Simultaneous determination of photometric accuracy during circular dichroism measurements
Ali A.
Dahab
*,
Dhia
El-Hag
and
Alex F.
Drake
Department of Pharmacy, King's College London, Franklin-Wilkins Building, 150 Stamford Street, London, UK SE1 9NH
First published on 27th May 2010
Abstract
Photometric accuracy is determined by the difference between the measured absorbance and the established standard value. Most quantitation applications using UV-Vis would involve the measurement of the standards and samples of comparable concentrations in rapid succession on the same instrument. Photometric accuracy is critical for measurement; it compares the extinction coefficients between different instruments, for any photometric inaccuracy will lead to errors in quantitation. In Circular Dichroism (CD) measurements it is important and often a requirement to also monitor the absorbance of the sample. However, it is common to make separate measurements to determine the absorbance which is less accurate and time consuming. This study shows that the simultaneous measurement of ordinary UV/vis absorbance spectra from the photomultiplier high voltage output during a circular dichroism measurement is accurate and reliable. The photometric accuracy and linearity of various spectrometers is determined using standard solutions of potassium dichromate. The mathematical treatment of the signal representing the high voltage applied to the detector is presented here. An ordinary UV-vis spectrophotometer was used to measure the direct UV absorbance for comparison and to assign a reference value (Z-Value) to each CD spectrometer. The results proved to be accurate and reliable.
1 Introduction
Molecules of biological interest, including drugs, are often chiral; absolute configuration and enantiomeric purity are important issues that can be addressed by optical activity measurement. Optical activity measurements, circular dichroism (CD) in particular, offer a convenient way of monitoring absolute stereochemistry and shape in solution at relatively low concentration, particularly with respect to solution variables such as temperature, concentration, pH, and additives. Measurements of absorption alongside CD measurements are important and often necessary. Drake et al.,1 have discussed the design of a polarisation modulation spectrometer extensively. Simultaneous monitoring of absorption and CD on-line during chromatography with a single flow cell enabled the calculation of the dissymmetry factor (CD/absorbance) as a measure of enantiomeric purity.A polarisation modulation spectrometer (CD spectrometer, spectropolarimeter) is capable2 of simultaneously monitoring light absorption, polarised spectroscopy including optical activity, turbidity, light scattering and fluorescence (Fig. 1).
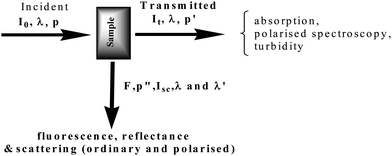 | ||
| Fig. 1 Parameters measured in a multimode optical spectrometer. | ||
CD gives a signal only in the vicinity of an optically active absorption band. Based on Beer's law, UV-vis absorption measurements normally provide the most convenient means of determining concentration often simply as a monitor for techniques such as chromatography, kinetics and titrations. Ordinary UV-vis absorption spectroscopy, although capable of identifying the chromophore in a molecular species, is used mainly to determine the existence/concentration of a molecular species. Circular Dichroism spectra are more generally analysed for molecular structure information, conformation and configuration.
In this study, only the simultaneous absorption measurements are made using potassium dichromate as a standard reference material with known values as assigned by British Pharmacopeia to assess the accuracy and reliability of the method as well as the photometric accuracy and linearity of the spectrometers. Potassium dichromate is a well established liquid reference material used in determining absorbance scale and linearity in the UV-vis region (with a usable range from 235 nm to 350 nm) as a measure of the photometric accuracy of spectrophotometers. The efficacy of potassium dichromate as a solution standard is well established.3,4 Potassium dichromate standards for this purpose are supplied in permanently heat sealed UV quartz cells accompanied by an accredited certificate of calibration. Potassium dichromate shows a spectrum with characteristic peak maxima at 257 nm and 350 nm, and minima at 235 nm and 313 nm. In this study, 5 sealed standards at different concentrations supplied by the national institute of standards in Germany, and 3 standards at the same concentration and different pathlengths (prepared at King's college London according to British Pharmacopeia recommendations) were measured. The simultaneous measurement of absorption during CD measurement proved to be a reliable method in determining photometric accuracy and linearity of spectrometers.
2 Theory
2.1 Relationship between photomultiplier high voltage and absorbance in the CD spectrometer
The incident radiation and the transmitted radiation after interacting with a sample are expressed as Io and It, respectively. The interaction of light with the photomultiplier (PM) cathode results in an output signal in the form of a current. The current resulting from the overall light intensity is expressed as iDC and the current that results from the differential transmitted intensity as iAC. This current will be amplified by the photomultiplier dynode chain and treated as a signal voltage after a pre-amplifier stage. Light incident on the sample with intensity Io will be transmitted as intensity It associated with a current from the photocathode of the photomultiplier which is designated here as it,a. The photocathode current that is associated with the incident light is expressed as io,a where a refers to the anode. According to Beer's law, absorbance is given as: | (1) |
Setting the photomultiplier gain due to the dynode chain as μ
| ia = icμ | (2) |
When measuring the CD spectrum the dynode voltage (HV) is varied to maintain a constant DC signal level.
ia,DC associated with no sample, incident intensity Io![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) io,a = μoIo,a io,a = μoIo,a | (3) |
| ia,DC associated with sample, transmitted intensity It: it,a = μtIt,a | (4) |
If the DC signal level is constant
| io,aμo = it,aμ | (5) |
 | (6) |
 | (7) |
The photomultiplier gain formula is:
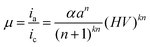 | (8) |
Simplifying:
| μ = K(HV)Z | (9) |
 | (10) |
Thus for a given photomultiplier and dynode chain:
The signal coming from the detector in a CD spectrometer has two parts (VDC and VAC). The CD is the ratio VAC/VDC. According to the Legrande and Grosjean procedure,5 for (AL − AR) < 0.01:
 | (11) |
The high voltage on the photomultiplier is varied to keep VDC constant, and VAC is amplified accordingly.
 | (12) |
To establish the relationship between the photomultiplier high voltage and the absorbance, standard materials with known absorbance values and peak positions are used to verify this relationship. In this study, potassium dichromate solutions of different concentrations were measured using the same cell pathlengths and solutions of the same concentration were studied with different cell pathlengths. From Beer's law, the ordinary absorption is based upon the transition electric dipole moment, whose magnitude can be judged by the value of the molar extinction coefficient. So, the larger the value of ε, the lower will be the limit of detection. However, spectroscopic transition in circular dichroism must have two characters, rotation and translation of charge, which can be quantified in terms of Kuhn6 dissymmetry factor (g-factor):
| g = ΔA/A | (13) |
From practical point of view, the lowest noise-to-signal is produced for A = 0.84 (assuming all other factors are constant during measurement). Therefore, this value should be the target absorption of the species being measured.
3 Experimental
3.1 Materials
In accordance with the British Pharmacopoeia, a single solution of 0.02% potassium dichromate in 0.05 M H2SO4 acid was measured as a function of pathlength (Standards: S1, S2 and S3 measured in 1.0, 0.5 and 0.2 cm pathlengths) to avoid dilution errors and to work in the manner often used in CD spectroscopy practice. A series of five dichromate solutions of concentrations spanning 0.002–0.011% with a fixed pathlength of 1 cm (Standards: S4–S8) was made available by colleagues in National Institute of Standards, Germany (Dr K. Andert, Berlin). The cells used to measure the in-house samples, standards S1–S3, (pathlengths 1.0, 0.5 and 0.2 cm) were specifically manufactured for circular dichroism measurements (Hellma GmbH & CO.KG, D-79-371, Mullheim, Germany). The in-house solution was prepared by dissolving ∼60 mg of potassium dichromate, previously dried to constant weight at 130 °C and dissolved in 0.05 M sulfuric acid to produce 1000 ml. Distilled water was purified in KCL (King's College London). Sulphuric acid was Analar grade from BDH laboratory suppliers, England.3.2 Instruments and measurement settings
The instruments used in this study were the APL π*–180 (Applied Photophysics Limited, Leatherhead, UK). The Jasco J720 & J600 (Jasco Inc., Tokyo, Japan) and the Aviv 17DS UV-VIS-IR, a dedicated high quality UV/vis spectrophotometer which provides reliable absorbance values, (Aviv, Lakewood, New Jersey, USA). These spectrometers were allowed to warm up for half an hour before measurements and were purged with nitrogen to overcome oxygen absorption below 200 nm and to avoid ozone development, which can affect the optics of the spectrometers and absorb in the far UV.The Jasco J720 and the Jasco J600 instruments were operated with a scan speed of 10 nm min−1, a sensitivity of 20 mdeg, a bandwidth of 1 nm, a wavelength step size of 0.2 nm and response time or time constant of 4 s. The spectral range of the dichromate standards measurements was 550–220 nm. The Aviv and APL π*–180 measurements were made using a 1 nm bandwidth, 0.2 nm step size and an effective 10 nm min−1 scan speed.
The dichromate standards were used to establish the relation between the photomultiplier high voltage and the absorbance. When measuring potassium dichromate standards, solvent spectra (0.05 M H2SO4) were measured and subtracted from the relevant solution spectra to compensate for any solvent absorbance.
3.3 Data processing and analysis
The Gram 32/AI (Thermo-Galactic), the JASCO standard analysis, Origin 6 Microcal, and Microsoft Excel software packages were used to compile and analyze the data.The following abbreviations were used in labeling the spectra: PM, photomultiplier, HV high voltage and S standard.
4 Results and discussion
4.1 Preliminary measurements using the Aviv spectrophotometer
As a preliminary to extensive measurements on the three CD spectrometers, absorbance measurements were made on the Aviv 17DS spectrophotometer. The absorbance spectra measured on the Aviv spectrophotometer are overlayed and presented in Fig. 2 (a and b). The linearity of the Aviv absorbance is presented in Fig. 2 (c and d). The plots represent the absorbances values for each wavelength plotted against pathlength and concentration respectively. Absorbances values measured on the Aviv spectrophotometer were used as references to absorbance to ensure a reliable HV converted absorbance and hence a reliable Z-value for each of the spectrometers. This was achieved by dividing the absorbance spectrum of a particular standard recorded on the Aviv instrument by the corrected log (HVt − HVo) of the relevant measurement. | ||
| Fig. 2 (a) Absorbance spectra of 0.02% K2Cr2O7 in 0.05 M H2SO4 with 1.0, 0.5 and 0.2 cm cells, (b) Absorbance spectra of a series of concentrations of K2Cr2O7(0.002%–0.011%) in 0.05 M H2SO4 in a 1.0 cm cell, (c) Linear plot of absorbance against cell pathlength and (d) against concentration. (Aviv) | ||
The British Pharmacopoeia (BP) recommendations7 for the calibration measurements and the values of the specific absorbance for 4 different wavelengths and the permitted limits are shown in Table 1, (The tolerance for absorbance is ±0.01)
| Recommended calibration values for UV/Vis absorbance measurements (BP) | ||||
|---|---|---|---|---|
| Wavelength/nm | 350 | 313 | 257 | 235 |
| A(1%, 1 cm) | 107.3 | 48.6 | 144.5 | 124.5 |
| Maximum tolerance | 105.6–109.0 | 47.0–50.3 | 142.8–146.2 | 122.9–126.2 |
| Calibration values for HV-derived UV absorbance and direct measurements | |||||
|---|---|---|---|---|---|
| Wavelength/nm | 350 | 313 | 257 | 235 | |
| J720 | A(1%, 1 cm) | 108.09 ± 0.8 | 49.5 ± 0.8 | 144.6 ± 1.5 | 125.3 ± 0.83 |
| J600 | A(1%, 1 cm) | 107.9 ± 0.9 | 49.1 ± 0.78 | 144.3 ± 1.2 | 124.6 ± 0.88 |
| APLp* | A(1%, 1 cm) | 107.2 ± 1.1 | 48.7 ± 0.5 | 144.8 ± 1.7 | 124.3 ± 0.56 |
| Aviv (Direct Abs) | A(1%, 1 cm) | 107.7 ± 1.3 | 47.9 ± 0.95 | 144.3 ± 1.9 | 124.5 ± 1.2 |
4.2 Absorbance measurements on the Jasco 720 CD spectrometer
Fig. 3 shows the processes by which the absorbance of the in-house solution in three different pathlengths (1.0, 0.5, 0.2 cm) is determined from the PM high voltage. The overlay of the direct measurement of absorbance (Aviv measurements, Fig. 2) and the HV-derived absorbance measurement from the PM HV of the J720 (Fig. 3d) shows a very good agreement.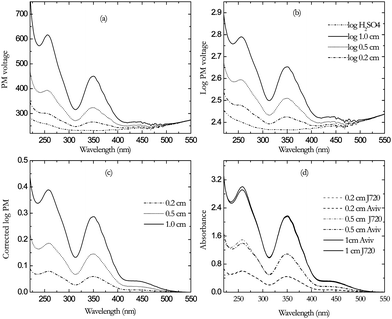 | ||
| Fig. 3 Spectra of 0.02% K2Cr2O7 in 0.05M H2SO4 with 1.0, 0.5 and 0.2 cm cells (a) PM HV (b) log PMHV (c) corrected log PMHV (d) Overlay of Aviv absorbance (in black) and absorbance from PMHV of Jasco 720 (in gray). | ||
The HV-derived absorbance of series of concentrations of potassium dichromate standard solutions S4, S5, S6, S7 and S8 (see materials section) are shown in Fig. 4. An overlay of the HV-derived absorbance and the direct measurement on the Aviv spectrophotometer is shown in (Fig. 4 d). It shows a very good agreement between the two measurements.
 | ||
| Fig. 4 (a) PM HV of a series of concentrations of K2Cr2O7 in 0.05 M H2SO4 in 1 cm cell. (b) Log PMHV(c) Corrected log PMHV (d) Overlay of Aviv absorbance (in black) and absorbance from PMHV of Jasco 720 (in gray). | ||
The values of the HV, log HV, absorbance and the resultant Z-values are presented in Table 2. Fig. 5 shows a plot of concentration using the set of 5 cells against HV-derived absorbance for each assigned wavelength to test the CD spectrometer for linearity through the UV range. The plot shows good linearity for the J720 CD spectrometer using the HV-derived absorbance values, indicating reliability of the method. The mean Z-value calculated for the J720 CD spectrometer is 7.47 with standard deviation and standard error of 0.1 and ±0.03 respectively. These values are based on the results obtained for the absorbance at each assigned wavelength for all the standards (N = 32).
| J720 | λ/nm | HV t | HV 0 | log HVt | logHV0 | C. log HVt | Abs | Z-value |
|---|---|---|---|---|---|---|---|---|
| S1 | 350 | 449 | 232.3 | 2.6521 | 2.3660 | 0.2860 | 2.1748 | 7.604 |
| 313 | 316 | 232.7 | 2.4991 | 2.3668 | 0.1323 | 0.9784 | 7.393 | |
| 257 | 616 | 251.9 | 2.7897 | 2.4012 | 0.3884 | 2.998 | 7.718 | |
| 235 | 577 | 266.7 | 2.7614 | 2.4260 | 0.3354 | 2.5723 | 7.670 | |
| S2 | 350 | 323 | 231.7 | 2.5096 | 2.3649 | 0.1447 | 1.086 | 7.506 |
| 313 | 270 | 232.3 | 2.4318 | 2.3660 | 0.0658 | 0.4897 | 7.443 | |
| 257 | 392 | 251.5 | 2.5931 | 2.4005 | 0.1925 | 1.4899 | 7.739 | |
| 235 | 389 | 266.7 | 2.5897 | 2.4260 | 0.1637 | 1.2747 | 7.787 | |
| S3 | 350 | 266 | 232.0 | 2.4252 | 2.3655 | 0.0597 | 0.4412 | 7.388 |
| 313 | 247 | 232.2 | 2.3932 | 2.3659 | 0.0274 | 0.2012 | 7.353 | |
| 257 | 299 | 250.0 | 2.4761 | 2.3979 | 0.0782 | 0.6097 | 7.800 | |
| 235 | 308 | 263.1 | 2.4880 | 2.4201 | 0.0679 | 0.5142 | 7.577 | |
| S4 | 350 | 336 | 232.2 | 2.5260 | 2.3659 | 0.1601 | 1.1858 | 7.407 |
| 313 | 275 | 232.8 | 2.4393 | 2.3670 | 0.0723 | 0.5327 | 7.363 | |
| 257 | 410 | 250.0 | 2.6128 | 2.3979 | 0.2148 | 1.61 | 7.494 | |
| 235 | 403 | 263.7 | 2.6053 | 2.4211 | 0.1842 | 1.3898 | 7.545 | |
| S5 | 350 | 293 | 232.2 | 2.4675 | 2.3659 | 0.1016 | 0.744 | 7.323 |
| 313 | 259 | 232.8 | 2.4133 | 2.3670 | 0.0463 | 0.3276 | 7.073 | |
| 257 | 341 | 250.0 | 2.5328 | 2.3979 | 0.1348 | 1 | 7.418 | |
| 235 | 344 | 263.7 | 2.5366 | 2.4211 | 0.1154 | 0.8629 | 7.474 | |
| S6 | 350 | 278 | 232.2 | 2.4440 | 2.3659 | 0.0782 | 0.5874 | 7.513 |
| 313 | 253 | 232.8 | 2.4033 | 2.3670 | 0.0363 | 0.269 | 7.409 | |
| 257 | 319 | 250.0 | 2.5043 | 2.3979 | 0.1064 | 0.797 | 7.491 | |
| 235 | 326 | 263.7 | 2.5138 | 2.4211 | 0.0926 | 0.6955 | 7.508 | |
| S7 | 350 | 266 | 232.2 | 2.4247 | 2.3659 | 0.0589 | 0.4142 | 7.038 |
| 313 | 247 | 232.8 | 2.3934 | 2.3670 | 0.0264 | 0.188 | 7.117 | |
| 257 | 297 | 250.0 | 2.4728 | 2.3979 | 0.0748 | 0.5584 | 7.464 | |
| 235 | 307 | 263.7 | 2.4869 | 2.4211 | 0.0657 | 0.4878 | 7.420 | |
| S8 | 350 | 250 | 232.2 | 2.3971 | 2.3659 | 0.0312 | 0.234 | 7.498 |
| 313 | 240 | 232.8 | 2.3809 | 2.3670 | 0.0140 | 0.105 | 7.526 | |
| 257 | 274 | 250.0 | 2.4376 | 2.3979 | 0.0397 | 0.3036 | 7.657 | |
| 235 | 286 | 263.3 | 2.4565 | 2.4205 | 0.0361 | 0.268 | 7.431 |
 | ||
| Fig. 5 Linear plots of HV-derived absorbance versus potassium dichromate concentration (0.002–0.011%) for the (Jasco720). | ||
4.3 Absorbance measurements on the Jasco 600 and APLπ* CD spectrometers
Similarly, the HV-derived absorbance of all standards was determined on both the Jasco 600 and the APLπ*, the spectra are not shown. There is a good agreement between the direct measurements performed on the Aviv spectrophotometer and the HV-derived measurements for both the in-house standard solutions (S1, S2, S3) and the sealed standard solutions (S4, S5, S6, S7, S8). This indicates that any difference in the obtained values is due to the spectrometer and not to the method. Fig. 6 shows plots of concentration versus HV-derived absorbance of the J-600 (left) and the APLπ* (right) CD spectrometers. Good linearity is observed through the UV range for both spectrometers. Similar tables were generated and the mean Z-value (N = 32) was calculated for both spectrometers and was found to be 7.79 for the Jasco 600 with standard deviation and standard error of 0.1 and ±0.03 respectively. The mean Z-value (N = 30, due to the instrument cut-off) for the APLπ* spectrometer was significantly different from the Jasco instruments and it was calculated to be 5.45 with standard deviation and standard error of 0.1 and ±0.01 respectively. However, because of the instrument cut-off at 280 nm for the S1 measurement this calculation of standard deviation for the APLπ* excluded the values of 257 nm and 235 nm in the 1 cm pathlength. The APLπ* instrument cut-off suggests that this concentration in a 1 cm cell is too high for this range (280–220 nm). However, the agreement between the direct measurement and the HV-derived measurement was excellent. Despite the instrument cut-off (Fig. 7), the APL π* spectrometer gave the best agreement, the resulting absorbance values were more accurate in relation to the direct measurements and the BP recommended values. In Fig. 7, there is a feature at ∼305–310 nm which appears in all pathlengths' spectra. This feature does not appear in the spectra produced by the Jasco instruments, which suggests an instrument artifact. The mean values of Absorbance (1%, 1 cm) derived from the PMHV for all CD spectrometers and the direct measurements on the Aviv spectrophotometer are in agreement with the BP recommended values (Table 2), indicating reliability of the method. | ||
| Fig. 6 Linear plots of HV-derived absorbance versus potassium dichromate concentration (%) for the Jasco600 (left) and the APLπ*(right). | ||
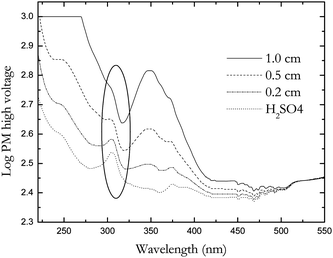 | ||
| Fig. 7 Spectra of log PM showing instrument cutoff in the 1 cm cell using APL π* and the feature at ∼305–310 nm (circled). | ||
5 Conclusion
This work describes how calibrated, routine, simultaneous measurements of absorption and CD can be made and used to determine photometric accuracy. Potassium dichromate measurements on the three spectrometers gave absorbance values within the limits set in the British pharmacopoeia (see Table 1). The results produced from the HV data before base line correction seem noisier (Fig. 3, 4 (a) and (b)) than the data produced by a direct absorbance measurement. This is largely due to sampling the feedback voltage directly. Proper electronic isolation of this signal monitor with the type of signal filtering imposed upon the CD signal will give better results. Sampling the light intensity before the sample as a reference will enable the compensation of intensity drifts due in the main to lamp performance.The absorbance of a sample can be worked out from the value of the applied HV using eqn (12) over the spectral regions of the measurements.
Simultaneous UV absorption and CD measurements generated a Z-value of ∼7.5, ∼7.7 and ∼5.45 for the Jasco 720, the Jasco 600 and the APL π* instruments respectively. Different detectors have distinct characteristics8 and for this reason the Z-value varies from one detector to another. Therefore, the differences in Z-value are mainly due to the detector characteristics. The photomultiplier characteristics that affect the Z-value are the quantum efficiency, the dynode chain characteristics and the spectral response.
In conclusion, the Z-value should be determined for any circular dichroism instrument before applying the principles of the simultaneous measurements. For routine calibration, it is an advantage to assess the Z-value using standard reference materials. This could be implemented as a part of the instrumental calibration and quality control.
The maximum and minimum absorbance that can be determined using the simultaneous measurements is set by the detector upper and lower limits. Absorbance spectra from photomultiplier high voltage are associated with noise; this could be rectified by proper electronic isolation of noise.
The high voltage is depicted in such a way the photocathode and each successive dynode are correctly biased with respect to one another. In order to obtain good correlation between the high voltage and absorbance, consideration must be given for the following:
i) The maximum and minimum voltage level.
ii) The maximum current from the supply; this current must be minimised in order to reduce heat dissipation across the high voltage resistors.
iii) The degree of filtering to eliminate low frequency noise or ripple at power line frequency.
iv) The degree of regulation against long term drift due to temperature change. For instance, a photomultiplier gain (G) is very sensitive to the applied voltage9 demanding precise control and stability. The overall photomultiplier gain can be represented as:
| G α Vn | (14) |
Differentiating the above equation gives
| dG/G = ndV/V | (15) |
In this expression and for n = 9, a change in the high voltage of 1% would result in 9% change in the photomultiplier gain. Investigation of the equation relating the high voltage and the direct absorbance (eqn (12)) reveals that this equation is concentration, pathlength and wavelength independent. The wavelength limitation is an instrumental factor and not related to the principles of simultaneous measurements.
The viability and reliability of the simultaneous measurements of absorbance and CD has been demonstrated. The light intensity measurements provide good criteria for instrument and lamp status. The simultaneous measurement of absorption provides important criteria for reliable CD measurement from an instrumental point-of-view and is an important feature of a protocol for CD measurements. Concentration variations can be monitored to better ensure that CD variations reflect conformational changes more precisely. Simultaneous monitoring of several optical parameters of the same sample on the same instrument is not only time saving but also ensures that any processes occurring in solution are monitored under the same conditions. The simultaneous photometric measurements made here can provide useful guide lines to ensure the measurement of trustworthy CD data of good quality and a protocol to describe how simultaneous, reliable CD and absorption measurements can be made.
The concentration/pathlength combinations indicated in this study are guidelines.10 The four standard wavelengths at which the transmittance/absorbance certification are provided for the potassium dichromate solutions correspond to absorption maxima and minima of their respective absorption spectra. Because of the lack of optical neutrality in their respective absorption spectra, the transmittance/absorbance certification for this photometric reference solution is restricted to their fixed standard wavelengths.
For greater penetration into the far-UV (<200 nm), shorter pathlengths (and lower concentrations) may be required. To measure minimum quantities without too much attenuation of the light intensity, a 1 ml sample accommodated in a 1 × 1 cm strain-free cell Hellma cell would seem to be a good compromise. However, it is important to ensure that suitable beam constriction (iris) is in place to avoid the solution meniscus being in the light path. In the far-UV, Hellma can now also produce guaranteed strain free short pathlength rectangular cells with low volume that may be more convenient than the traditional cylindrical ones. For critical quality assessments, the CD and log (HV) data for the solvent should be measured for each sample individually, rather than producing one set of data for the solvent. In this way, two sets of two spectra per sample/pathlength combination are generated; the CD and absorption spectra of the solvent and the CD and absorption spectra of the sample solution.
Factors such as accuracy, precision, reproducibility, repeatability, and other issues related to the robustness of the polarization modulation technique, noise, time scale of data collection (cf. time constant), spectrum scan speed, and spectral bandwidth will be the subject of future work.
Acknowledgements
We thank National Institute of Standards, Germany and in particular Dr K. Andert for supplying the standard reference solutions and we also thank Applied Photo Physics (APL) for their support.References
- A. F. Drake, J. M. Grould and S. F. Mason, Simultaneous monitoring of light-absorption and optical activity in the liquid chromatography of chiral substance, J. Chromatogr., A, 1980, 202, 239 CrossRef CAS.
- T. Arvinte, T. Bui, A. Aboel Dahab, B. Demeule, A. F. Drake, D. Elhag and P. King, The multi-mode polarization modulation spectrometer: part 1: simultaneous detection of absorption, turbidity, and optical activity, Anal. Biochem., 2004, 332, 46 CrossRef CAS.
- R. W. Burke, E. R. Deardorff and O. Menis, in Accuracy in Spectrophotometry and Luminescence Measurements (R. Mavrodineanu, J. I. Shultz and O. Menis, eds), NBS Special Publication No. 378, 1973, 95 Search PubMed.
- Anonymous, National Bureau of Standards, Letter Circular No. 1017 (1955).
- M. Grosjean and M. Legrand, Compt. rend. Acad. Sci., (Paris), 1960, 251, 2150 Search PubMed.
- W. Kuhn, The physical significance of optical rotatory power, Trans. Faraday Soc., 1930, 26, 293 RSC.
- British Pharmacopoeia Commission BP. British Pharmacopoeia, Vol. II. London: The Stationary Office, 2002 Search PubMed.
- R. D. Sacks, “Emission spectroscopy”, Wiley-Interscience, New York, 1981 Search PubMed.
- W. R. Leo. Techniques for Nuclear and Particle Physics Experiments. Springer-Verlag, Heidelberg, 1987 Search PubMed.
- A. Shultz, D. Campbell and J. Messman, Reference Material Standardization Guidelines for Quality Control and Validation of UV/VIS Absorption Spectrophotometers, The International Journal of Metrology, December 1998, 27 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
