13C NMR spectroscopy for determining the acylglycerol positional composition of lampante olive oils. Chemical shift assignments and their dependence on sample concentration
Giovanna
Vlahov
*,
Angela Alessia
Giuliani
and
Paolo
Del Re
CRA Centro per l'Olivicoltura e l'Industria Olearia, Sede Scientifica di Città S. Angelo, Viale Petruzzi n. 75, 65013, Città S. Angelo, Pescara, Italy. E-mail: g.vlahov@tiscali.it; Fax: +39(0) 85 959518; Tel: +39 (0) 85 95212
First published on 5th May 2010
Abstract
Two Lampante olive oils with free acidity expressed as oleic acid of 25.9 and 8.4 g per 100 g were used to carry out the 13C NMR study for the assignment of all resonances of free fatty acid fraction and of the acylglycerol fraction comprising 1(3)-mono- (1(3)-MAG), 1,3-di- (1,3-DAG), 1,2-di- (1,2-DAG) and triacylglycerols (TAG). The glycerol carbon resonances were found to be resolved according to the esterification degree of the glycerol molecule but no data were available on the length and the unsaturation pattern of the acyl chains. It was the spectral region where the C-1 carboxy carbons resonate that enabled the determination of free fatty acids and of different acylglycerol species. Moreover, within each acylglycerol class, the acyl chain distribution between the 1,3- and 2- glycerol positions was also calculated where the saturated, vaccenate, oleate and linoleate chains were detected as baseline resolved resonances in 1(3)-MAG, 1,3-DAG, 1,2-DAG and TAG. The variability of chemical shifts to sample concentration changes was also evidenced in all the frequency ranges being studied where the methylene C-2 and C-3 carbons underwent the most dramatic changes in their resonance patterns which prevented any resonance assignment.
Introduction
Lampante Olive Oil, it is designated “Lampante” because of its ancient use as fuel in oil burning lamps, is a Virgin Olive Oil which is not fit for human consumption without further processing. According to the International Olive Oil Council and the current European Union legislation, Lampante Olive Oil is the lowest grade oil within the group of Virgin Olive Oils. They are all obtained from the fruit of olive tree solely by mechanical or other physical means under conditions that do not lead to any chemical change.1,2 However, considering that Lampante Olive Oil has a free acidity expressed as oleic acid of more than 3.3 g per 100 g and/or an unpleasant flavor,1 it has to be refined to become edible.So Lampante Olive Oils unlike Virgin olive oils, are characterized from both, high free acidity and total amount of diacylglycerols (they originate from triacylglycerols hydrolysis) which depend on raw material quality and product storage conditions. Total diacylglycerols in virgin olive oils freshly made from healthy olive fruits do not exceed 1–3%, but their content is higher (4–5%) in olive oils obtained from damaged olive fruits or from olives stored under unsuitable conditions.3
In particular, sn-1,2-diacyglycerols (they are stereospecifically numbered) are the biosynthetic intermediate of triacylglycerol formation,4 whereas sn-2,3- and sn-1,3-diacylglycerols arise from enzymatic hydrolysis of triacylglycerols and from isomerization processes5,6 by transposition of an acyl group,7 respectively. As a result, diacylglycerol fraction can be a useful analytical parameter to determine olive oil grades and detect extra virgin olive oil adulteration with Lampante Olive Oil.5–7
Quantitative determination of olive oil diacylglycerols has been carried out by using gas chromatography,8,9 high performance size exclusion chromatography,10,11 and high performance liquid chromatography.12
However, a new trend emerged in the research field of olive oil chemistry which encouraged the use of spectroscopic techniques such as vibrational spectroscopy,13–15 synchronous fluorescence and total luminescence spectroscopy,16 along with nuclear magnetic resonance (NMR) spectroscopy.17–20 Spectroscopic techniques reduce up to zeroing sample handling and improve the repeatability and reproducibility of quantitative data as compared to those obtained by classical analytical methods.
In particular, 13C NMR spectroscopy was applied to detect partial acylglycerols and free fatty acids in palm oil by using carbonyl carbon (C-1), C-2 and C-3 carbons of acyl chains and glycerol carbons.21
The characterization of partial acylglycerols of olive oil samples has been carried out by using 1H, 13C and 1P NMR spectroscopy.22–27
This paper aims to demonstrate that 13C NMR succeeds in determining the full pattern of acylglycerol species of lampante olive oil samples along with the positional composition of fatty acids at the 1,3- and 2- glycerol backbone positions.
Experimental
Conventional 13C 1D-NMR (one dimensional) spectra were run at 25 °C on a Unity Inova Narrow Bore 500 MHz spectrometer equipped with a UNIX-based Sun Microsystems workstation (Varian NMR Instruments, Palo Alto, CA), a pulse field gradient unit and a 5 mm probe. Proton-decoupled 13C spectra were obtained at 125 MHz, and chemical shifts in parts per million (ppm) were measured relative to tetramethylsilane by using the residual 1H and 13C peaks of the solvent as internal standards. One-dimensional 13CNMR spectra were obtained using a spectral window of 26 kHz with 128 K data point.Considering that the compositional profiles of acylglycerols were calculated on the basis of carboxy carbon resonances which are characterized by long T1 longitudinal relaxation times, intensity distortions of 13C resonances due to signal saturation which occurs when insufficient relaxation delay d1 between pulses is allowed, were overcome by reducing the flip angle α of the pulses down to 45° and applying a delay d1 of 20 s. The d1 value was five times longer than the longest T1 which was measured for C-1 carboxy carbon of glycerol 1(3)-oleate.28
The NOE enhancement factors demonstrated that the carboxy carbons of different chains in different acylglycerols were affected by proton decoupling to the same extent. As a result, the 13C spectra can be acquired under full NOE conditions with the benefit of working with higher signal-to-noise ratios in shorter experiment times.28
The spectra were processed by applying a resolution enhancement function both, to improve resolution and optimize signal-to-noise ratio. Time domain signal was zerofilled prior to Fourier transformation. Zeros were added to the end of the time domain that makes the effective zerofilled acquisition time ATz longer with the consequent decrease of the spacing in the discrete frequency spectrum from 1/AT to 1/ATz. Zerofilling to the power of 2^2 was applied thus giving a digital resolution of 0.10 Hz.
Results and discussion
Lampante olive oil samples with free acidity expressed as oleic acid of 25.9 and 8.4 g per 100 g (% w/w), were used to carry out the 13C NMR study for the assignment of all resonances.Considering that lampante olive oil samples exhibited both a free fatty acid fraction and an acylglycerol fraction comprising triacylglycerols and partial acylglycerols, i.e. 1(3)- and 2- mono-, 1,2-di- and 1,3- diacylglycerols, five frequency regions were selected to determine the fatty acid composition also on the basis of their position on the glycerol backbone in different acylglycerol species.
In particular, the frequency range from 60 to 74 ppm where the carbons of glycerol backbone moiety resonate, and the ranges where the carboxy carbons C-1 (from 172.0 to 180.0 ppm) and the methylene carbons C-2 (from 33.8 to 34.3 ppm), C-3 (from 24.7 to 25.0 ppm) and C-16 (from 31.40 to 31.95 ppm) resonate, were selected.
13C chemical shifts of carbons of glycerol backbone moiety (60–74 ppm)
The structures of acylglycerols as a function of their esterification degree were successfully studied by means of 13C nuclei of glycerol backbone which resonate in the spectral region from 60 to 74 ppm. The carbons of acylglycerol backbone moiety were fully assigned29and the chemical shifts are reported in Table 1.| Carbon Resonances | Acylglycerols | ||||
|---|---|---|---|---|---|
| Free Fatty Acids | 1(3)-MAG | 1,3-DAG | 1,2-DAG | TAG | |
| Glycerol moiety | |||||
| CH2OCOR 1,3-pos | — | 63.27 | 64.95 | 61.31 | 62.08 |
| CH2OCOR 2-pos | — | — | — | 72.07 | 68.90 |
| CH2OH 1,3-pos | — | 65.00 | — | 62.14 | — |
| CH2OH 2-pos | — | 70.24 | 68.19 | — | — |
| Acyl chain moiety | |||||
| Carboxy C-1 | |||||
| Saturated acid, 1,3-pos | 179.60 | 174.21 | 173.86 | 173.70 | 173.24 |
| Vaccenate 1,3-pos | — | — | 173.84 | — | 173.23 |
| Oleic acid, Oleate 1,3-pos | 179.60 | 174.18 | 173.82 | 173.66 | 173.20 |
| Linoleate 1,3-pos | — | — | 173.81 | — | 173.19 |
| Oleate 2-pos | — | — | — | 173.38 | 172.80 |
| Linoleate 2-pos | — | — | — | — | 172.79 |
| Methylene C-2 | |||||
| Saturated | — | — | — | — | 33.97 |
| Oleate 1,3-pos | — | — | — | — | — |
| Oleate,Linoleate 1,3-pos | — | — | — | — | 33.95 |
| Oleate, Linoleate 2-pos | — | — | — | — | 34.11 |
| Methylene C-3 | |||||
| Saturated | 24.69 | — | — | — | — |
| Oleic acid | 24.67 | — | — | — | — |
| Oleate, Linoleate 1,3-pos | — | — | — | — | 24.77 |
| Oleate, Linoleate 2-pos | — | — | — | — | 24.81 |
| Methylene C-16 | |||||
| Saturated | — | — | — | — | 31.86 |
| Oleate | — | — | — | — | 31.84 |
| Vaccenate | — | — | — | — | 31.72 |
| Linoleate | — | — | — | — | 31.45 |
The symmetrical 1,3-DAG (diacylglycerol) and TAG (triacylglycerol) give 2 signals for the glycerol moiety with an intensity ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the asymmetrical 1(3)-MAG (monoacylglycerol) and 1,2-DAG(diacylglycerol) give 3 signals with intensities in a 1
2, the asymmetrical 1(3)-MAG (monoacylglycerol) and 1,2-DAG(diacylglycerol) give 3 signals with intensities in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The C-2 signals of glycerol resonate always downfield compared to C-1 and C-3 of glycerol backbone. No data were available on the length and unsaturation pattern of glyceride acyl chains.
1 ratio. The C-2 signals of glycerol resonate always downfield compared to C-1 and C-3 of glycerol backbone. No data were available on the length and unsaturation pattern of glyceride acyl chains.
The lampante olive oil samples with 25.9 and 8.4% free acidity confirmed the presence of 1(3)- mono-, 1,2- and 1,3- di- and triacylglycerols (Fig. 1).
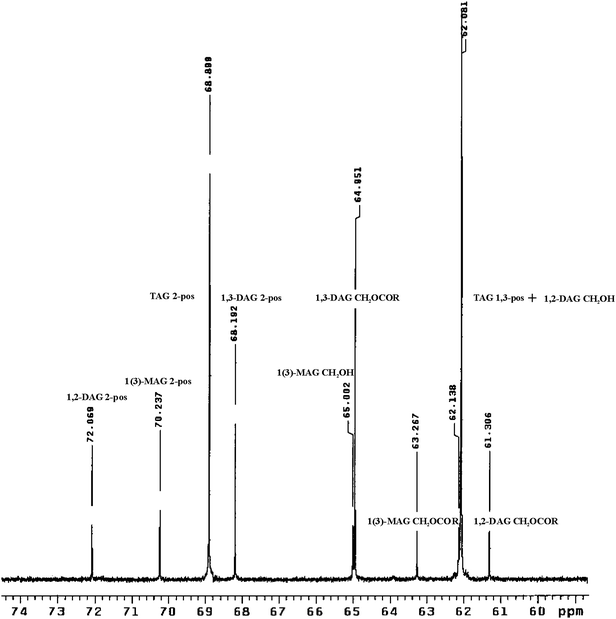 | ||
| Fig. 1 The glycerol backbone carbon region 60–74 ppm of the 500 MHz 13C spectrum of the lampante olive oil sample with 25.9% free acidity. The resonances of 1(3)-MAG, 1,3-DAG, 1,2-DAG and TAG were assigned. | ||
The integration of glycerol backbone resonances enabled the calculation of full glyceride composition of lampante olive oil samples. In particular, the C-2 glycerol signals of different acylglycerol species were integrated because they were baseline resolved. The results were reported in Table 2.
| Carbon Resonance | Acylglycerols | |||||||
|---|---|---|---|---|---|---|---|---|
| 1(3)-MAG (%) | 1,3-DAG (%) | 1,2-DAG (%) | TAG (%) | |||||
| 8.4% | 25.9% | 8.4% | 25.9% | 8.4% | 25.9% | 8.4% | 25.9% | |
| Glycerol backbone | 1.1 | 4.3 | 10.8 | 17.1 | 3.9 | 6.7 | 84.2 | 71.9 |
| Carboxy C-1 | ||||||||
| Total Composition | ||||||||
| Saturated | — | 28 | 13.8 | 14.4 | 6.5 | 7.9 | 15.5 | 17.2 |
| Vaccenate | — | — | 3.8 | 4.5 | — | — | 3.2 | 3.6 |
| Oleate | 100 | 72 | 68.8 | 73.0 | 74.2 | 81.6 | 69.1 | 71.4 |
| Linoleate | — | — | 13.8 | 8.1 | 19.4 | 10.5 | 12.2 | 7.7 |
| 1,3-pos Composition | ||||||||
| Saturated | — | 28.0 | 13.8 | 14.4 | 11.8 | 14.3 | 22.9 | 25.2 |
| Vaccenate | — | — | 3.8 | 4.5 | — | — | 4.7 | 5.3 |
| Oleate | — | 72.0 | 68.8 | 73.0 | 70.6 | 76.2 | 63.3 | 63.7 |
| Linoleate | — | — | 13.8 | 8.1 | 17.6 | 9.5 | 8.9 | 5.8 |
| 2-pos Composition | ||||||||
| Oleate | — | — | — | — | 78.9 | 88.2 | 81.0 | 88.0 |
| Linoleate | — | — | — | — | 21.4 | 11.8 | 19.0 | 12.0 |
| 2-pos Specificity | ||||||||
| Oleate | — | — | — | — | 47.8 | 48.4 | 38.1 | 39.0 |
| Linoleate | — | — | — | — | 50.0 | 50.0 | 50.4 | 48.9 |
| Methylene C-16 | ||||||||
| Composition | ||||||||
| Saturated | — | — | — | — | — | — | 17.6 | 18.8 |
| Vaccenate | — | — | — | — | — | — | 4.6 | 4.3 |
| Oleate | — | — | — | — | — | — | 68.9 | 70.1 |
| Linoleate | — | — | — | — | — | — | 8.8 | 6.8 |
The ratios between 1,2- and 1,3-diacylglycerols were also calculated. Both lampante olive oil samples from Leccino and Dritta cultivars exhibited the same ratio value of 0.4. Considering that according to the Kennedy pathway, the plant tissues sinthesize TAG by acylation of DAG at the sn-3-position, the sole presence of 1,2-DAG is expected to occur in the glyceride fraction of olive oil. So, the increase of 1,3-DAG and the correspondent decrease of 1,2-DAG/1,3-DAG ratio can be explained in term of isomerization of 1,2-DAG into the most stable 1,3-DAG due to a number of interacting factors.29,30
13C chemical shifts of C-1 carboxy carbons of glyceride acyl chain moieties (172–180 ppm)
The partial results obtained by the glycerol backbone resonances where no information can be achieved on the glyceride acyl chains, found their completeness in the frequency region from 172 to 180 ppm where the carboxy carbons resonate.The spectrum of the lampante olive oil sample 25.9% free acidity showed two well defined sets of resonances. The high frequency set comprised the carboxy carbon resonances of free fatty acids which, however, were not resolved according to the chain unsaturation degree. The low frequency set spread over a shift range of about 2 ppm from 172.6 to 174.6 ppm and grouped into four clusters corresponding to 1(3)- mono-,1,3- di-, 1,2-di- and triacylglycerols from a higher to a lower frequency in that order (Fig. 2).28
 | ||
| Fig. 2 The carboxy carbon region 172.7–174.3 ppm of the 500 MHz 13C spectrum of the lampante olive oil sample with 25.9% free acidity. The resonances of Saturated (S), Vaccenate (V), Oleate (O), and Linoleate (L) chains were assigned within each glyceride species comprising 1(3)-MAG, 1,3-DAG, 1,2-DAG and TAG. | ||
The 1(3)-monoacylglycerol cluster comprised the resonances of saturated (174.21 ppm) and oleate chains (174.18 ppm), whereas the 1,3-diacylglycerol cluster exhibited the saturated (173.86 ppm), vaccenate (173.84 ppm, vaccenate and eicosenate chain overlapped in this frequency region) oleate (173.82 ppm) and linoleate (173.81 ppm) chains.
Saturated (173.70 ppm) and oleate (173.66 ppm) chains at 1,3-positions of 1,2-DAG resonated at a higher frequency from the chains at 2-position where the sole oleate chain (173.38) was detected thus confirming that saturated chain esterify only the 1,3-positions of glycerol backbone in triglycerides.
The triacylglycerol cluster showed the acyl chain resonances at 1,3-positions which were higher frequency shifted from the chains at 2-positions. A constant chemical shift difference of 0.41 ppm separated saturated (173.24 ppm), vaccenate (173.23 ppm), oleate (173.20 ppm) and linoleate (173.19 ppm) chains at 1,3-positions from the same chains detected at 2-position, in particular oleate (172.80 ppm) and linoleate (172.79 ppm) chains.These results confirmed that saturated chains were constantly absent in the acylglycerol 2-position in agreement with the most widely accepted theory of distribution of fatty acids among the glycerol positions, i.e. the 1,3-random-2-random theory. The theory predicts the characteristic placement of saturated acids in the 1,3-positions and of unsaturated acids in the glycerol 2-positions. Whenever the saturated acids in the 2-positions exceed the 1.5% level, the olive oil is likely to be adulterated by mixture with esterified olive oil.28
The chemical shift pattern was confirmed according to which the introduction of an increasing double bond number in the chains made them shift at a lower frequency, namely saturated chains resonated at a higher frequency from oleate and linoleate chains, in that order. The pattern was explained in terms of the σ-inductive theory for the transmission of the effect of a double bond dipole through the C–C bonds up to the polarisation of the π electrons of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.31
O group.31
The chemical shift difference of 0.41 ppm between the chains at 1,3- and 2-positions was likely to be due to two γ-gauche interactions which affected the carboxy carbons of a chain at the 2-glycerol positions making them be shifted at a higher frequency, as compared to one γ-gauche interaction for the chains at 1,3-glycerol positions.31
Based on the high resolution of carboxy carbons according to glyceride esterification degree, to chain unsaturation degree and their position on glycerol backbone, the full chain composition of the glyceride species, the chain compositions of the two different pools of fatty acids esterifying the 1,3- and 2- glycerol positions, and the chain specificity for 2-position were calculated. The data are reported in Table 2.
13C chemical shifts of C-16 methylene of glyceride acyl chain moieties (31.4–31.9 ppm)
The spectral region from 31.4 to 31.9 ppm detected as baseline resolved signals, the C-16 of saturated (31.86 ppm), oleate (31.84 ppm), vaccenate (31.72 ppm) and linoleate (31.45 ppm) chains. This region was the only spectral range which enabled the unambiguous detection of vaccenate chain which cannot be resolved from eicosenate chain in the carboxy carbon region.32The chain composition was calculated by integrating C-16 carbon resonances. They confirmed the values obtained by carboxy carbon resonances (Table 2).
13C chemical shifts of C-2 methylene of glyceride acyl chain moieties (33.8–34.3 ppm)
The natural line widths of methylene C-2 of triacylglycerol acyl chain moieties were found to broaden (1.1 Hz) in correspondence of shorter effective transverse relaxation times T2* thus giving poorly resolved 13C NMR resonances in this frequency region.33Methylene C-2 carbons of triglycerides of the Lampante olive oil sample 25.9% free acidity (Fig.3, lower trace) were assigned on the basis of the chemical shift difference of 0.16 ppm which was detected in common C-18 acyl chains between the overlapping oleate and linoleate chains at 2-position (34.11 ppm) and at 1,3-positions (33.95 ppm), the saturated chains resonated at 33.97 ppm. Two major resonances which were detected at 34.08 ppm and at 34.00 ppm were only tentatively assigned to 1(3)-MAG and 1,3- DAG, respectively, also on the basis of quantitative ratios as determined in the C-1 frequency region.
 | ||
| Fig. 3 The methylene C-2 carbon region 33.8–34.3 ppm of the 500 MHz 13C spectrum of the lampante olive oil sample with 25.9% free acidity at a concentration of 200 mg/0.7 ml CDCL3 (lower trace) and of 50 mg/0.7 ml CDCL3 (upper trace). | ||
13C chemical shifts of C-3 methylene of glyceride acyl chain moieties (24.6–24.9 ppm)
The spectral region from 24.6 to 25.0 ppm where the methylene C-3 carbons resonate, detected as baseline resolved resonances free saturated and oleic acids at 24.69 and 24.67 ppm, respectively (Fig. 4, lower trace). However, an extensive overlapping prevents any determination of the differentiated acylglycerol resonances. When measuring the 13C spectrum of an extra virgin olive oil sample, the oleate and linoleate chains at 2-position of triglycerides overlapped at a higher frequency (24.81 ppm, tentative assignment, Fig.4, lower trace) from the overlapping oleate and linoleate chains at 1,3-positions of triacylglycerols (24.77 ppm, tentative assignment, Fig.4, lower trace). | ||
| Fig. 4 The methylene C-3 carbon region 24.6–24.9 ppm of the 500 MHz 13C spectrum of the lampante olive oil sample with 25.9% free acidity at a concentration of 200 mg/0.7 ml CDCL3 (lower trace) and of 50 mg/0.7 ml CDCL3 (upper trace). | ||
The integration of free fatty acids resonances enabled the calculation of free fatty acid composition which could not be measured in the carboxy carbon region where saturated and oleate chains overlapped.
13C chemical shift sensitivity to oil sample concentration
Chemical shift assignments of C-2 and C-3 methylenes in lampante olive oil spectra were made still more difficult at the high sample concentration used to improve spectrum sensitivity and assure accurate integration of C-1 resonances. However, the high sample concentration did not compromise the resolution of C-1 carboxy carbons29 in agreement with the electronic excitation energy of carboxy carbons which is lower than the energy associated with C-2 and C-3 methylenes thus increasing the paramagnetic shielding term σpara.34The concentration effect on 13C chemical shifts of glycerol and acyl chain moieties of glycerides was checked by measuring the chemical shift differences among two Lampante olive oil (25.9% free acidity) samples containing 50 and 200 mg of oil diluted with 0.7ml CDCl3. The results are reported in Table 3 whereas Fig. 3 and 4 showed the effect of sample dilution from 200 (lower trace) down to 50 mg/0.7 ml CDCl3 (upper trace) on the resonance patterns of C-2 and C-3 carbons, respectively.
| Carbon Resonances | Chemical Shift | Chemical Shift | Difference |
|---|---|---|---|
| 50 mg | 200 mg | Δδ50–200 | |
| Glycerol moiety | |||
| 1,2-DAG 2-pos CH2OCOR | 72.093 | 72.026 | 0.067 |
| 1(3)-MAG 2-pos CH2OH | 70.267 | 70.196 | 0.071 |
| TAG 2-pos | 68.864 | 68.845 | 0.019 |
| 1,3-DAG 2-pos CH2OH | 68.361 | 68.174 | 0.187 |
| 1(3)-MAG 1(3)-pos CH2OH | 65.112 | 64.969 | 0.143 |
| 1,3-DAG 1,3-pos CH2OCOR | 65.013 | 64.913 | 0.100 |
| 1(3)-MAG 1(3)-pos CH2OCOR | 63.281 | 63.222 | 0.059 |
| TAG 1,3-pos | 62.085 | 62.035 | 0.050 |
| 1,2-DAG 1(3)-pos CH2OH | 62.085 | 62.035 | 0.050 |
| 1,2-DAG 1(3)-pos CH2OCOR | 61.493 | 61.289 | 0.204 |
| Acyl chain moiety | |||
| Carboxy C-1 | |||
| Free Fatty Acids | 179.553 | 179.604 | −0.051 |
| 1(3)-MAG Saturated | 174.345 | 174.198 | 0.147 |
| 1(3)-MAG Oleate | 174.309 | 174.165 | 0.144 |
| 1,3-DAG Saturated | 173.915 | 173.831 | 0.084 |
| 1,3-DAG Oleate | 173.880 | 173.799 | 0.081 |
| 1,3-DAG Linoleate | 173.868 | 173.787 | 0.081 |
| 1,2-DAG 1,3-pos Oleate | 173.741 | 173.641 | 0.100 |
| 1,2-DAG 2-pos Oleate | 173.402 | 173.347 | 0.055 |
| TAG 1,3-pos Saturated | 173.286 | 173.214 | 0.072 |
| TAG 1,3-pos Oleate | 173.252 | 173.18 | 0.072 |
| TAG 1,3-pos Linoleate | 173.241 | 173.170 | 0.071 |
| TAG 2-pos Oleate | 172.834 | 172.766 | 0.068 |
| TAG 2-pos Linoleate | 172.822 | 172.754 | 0.068 |
| Methylene C-16 | |||
| TAG Saturated | 31.909 | 31.873 | 0.036 |
| TAG Oleate | 31.889 | 31.852 | 0.037 |
| TAG Vaccenate | 31.768 | 31.731 | 0.037 |
| TAG Linoleate | 31.508 | 31.465 | 0.043 |
| Methylene C-3 | |||
| Free Fatty Acid Saturated | 24.723 | 24.689 | 0.034 |
| Free Fatty Acid Oleic | 24.706 | 24.671 | 0.035 |
C-1 carbon resonances of different acylglycerols were shifted to higher frequencies upon decreasing the oil concentration from 200 to 50 mg/0.7 ml CDCl3 in agreement with those detected with standard glycerides but in disagreement with the shifts of methylene C-16 and C-1 (3) and C-2 carbons of glycerol.35
The highest shift difference was detected in 1(3)-MAG where the chemical shift of saturated chain increased from 174.198 to 174.345 ppm in correspondence of 200 mg and 50, respectively, where the shift difference was 0.147 ppm.
The highest downfield shift of C-1 carbons of free fatty acids in the most concentrated oil sample was explained with the strong intermolecular hydrogen bonding which predominates at a higher concentration forming carboxylic acid dimers whereas acid monomers predominates at lower concentrations.28
13C NMR to determine fatty acid composition, their 2-position specificity,and composition of two fatty acid pools at 1,3- and 2-glycerol positions of glyceride species
Two lampante olive oil samples based on Leccino and Dritta cultivars with 25.9 and 8.4% free acidity were studied by 13CNMR and the results were reported in Table 2.Fatty acid composition of different acylglycerol species were calculated on the basis of integral values measured in correspondence of C-1 resonances and confirmed by C-16 integrals.
C-1 resonances enabled for the first time unlike the chromatographic techniques, the experimental determination of composition of two fatty acid pools esterifying the 1,3- and 2- glycerol positions in all glyceride species.
As the fatty acid pool at 1,3-positions is concerned, both in Leccino (25.9%) and Dritta (8.4%) lampante olive oil samples, the percentages of saturated chains which are esterified at the 1,3-glycerol positions, decreased from TAG (25.2, 22.9) to 1,2-DAG (14.3, 11.8) and to 1,3-DAG (14.4, 13.8) indicating that an enzymatic hydrolysis of triacylglycerols regiospecific for 1(3)- positions operated.
In correspondence, the percentages of oleate and linoleate chains increased from TAG (Oleate: 63.7, 63.3; Linoleate: 5.8, 8.9) to 1,2-DAG (Oleate: 76.2, 70.6; Linoleate: 9.5, 17.6) and slightly decreased in 1,3-DAG (Oleate: 73.0, 68.8; Linoleate: 8.1, 13.8). The patterns evidenced that the largest variation of 1,3-distribution values occurred for saturated chains which halved from TAG to 1,2-DAG, and that saturated chains were replaced by oleate and linoleate chains at 1,3-positions where linoleate almost doubled from TAG to 1,2-DAG.
The specificities of oleate and linoleate chains for 2-positions were calculated by normalizing the 2-position resonance value to both 1,3- and 2-position values for each chain and the data are reported in Table 2.
The results confirmed that saturated chains are esterified only at 1,3-positions and unsaturated chains preferentially entered glycerol 2-position.36
In particular, oleate chain with a specificity for the 2-position of triglycerides of 39.0 and 38.1% moved away from a pure random distribution model (33.3%) less than linoleate chain with a 2-specificity of 48.9 and 50.4% in lampante olive oil samples with 25.9 and 8.4% free acidity, respectively.
The 2-specificity data of two lampante olive oil samples also evidenced that hydrolysis of triglycerides at the 1,3-positions where more oleate than linoleate was present, made the oleate at 1,3-positions lower and consequently the 2-specificity of oleate increased from 39.0 to 48.4 and from 38.1 to 47.8 in 1,2-diglycerides detected in the lampante olive oil samples with 25.9% and 8.4% free acidity, respectively.
Conclusions
13C NMR confirmed to be the only technique to be able to determine the full pattern of different acylglycerol species in Lampante olive oil samples.The acylglycerols were determined according to the esterification degree in the shift range from 60 to 74 ppm where the carbons of glycerol backbone resonate. The quantitative ratio of 1,2- and 1,3- diacylglycerol positional isomers was calculated thus accounting for the isomerization of 1,2-diacylglycerols into the most stable form 1,3-diacylglycerols that is indicative of long storage time of the oil and of the processing conditions as high temperatures and three phase extraction with water dilution.
The compositions of the two different pools of fatty acids esterifying the 1,3- and 2-glycerol positions were also determined in the shift range from 172 to 180 ppm where the carboxy carbons resonate. The positional distribution of fatty acids between the 1,3- and 2- positions can account for the enzyme regiospecificity during the enzymatic hydrolysis of lampante olive oil samples thus opening new application fields for 13C NMR.
As an example, a reply should be given to the question whether the 1,3-DAG found in commercial oil samples may not be the results of artefacts but that a possibility exists that part of 1,3-DAG may form in the fruit during the maturation.
Furthermore, a reply should be given to the demand for stereospecific analysis of triacylglycerols where the fatty acid positional analysis of acylglycerols by applying 13C NMR directly on the oil sample without any further chemical treatment is a fundamental starting point.
References
- IOOC, Trade Standard Applying to Olive Oil and Olive-Pomace Oil, COI/T.15/NC no. 3, 1 Search PubMed.
- Characteristics of Olive Oil in Commission Regulation EEC no. 2568/91, J. Eur. Commun., no L248, 5 September 1991, 1 Search PubMed.
- M. C. Perez-Camino, W. Modera and A. Cert, J. Agric. Food Chem., 2001, 49, 699 CrossRef CAS.
- C. Kitchcock and B. W. Nichols, Plant Lipid Biochemistry, Academic Press, New York, 1971, 176 Search PubMed.
- L. Cossignani, R. Luneia, P. Damiani, M. S. Simonetti, R. Riccieri and E. Tiscornia, Eur. Food Res. Technol., 2007, 224, 379 CAS.
- O. Rosati, S. Albrizio, D. Montesano, R. Riccieri, L. Cossignani, M. Curini, M. S. Simonetti, L. Rastrelli and P. Damiani, J. Agric. Food Chem., 2005, 55, 191.
- G. Fragaki, A. Spyros, G. Siragakis, E. Salivaras and P. Dais, J. Agric. Food Chem., 2005, 53, 2810 CAS.
- D. Firestone, J. AOAC Int., 1994, 776, 677.
- C. Planck and E. Lorbeer, J. Chromatogr., A, 1995, 697, 461 CrossRef CAS.
- T. Gomes, J. Am. Oil Chem. Soc., 1992, 69, 1219 CrossRef CAS.
- G. Marquez Ruiz, N. Jorge, M. Martin Polvillo and M. C. Dobarganes, J. Chromatogr., A, 1996, 749, 55 CrossRef CAS.
- J. Liu, T. Lee, E. Jr. Bobik, M. Guzman-Harty and C. Hastilow, J. Am. Oil Chem. Soc., 1993, 70, 343 CrossRef CAS.
- G. Downey, P. McIntyre and A. N. Davies, J. Agric. Food Chem., 2002, 50, 5520 CrossRef CAS.
- V. Baeten, M. Meurens, M. Morales and R. Aparicio, J. Agric. Food Chem., 1996, 44, 2225 CrossRef CAS.
- N. A. Marigheto, E. Kemsley, M. Defernez and R. H. Wilson, J. Am. Oil Chem. Soc., 1998, 75, 987 CAS.
- K. I. Poulli, G. A. Mousdis and C. A. Georgiou, Anal. Chim. Acta, 2005, 542, 151 CrossRef CAS.
- F. D. Gunstone, Advances in lipid methodology-two In W. W. Christie (Ed.), The Oily Press, UK, 1993 Search PubMed.
- K. F. Wollenberg, J. Am. Oil Chem. Soc., 1990, 67, 487 CrossRef CAS.
- L. Mannina, M. Patumi, P. Fiordiponti, M. C. Emanuele and A. L. Segre, Italian J. Food Sci., 1999, 2, 139 Search PubMed.
- G. Vlahov, A. D. Shaw and D. B. Kell, J. Am. Oil Chem. Soc., 1999, 76, 1223 CrossRef CAS.
- Soon Ng, J. Am. Oil Chem. Soc., 2000, 77, 749 CrossRef CAS.
- F. D. Gunstone, Chem. Phys. Lipids, 1991, 58, 159 CrossRef CAS.
- P. Sacchi, F. Addeo, I. Giudicianni and L. Paolillo, La Rivista Italiana delle Sostanze Grasse, 1990, 67, 245 Search PubMed.
- F. D. Gunstone, Chem. Phys. Lipids, 1991, 58, 219 CrossRef CAS.
- O. Rosati and S. Albrizio, J. Agric. Food Chem., 2007, 55, 191 CrossRef CAS.
- A. Spyros and P. Dais, J. Agric. Food Chem., 2000, 48, 802 CrossRef CAS.
- P. Fronimaki, A. Spyros, S. Christophoridou and P. Dais, J. Agric. Food Chem., 2002, 50, 2207 CrossRef CAS.
- G. Vlahov, Triglycerides and Cholesterol Research, Nova Science Publishers Inc., 2006 Search PubMed.
- G. Vlahov, J. Am. Oil Chem. Soc., 1996, 73, 1201 CAS.
- H. R. Barton and C. J. O'Connor, Aust. J. Chem., 1997, 50, 355 CrossRef.
- O. W. Howarth, C. J. Samuel and G. Vlahov, J. Chem. Soc., Perkin Trans. 2, 1995, 2307 RSC.
- G. Vlahov, P. K. Chepkwony and P. K. Ndalut, J. Agric. Food Chem., 2002, 50, 970 CrossRef CAS.
- G. Vlahov, C. Schiavone and N. Simone, Magn. Reson. Chem., 2001, 39, 689 CrossRef CAS.
- E. Breitmaier and W. Voelter, Carbon-13 NMR Spectroscopy, VCH, Weinheim, 1990 Search PubMed.
- L. Mannina, C. Luchinat, M. Patumi, M. C. Emanuela, E. Rossi and A. Segre, Magn. Reson. Chem., 2000, 38, 886 CrossRef CAS.
- F. H. Mattson and R. A. Volpenheim, J. Lipid Res., 1963, 4, 392 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
