 Different concentrations of antibodies (5, 10 and 20 μg mL−1 of sepharose) were immobilized on activated sepharose separately in a micro-immunoreactor. Each concentration of immobilized antibodies was observed for maximum binding with MP-HRP conjugate in the micro-immunoreactor. The concentration of MP-HRP conjugate passed through the column was kept constant (25 μL from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilution of 1 mg mL−1 stock). Anti-MP IgY antibodies were incubated with 1 mL of activated sepharose at 4 °C for two hours with intermittent shaking. The resultant Schiff base products were reduced using sodium cyanoborohydride. The immobilized antibodies were then thoroughly washed with distilled water followed by PBS and stored in 0.01% sodium azide at 4 °C until further use. Efficient binding of antigen (MP) or conjugate (MP-HRP) with immobilized anti-MP IgY antibodies in the micro-immunoreactor requires optimal residence time. Therefore, experiments were conducted to optimize flow rate of conjugate by adjusting flow of the peristaltic pump. The reagent flow rate through the column using a peristaltic pump was varied between 25, 50 and 55 μl min−1. A flow rate of 50 μL min−1 was selected for further experiments.
5000 dilution of 1 mg mL−1 stock). Anti-MP IgY antibodies were incubated with 1 mL of activated sepharose at 4 °C for two hours with intermittent shaking. The resultant Schiff base products were reduced using sodium cyanoborohydride. The immobilized antibodies were then thoroughly washed with distilled water followed by PBS and stored in 0.01% sodium azide at 4 °C until further use. Efficient binding of antigen (MP) or conjugate (MP-HRP) with immobilized anti-MP IgY antibodies in the micro-immunoreactor requires optimal residence time. Therefore, experiments were conducted to optimize flow rate of conjugate by adjusting flow of the peristaltic pump. The reagent flow rate through the column using a peristaltic pump was varied between 25, 50 and 55 μl min−1. A flow rate of 50 μL min−1 was selected for further experiments.
CL based FIA
Anti-MP IgY at the optimized concentrations were immobilized and packed into a micro-immunoreactor of 3 cm height and internal diameter of 0.2 cm (glass capillary column). The column was equilibrated with 2 mL of PBS at a flow rate of 50 μL min−1 using a peristaltic pump. The MP samples obtained from water and fruit beverages were passed through the micro-immunoreactor sequentially. Again the column was washed with PBS. MP-HRP conjugate 25 μL was passed through the column and excess conjugate coming out of the column as a result of competitive binding was collected as a non-retained effluent in the flow through fraction. These fractions were reacted with 0.1 mM U–H2O2 and 1 mM luminol to produce photons in a PMT based detector system and signals generated were recorded in terms of CL units (CLU).Indirect competitive (IC) ELISA for the determination of IC50 of spiked MP samples
IC-ELISA was performed to determine the IC50 of spiked MP samples.28 ELISA plate was coated with 100 μl of MP-HRP conjugate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilutions from 1 mg mL−1 stock) in carbonate buffer (pH-9.6) overnight at 4 °C. The plate was washed thoroughly with 0.05% PBS-Tween 20 (PBST) followed by blocking with 1% BSA in PBS. After washing, 100 μL of anti-MP IgY (0.5 μg/well) along with free MP at the range of 1000–0.1 ng/well was added in the wells and incubated for 2 h at 37 °C. The plate was washed as described above and further incubated with 100 μL of rabbit anti-chicken IgY secondary antibody (1
2000 dilutions from 1 mg mL−1 stock) in carbonate buffer (pH-9.6) overnight at 4 °C. The plate was washed thoroughly with 0.05% PBS-Tween 20 (PBST) followed by blocking with 1% BSA in PBS. After washing, 100 μL of anti-MP IgY (0.5 μg/well) along with free MP at the range of 1000–0.1 ng/well was added in the wells and incubated for 2 h at 37 °C. The plate was washed as described above and further incubated with 100 μL of rabbit anti-chicken IgY secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10000) conjugated to alkaline phosphatase (ALP) at 37 °C. The plates were washed followed by addition of 100 μL of ALP substrate solution (1 mg mL−1 of p-nitrophenylphosphate) to each well. The absorbance at 405 nm was recorded using a tunable micro plate reader after 30 min of incubation at 37 °C.
10000) conjugated to alkaline phosphatase (ALP) at 37 °C. The plates were washed followed by addition of 100 μL of ALP substrate solution (1 mg mL−1 of p-nitrophenylphosphate) to each well. The absorbance at 405 nm was recorded using a tunable micro plate reader after 30 min of incubation at 37 °C.
Results and discussion
Synthesis of hapten, immunization and antibody (IgY) generation
The hapten was synthesized from aminophenol to obtain O-4-aminophenyl O,O-dimethyl phosphorothioate followed by its reaction with succinic anhydride in dry dioxane to provide a spacer arm for MP which has a free –COOH group to facilitate of its conjugation with BSA.The MP-IgY antibodies produced in white leghorn hen eggs were tested for both protein and antibody titer. Protein level increased with booster injections. Anti-MP IgY was extracted from egg yolk by water dilution method, which was preferred over other extraction procedures in order to get high yield and pure IgY antibodies with high titer values. The extracted anti-MP IgY antibodies were purified by BSA affinity column to remove any anti-BSA antibodies that would have generated as a result of immunization with MP-BSA. Indirect non-competitive ELISA was tested on isolated antibodies for better titer values and it was observed that antibodies showed higher titer values after third and fourth booster doses. After purification, native-PAGE and indirect competitive ELISA showed the antibodies to be highly purified and became more specific and sensitive after affinity purification through the BSA column.
Optimization of anti-MP IgY antibodies for immobilization
Immobilization of anti-MP IgY antibodies was found to be a very critical factor as the technique relies on specific interactions between immobilized antibodies and MP pesticide in a flow through micro-immunoreactor. The concentration of antibodies for immobilization was optimized based on the MP-HRP conjugate (25 μL from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilution of 1 mg mL−1 concentration) to be passed through the micro-immunoreactor. Conjugate concentration was fixed based on the generation of photons in presence of 0.1 mM U–H2O2 and 1 mM luminol at optimized concentrations.
5000 dilution of 1 mg mL−1 concentration) to be passed through the micro-immunoreactor. Conjugate concentration was fixed based on the generation of photons in presence of 0.1 mM U–H2O2 and 1 mM luminol at optimized concentrations.
Different concentrations of antibodies (5, 10, 20 μg mL−1) were immobilized sequentially and optimized by passing known amount of MP-HRP conjugate through the antibody immobilized micro-immunoreactor. The percentage binding of MP-HRP was monitored based on the biochemical reactions driven by excess MP-HRP conjugate that came out of the micro-immunoreactor column. The antibody concentration for immobilization was optimized keeping MP-HRP conjugate constant (25 μL from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilution of 1 mg mL−1 concentration). At concentrations lower than 5 μg mL−1, more unbound MP-HRP came out of the column and maximum light production was observed. With concentration higher than 20 μg mL−1 of antibody, the maximum binding of conjugate and minimum light production (CLU) was observed for the conjugate coming out of the column. Therefore, 20 μg mL−1 of antibodies was selected as the optimum concentration for binding, and this condition was used in all further chemiluminescence studies as shown in Fig. 2.
5000 dilution of 1 mg mL−1 concentration). At concentrations lower than 5 μg mL−1, more unbound MP-HRP came out of the column and maximum light production was observed. With concentration higher than 20 μg mL−1 of antibody, the maximum binding of conjugate and minimum light production (CLU) was observed for the conjugate coming out of the column. Therefore, 20 μg mL−1 of antibodies was selected as the optimum concentration for binding, and this condition was used in all further chemiluminescence studies as shown in Fig. 2.
 | ||
| Fig. 2 Optimization of anti-MP IgY antibodies concentration for immobilization in micro-immunoreactor column. (●) 5 μg mL−1 (▲) 10 μg mL−1 (■) 20 μg mL−1. | ||
Detection of MP by CL based FIA
In the present investigation, the previously mentioned CL technique was applied for the sensitive detection of simulated real samples by spiking different concentrations of MP in water and fruit beverages. Competitive binding between the extracted MP and a known concentration of MP-HRP conjugate for immobilized anti-MP IgY antibody was used to measure the concentration of extracted MP from spiked samples. As the concentration of immobilized antibody remains constant, variation in the concentration of extracted MP passed through antibody immobilized immunoreactor column will affect the binding of MP-HRP conjugate. Thus, the elution of unbound MP-HRP conjugate from immunoreactor column depends on free MP concentration as the binding of MP-HRP conjugate was depended on the left over antibody after the binding of extracted MP. Higher the extracted MP passed lower will be the binding of MP-HRP conjugate and the conjugate coming out of the column will be more. Therefore, the unbound MP-HRP conjugate gives maximum signals in the Luminometer. Hence the signal obtained from luminometer has been correlated to the concentration of MP as shown in Fig. 3.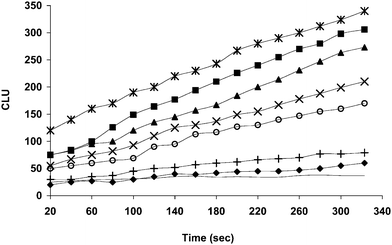 | ||
Fig. 3 CL response graph obtained for different concentrations of MP extracted from water. ( ) 1 ng mL−1 (■) 0.5 ng mL−1 (▲) 0.250 ng mL−1 (×) 0.1 ng mL−1 (○) 0.050 ng mL−1 (+) 0.010 ng mL−1 (◆) 0.005 ng mL−1 (-) Control. ) 1 ng mL−1 (■) 0.5 ng mL−1 (▲) 0.250 ng mL−1 (×) 0.1 ng mL−1 (○) 0.050 ng mL−1 (+) 0.010 ng mL−1 (◆) 0.005 ng mL−1 (-) Control. | ||
Calibration curves and detection limits
Calibration curves for MP were obtained under optimized conditions by plotting the graph of ΔI (relative CL intensity) vs. concentration. Here, ΔI is the measure of CL response over time (sec), obtained as a result of the biochemical reaction to generate photons by oxidation of luminol catalysed by the unbound MP-HRP eluted out of the immunoreactor column. The measurable range of MP concentration was of 0.001–500 ng mL−1 using a regression equation for MP in water, ΔI = 40.487 ln(C) − 22.045 (C, ng mL−1; r = 0.9943). A series of experiments were carried out in quadruplicate for each concentration, with LOQ at 0.005 ng mL−1 for fruit beverages. The CL detection of MP in different fruit beverages showed a LOQ 0.005 ng mL−1. The LOQ is slightly higher for fruit beverages compared to water. The recovery was not satisfactory at concentrations lower than 0.005 ng mL−1 for fruit beverages suggesting the possibility of matrix effect. Hence the LOQ was 0.005 ng mL−1. The regression equations for MP in mango, apple and grape beverages are, ΔI = 37.25 ln(C) − 29.50 (C, ng mL−1; r = 0.99), ΔI = 44.887 ln(C) − 43.42 (C, ng mL−1; r = 0.9927) and ΔI = 37 ln(C) − 29.9 (C, ng mL−1; r = 0.99) respectively.Analysis of recoveries for spiked MP in water and fruit beverages
The fruit beverages samples such as apple, grape and mango along with water were tested and found to be free of MP contamination by HPLC analysis. These samples were later spiked with different concentrations of MP. Extraction of these samples showed MP recoveries in the range 84.3–103.1% (RSD of 0.22–7.03%, Table 1) followed by analysis of different concentrations of spiked MP (ng mL−1) represented by positive and negative symbols shows the possibility of detection (+), non possibility of detection (—) and may or may not be detected (±) by CL method and ELISA technique. Indirect competitive ELISA was used as a reference method for comparison with the CL method in terms of sensitivity and specificity. The IC50 values obtained for different spiked samples were 53 ng mL−1 for water, 62 ng mL−1 for apple juice, 67 ng mL−1 for grape juice and 72.37 ng mL−1 for mango juice as shown in Fig. 4.| Samples | Spiking level/ng mL−1 | Recovery (n = 4), (%) | CV (%) | CL Method | ELISA Method |
|---|---|---|---|---|---|
| Water | 500 | 99.7 ± 0.2 | 0.01 | + | + |
| 50 | 98.4 ± 0.8 | 0.01 | + | + | |
| 0.5 | 103.1 ± 4.6 | 1 | + | ± | |
| 0.05 | 98.9 ± 1.3 | 0.01 | + | — | |
| 0.005 | 99.1 ± 1.9 | 0.01 | + | — | |
| 0.001 | 99.24 ± 1.8 | 0.01 | + | — | |
| Apple beverage | 500 | 97.5 ± 2.5 | 0.73 | + | + |
| 50 | 99.6 ± 0.3 | 0.01 | + | + | |
| 0.5 | 99.6 ± 1.2 | 0.02 | + | — | |
| 0.05 | 99.3 ± 1.2 | 0.02 | + | — | |
| 0.005 | 89.6 ± 9.2 | 0.01 | + | — | |
| 0.001 | 70.3 ± 8.7 | 0.02 | + | — | |
| Grape beverage | 500 | 97.5 ± 2.4 | 0.01 | + | + |
| 50 | 97.9 ± 1.9 | 0.01 | + | + | |
| 0.5 | 99.5 ± 1.5 | 0.02 | + | — | |
| 0.05 | 99.0 ± 1.9 | 0.01 | + | — | |
| 0.005 | 99.0 ± 1.3 | 0.01 | + | — | |
| 0.001 | 67.2 ± 7.9 | 0.04 | + | — | |
| Mango beverage | 500 | 84.3 ± 7.0 | 0.07 | + | + |
| 50 | 98.6 ± 1.3 | 0.02 | + | + | |
| 0.5 | 99.5 ± 0.8 | 0.01 | + | — | |
| 0.05 | 99.0 ± 2.5 | 0.01 | + | — | |
| 0.005 | 89.2 ± 9.1 | 0.01 | + | — | |
| 0.001 | 59.2 ± 5.6 | 0.05 | + | — |
 | ||
| Fig. 4 Indirect competitive ELISA of methyl parathion spiked in different beverages to determined the IC50. (■) Water (▲) Apple juice (●) Mango juice (◆) Grape juice. | ||
The recoveries of MP spiked in different fruit beverages, determined by the ELISA and CL methods, are presented in Table 1. The recovery values were satisfactory ranging between 84% and 103%. The results clearly indicate that there is no significant matrix effect in the CL analysis of MP in fruit beverages in the range 0.005–500 ng mL−1. This method is on comparable with the existing protocols with respect to its accuracy and credibility.
Conclusions
Quantitative determination of MP by CL method using a micro-immunoreactor column showed a high sensitivity level with LOQ of 0.005 ng mL−1 for the samples. This technique offers several advantages over standard HPLC and ELISA methods as it is; rapid, reliable, specific based on antigen-antibody interactions and highly efficient photon generation which can detect the pesticide at trace level. Application of IgY in present study is advantageous compared to IgG in terms of its stability, high antibody production capacity and cost effectiveness. The present method is quite promising and has the potential application for different pesticides and toxins.Acknowledgements
R. S. Chouhan and A. C. Vinayaka are thankful to Council of Scientific and Industrial Research for providing Senior Research Fellowship. Financial support from Department of Biotechnology, India and Swiss Development Cooperation, Switzerland for the investigation under an Indo-Swiss Biotechnology collaboration project (BR 2.2) is gratefully acknowledged. The authors thank the Director, CFTRI, Mysore, for providing facilities and Dr N. G. Karanth, Former Deputy Director, CFTRI, Mysore for his valuable suggestions.References
- C. J. Pappas, N. B. Kyriakidis and P. E. Athanasopoulos, J. AOAC, 1999, 82, 359 CAS.
- J. Oliva, A. Barba, N. Vela, F. Melendreras and S. Navarro, J. Chromatogr., A, 2000, 882, 213 CrossRef CAS.
- S. K. Rastogi, V. K. Singh, C. N. Kesavachandran, Jyoti, M. K. J. Siddiqui, N. Mathur and Bharti, Indian J. Occup. Environ. Med, 2008, 12, 29 Search PubMed.
- US EPA, 2000, Summary of the risks and uses of organophosphate Methylparathion, Available: http://www.epa.gov./pesticides/opmethyl_parathion/methylsum.htm Search PubMed.
- M. S. Thakur and N. G. Karanth, Advances in biosensors: ed. B. D. Malhotra and A. P. F. Turner, 2003, Elsevier, Amsterdam, 5, 131 Search PubMed.
- K. Sasaki, K. Suzuki and Y. Sito, J. AOAC, 1987, 70, 460 CAS.
- M. Fernandez, Y. Pico, S. Girotti and J. Manes, J. Agric. Food Chem., 2001, 49, 3540 CrossRef CAS.
- S. J. Lehotay, J. AOAC, 2002, 83, 680.
- J. H. Skerritt, S. L. Guihot, M. B. Asha, B. E. A. Rani and N. G. K. Karanth, Food Agric. Immunol., 2003, 151, 1 CrossRef.
- P. Moris, I. Alexander, M. Roger and J. Remacle, Anal. Chim. Acta, 1995, 302, 53 CrossRef CAS.
- M. D. Gouda, M. S. Thakur and N. G. Karanth, Biotechnol. Tech., 1997, 11, 653 CrossRef CAS.
- P. Mulchandani, A. Mulchandani, I. Kaneva and W. Chen, Biosens. Bioelectron., 1999, 14, 77 CrossRef CAS.
- K. Rekha, M. S. Thakur and N. G. Karanth, Crit. Rev. Biotechnol., 2000, 20, 213 CrossRef CAS.
- K. Ramanathan, A. Dzgoev, J. Svitel, J. B. Rees and B. Danielsson, Biosens. Bioelectron., 2002, 17, 283 CrossRef CAS.
- K. Rekha, M. D. Gouda, M. S. Thakur and N. G. Karanth, Biosens. Bioelectron., 2000, 15, 499 CrossRef CAS.
- S. Kroger, S. J. Setford and A. P. F. Turner, Anal. Chem., 1998, 70, 5047 CrossRef CAS.
- T. E. Plowman, J. D. Durstchi, H. K. Wang, D. A. Christensen, J. N. Derron and W. N. Reichert, Anal. Chem., 1999, 71, 4344 CrossRef CAS.
- P. Skladal, A. Deng and V. Kolar, Anal. Chim. Acta, 1999, 399, 29 CrossRef CAS.
- H. Schmid, M. S. Thakur, K. R. Thampi and C. R. Suri, Sens. Actuators, B, 2006, 113, 297 CrossRef.
- E. M. Akita and S. Nakai, J. Food Sci., 1992, 57, 629 CrossRef CAS.
- E. M. Akita and S. Nakai, J. Immunol. Methods, 1993, 160, 207 CrossRef CAS.
- E. M. Akita and E. C. Y. Li-Chan, J. Dairy Sci., 1998, 81, 54 CrossRef CAS.
- R. S. Chouhan, K. V. Babu, M. A. Kumar, N. S. Neeta, M. S. Thakur, B. E. A. Rani, P. Akmal, N. G. K. Karanth and N. G. Karanth, Biosens. Bioelectron., 2006, 21, 1264 CrossRef CAS.
- L. Rose and T. D. Waite, Anal. Chem., 2001, 73, 5909 CrossRef CAS.
- J. Wang, C. Zhang, H. Wang, F. Yang and X. Zhang, Talanta, 2001, 54, 1185 CrossRef CAS.
- AOAC international, Official methods of analysis, 1995, 18th ed. Section 970, 52, Arlington, VA Search PubMed.
- G. T. Hermanson, A. K. Mallia and P. K. Smith, 1992. Academic press, San Diego, 69.
- Y. Kolosova, H. J. Park, S. A. Eremin, S. J. Park, S. J. Kang, W. B. Sim, Y. T. Lee and D. H. Chung, Anal. Chimica. Acta, 2004, 511, 323 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
