Specific assay of carboxyl ester hydrolase using PEG esters as substrate
Sylvie
Fernandez
ab,
Amal
Najjar
b,
Sylvie
Robert
b,
Jean-David
Rodier
a,
Bruno
Mahler
a,
Frédéric
Demarne
a,
Frédéric
Carrière
*b and
Vincent
Jannin
*a
aGattefossé SAS, 36 chemin de Genas, 69804, Saint-Priest, France. E-mail: vjannin@gattefosse.com; Fax: (+33) 4 78 90 45 67; Tel: (+33) 4 72 22 98 38
bCNRS, Aix-Marseille Université, Enzymologie Interfaciale et Physiologie de la Lipolyse - UPR 9025, 31 chemin Joseph Aiguier, 13402, Marseille cedex 20
First published on 21st May 2010
Abstract
The bile salt-stimulated lipase or carboxyl ester hydrolase (CEH) is a non-specific enzyme secreted by the exocrine pancreas and mammary glands. Recently we demonstrated that PEG esters were good substrates for CEH as it exhibited the highest specific activity ever recorded for this enzyme on PEG-8 monocaprylate. The aim of this study was to develop a specific and sensitive method for assaying CEH in biological samples in which several lipases are contained. Eight different PEG-n mono and dicaprylates with ethylene oxide units ranging from n = 6 to 32 were tested using the pH-stat technique. PEG-20 dicaprylate was the best substrate to discriminate CEH from other digestive lipases. Since pancreatic lipase related-protein 2 (PLRP2) was also found to display a significant activity on PEG-20 dicaprylate, experiments were designed to optimize the specificity of the assay for CEH. The main parameters for increasing CEH activity, while reducing that of PLRP2, were the pH and the concentration of bile salts. A linear relationship between the enzyme activity and the mass of CEH was established. The specificity of this assay was validated using gastrointestinal fluids containing CEH, PLRP2, gastric and pancreatic lipases.
1. Introduction
Carboxyl Ester Hydrolase (CEH,1 EC 3.1.1.1) is a non-specific esterase hydrolyzing many substrates, which had led to the attribution of different names for the same enzyme: Bile Salt-Stimulated Lipase (BSSL2), cholesterol esterase,3,4 Carboxyl Ester Lipase (CEL)5 and lysophospholipase.6In humans, CEH is secreted by the acinar cells of the exocrine pancreas and represents 4% of the total proteins in the human pancreatic juice.7 It is also produced by the mammary glands where it represents 1% of proteins in mother milk8,9 and is often named in that case BSSL. Although it was shown that human CEH and BSSL are encoded by a single gene,10,11,12 the proteins differ in their glycosylation.
Rudd and Brockman5 listed all the substrates that could be hydrolyzed with a significant activity (at least 10% of the specific activity measured on cholesterol oleate i.e., 2780 μmol h−1 mg−1) by CEH purified from various species. All these CEH hydrolyze cholesterol esters (cholesteryl palmitate, oleate, stearate, linoleate13), phospholipids (dihexanoyl dioctanoyl, didecanoyl phosphatidylcholine, 1-palmitoylthio-2-ethylphosphatidylcholine1), acylglycerols (tributyroylglycerol, mono-, di-, tri(cis-9-octadecenoyl)glycerol, triacetylglycerol1), vitamin esters (vitamin E and D3 acetate,14 vitamin A palmitate15), and substituted aryl acetates (nitrophenylacetates, bromo- and chlorophenylacetates, methyl-, tert-butyl-, phenyl-, and naphtylacetate16). More recently, it was shown that CEH also hydrolyzes carotenoid esters (lutein and capsanthin diesters, esters of β-cryptoxanthin17).
Considering the large variety of substrates which can be hydrolyzed by CEH, this enzyme combines properties of esterases (activity on esters soluble in water) and lipases (activity on insoluble long chain acylglycerols). The lipase activity of CEH is absolutely dependent on bile salts. When the fatty acid chain length is shorter than 8 carbons, CEH can however hydrolyze ester bonds of acylglycerols in the absence of bile salts. CEH does not present regiospecificity on triacylglycerols and can thus hydrolyze all three esters bonds whatever their position.18
Beyond its absence of specificity for a particular substrate, it is also difficult to compare the activities of CEH measured by different laboratories since several substrates (p-nitrophenol esters, triolein,…), enzymes (human and bovine CEH purified from pancreatic juice, BSSL purified from mother milk, recombinant BSSL,…), bile salts and analytical methods are used, such as the measurement of the absorbance of lipolysis products like p-nitrophenol,19 chromatographic analysis of lipolytic products,20 or potentiometric titration of free fatty acids by the pH-stat technique.1
The esterase activity of CEH is often measured using p-nitrophenyl acetate. Wang et al.21 measured a specific activity of 50 to 60 U/mg using this substrate.20 The lipase activity of CEH can be measured using tri(cis-9-octadecenoyl)glycerol as substrate, and specific activities of about 100 to 110 U/mg were reported by Murasugi et al.22
Recently it has been demonstrated that mono and diesters of PEG were also good substrates for CEH.23,24 The specific activity of CEH on PEG-8 monocaprylate was 325 U/mg. To our knowledge, this is the highest specific activity ever measured with CEH on any substrate.
It seems necessary today to develop a more specific and sensitive assay method for quantifying CEH, given the abundance of CEH in various species from fish to mammals, its presence in various tissues, cell types and secretions (pancreatic juice, milk), and its putative roles in various physiological processes like dietary lipids hydrolysis. CEH or its mRNA was identified in human macrophages,25 granulocytes eosinophils26 and even in the placenta.27
Recently, Lombardo reported the physiopathological implications of CEH28 and evoked the relation between CEH and pathologies like pancreatitis, pancreatic cancer and diabetes. Nowadays the physiological role of CEH remains under discussion but it seems that CEH has a major role in the digestion of cholesterol esters and lipophilic vitamins in adults and probably of acylglycerols in newborn children.29 Whatever the tissues or the biological fluids in which CEH is present, it is always mixed with other lipases and esterases. This is particularly true in humans during digestion of a meal. An assay of CEH will therefore have to be discriminative towards these other enzymes. It was shown that the main digestive lipases are secreted in varying amounts during a meal: 10–25 mg of gastric lipase (GL), 80–400 mg of pancreatic lipase (PL). From these values and the known pancreatic lipase related-protein 2 (PLRP2) to PL ratio and CEH to PL ratio in human pancreatic juice,30,7 the amounts of PLRP2 and CEH secreted during a meal were estimated to be 5–25 mg and 40–200 mg, respectively. When expressed as mass equivalents of the pancreatic juice proteins, GL, PL, PLRP2 and CEH represent 0.5%, 8%, 0.5%, and 4%, respectively.24 Contrary to GL and PL which present a clear preference for triacylglycerols, PLRP2 and CEH are unspecific lipases that are able to hydrolyze various substrates such as monoacylglycerols, phospholipids, and galactolipids. It is therefore difficult to find a specific substrate for CEH among these lipid substrates.
In the present study, we used PEG esters as the substrate to establish a sensitive and discriminative assay of CEH. This assay was optimized by varying pH, bile salt and NaCl concentrations and by comparing simultaneously the activities of CEH, PLRP2, PL and GL. Conditions for which CEH showed a high specific activity and the other lipases/esterases no significant activity were selected. The assay was then validated using human duodenal samples containing CEH and other lipases.
2. Materials and methods
2.1. Chemicals
Sodium taurodeoxycholate (NaTDC, 97% TLC), bovine serum albumin (BSA), and sodium chloride (NaCl) were purchased from Euromedex (Mundolsheim, France) and from VWR International (Fontenay-sous-Bois, France), respectively. PEG-20 dicaprylate (90%), was provided by Gattefossé SAS (Saint-Priest, France). A stock solution of 1 M NaOH (Tritrisol, Merck, Darmstadt, Germany) was diluted with water to obtain 0.1 M NaOH titration solution.2.2. Enzymes
Recombinant Dog Gastric Lipase (rDGL) was a generous gift from Meristem Therapeutics (Clermont-Ferrand, France). Bovine Carboxyl Ester Hydrolase (CEH) was purchased from Sigma-Aldrich-Fluka (Saint-Quentin-Fallavier, France). Recombinant Human Pancreatic Lipase (rHPL) and Pancreatic Lipase-Related Protein 2 (rHPLRP2) were produced in Pichia pastoris and purified from culture media at our laboratory, as described in ref. 31 and ref. 32 respectively. Porcine colipase was prepared from lipid-free pancreatic powder using the procedure described in ref. 23. Protein concentrations were determined using Bradford's procedure33 with Bio-Rad dye reagent, using BSA as the standard protein.2.3. Preparation of semi-solid substrate samples
PEG-20 dicaprylate was first melted for one minute at 800 W using a microwave oven (Samsung). After being homogenized, 500 ± 2 mg liquid samples were weighed and cooled to room temperature. Samples were then stored at room temperature for at least 24 h before use to ensure that a complete and homogeneous crystallization of the product had occurred.2.4. Assay of CEH activity and optimisation of the method
The objective of the design of experiments was to determine the optimal assay conditions where bovine CEH shows a high specific activity and other lipases do not present significant activity on the selected substrate. Three parameters were selected to adjust the assay solution based on data from previous studies in which the specific activities of CEH, rHPL, rHPLRP2 and rDGL were measured using various PEG esters as substrates23,24 (see Fig. 1): (1) pH value ranging from 6.0 to 9.0, (2) the ionic force induced by the concentration of NaCl (0.15 to 1.0 M) and (3) the concentration of NaTDC ranging from 0.5 to 15.0 mM.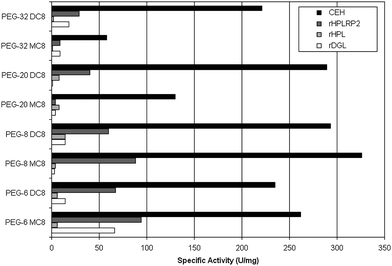 | ||
| Fig. 1 Specific activities of various lipolytic enzymes on PEG-6 monocaprylate (PEG-6 MC8), PEG-6 dicaprylate (PEG-6 DC8), PEG-8 monocaprylate (PEG-8 MC8), PEG-8 dicaprylate (PEG-8 DC8), PEG-20 monocaprylate (PEG-20 MC8), PEG-20 dicaprylate (PEG-20 DC8), PEG-32 monocaprylate (PEG-32 MC8), and PEG-32 dicaprylate (PEG-32 DC8). | ||
The design of experiments selected was a Central Composite Face design composed of a full factorial design and star points placed on the faces of the sides (Table 1). The centre point was repeated three times. The only responses used were the specific activities of the CEH and rHPLRP2, since the selected conditions already excluded significant activities of rHPL and rDGL on PEG-20 dicaprylate.
| Experiment number | pH | NaCl/M | NaTDC/mM | Specific activity of bovine CEH (U/mg) | Specific activity of rHPLRP2 (U/mg) |
|---|---|---|---|---|---|
| 1 | 6.0 | 0.150 | 0.50 | 102 | 9 |
| 2 | 9.0 | 0.150 | 0.50 | 57 | 12 |
| 3 | 6.0 | 1.000 | 0.50 | 182 | 12 |
| 4 | 9.0 | 1.000 | 0.50 | 0 | 6 |
| 5 | 6.0 | 0.150 | 15.00 | 68 | 27 |
| 6 | 9.0 | 0.150 | 15.00 | 0 | 48 |
| 7 | 6.0 | 1.000 | 15.00 | 80 | 30 |
| 8 | 9.0 | 1.000 | 15.00 | 0 | 42 |
| 9 | 6.0 | 0.575 | 7.75 | 273 | 27 |
| 10 | 9.0 | 0.575 | 7.75 | 0 | 30 |
| 11 | 7.5 | 0.150 | 7.75 | 159 | 54 |
| 12 | 7.5 | 1.000 | 7.75 | 227 | 65 |
| 13 | 7.5 | 0.575 | 0.50 | 227 | 27 |
| 14 | 7.5 | 0.575 | 15.00 | 159 | 71 |
| 15 | 7.5 | 0.575 | 7.75 | 227 | 60 |
| 16 | 7.5 | 0.575 | 7.75 | 205 | 54 |
| 17 | 7.5 | 0.575 | 7.75 | 205 | 54 |
Experiments were performed with a pH-stat apparatus equipped with a temperature-controlled reaction vessel at 37 °C. Five hundred mg of PEG-20 dicaprylate were dispersed in 30 mL of assay solution under mechanical stirring. After 5 min, the time necessary for the complete dispersion of the substrate in the assay solution, pH was adjusted to the predefined end-point value and the enzyme solution was added (bovine CEH or rHPLRP2 with colipase in 2-fold molar excess). The pH was kept constant by titrating FFA with a 0.1 M NaOH solution using an automated burette and the volume of NaOH added was recorded as a function of time. Activities were expressed in enzyme units: 1 U corresponds to 1 μmol of FFAs released per minute. Specific activities were expressed as U per mg of pure enzyme.
Depending on the pH of the assay, FFAs are sometimes only partly ionized and lipase activity can be underestimated due to the incomplete titration of these FFA by NaOH. Back-titration experiments can however be performed using a pH-stat in order to titrate all the FFAs released during a given period of time.23,32,34,35 When back titration was required, PEG esters dispersion in the assay solution was incubated with the lipase for 3 min at a given pH. The pH end-point value was then shifted to pH 9.0 using the pH-stat device and the volume of NaOH required to reach this pH value was measured using the automated burette. Control experiments without any enzyme were performed to determine the amounts of NaOH required reaching pH 9.0 in the absence of FFAs released by the lipase. Subtraction of these amounts of NaOH to the total amounts of NaOH delivered in the presence of the lipase allows us to determine the amounts of FFAs released by the lipase, and therefore, lipase activity.
The modelling was performed using the software Modde 5.0 (Umetrics, Kinnelon, NJ, USA). Statistical analysis was performed using ANOVA with an interval confidence of 0.95.
After determination of the optimum conditions, i.e. the conditions where CEH presented a high activity and where rHPLRP2 showed no or low activity on PEG-20 dicaprylate, additional lipolysis experiments with bovine CEH, rDGL, rHPL, and rHPLRP2 were performed to check the specificity of the method.
2.5. Linearity and range of the CEH assay method
The linear range of the optimized method was determined by establishing calibration curves with a series of standards prepared using a CEH stock solution (1.1 μg CEH/mL in 0.5 mM NaTDC, 0.575 M NaCl, 1.4 mM CaCl2, and 1 mM Tris-HCl at pH 6.0). Two sets of 6 volumes of the CEH solution (5, 10, 30, 45, 60, and 80 μL) were tested each day and these assays were repeated for three days. The quantification plot was determined by least squares linear regression. Samples containing precisely weighted amounts of CEH were then assayed to establish the precision of the method.2.6. Quantification of active CEH in human gastro-intestinal fluid (GIF)
GIF samples were collected using a duodenal tube from patients involved in clinical experiments performed at Centre de Pharmacologie Clinique et d'Etudes Thérapeutiques (Hôpital d'adultes de la Timone, Marseille), after the clinical protocol (S245.2.003) had been approved by the Institutional review board of the local Ethics Committee (CPP II, Comité de Protection des Personnes, Hôpital Salvator, 249 Boulevard de Sainte Marguerite, 13009 Marseille). The study was conducted in keeping with the EU Clinical Trial Directive (2001/20/EC), the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice (GCP) dated July 1996, and the ethical principles laid down in the Helsinki Declaration. Only the basal GIF samples aspirated by the duodenal tube were pooled and used for the validation of CEH assay. The pH value of the pooled GIF samples was 6.25 and a yellow colour attested the presence of bile. The pool of GIF samples was first heated at 90 °C for 5 min in order to inactivate the endogenous lipolytic enzymes and the absence of residual activity was checked using tributyrin as substrate after cooling down the samples to room temperature. Pure lipases were precisely weighted and then mixed with GIF based on the mean concentration of human pancreatic lipase (HPL) in duodenal contents during a test meal (250 μg mL−1)36 and the respective proportions of all lipases expressed in % w/w of pancreatic juice proteins (GL, 0.5; PL, 8; PLRP2, 0.5; CEH, 4).24 Of course, GL is produced in the stomach independently from pancreatic enzymes, but its remains present and active in duodenal contents where it is mixed with pancreatic enzymes. The final concentrations of gastric lipase (rDGL), pancreatic lipase (HPL), PLRP2 (rHPLRP2) and bovine CEH in GIF were 16, 250, 16 and 125 μg mL−1, respectively. The activity of this mixture on PEG-20 dicaprylate was then tested and the amounts of active CEH re-estimated based on the known specific activity of bovine CEH on PEG-20 dicaprylate.3. Results and discussion
3.1. Measurement of lipase specific activities on various PEG esters and choice of the best substrate for CEH
Specific activities of rHPL, rHPLRP2, bovine CEH and rDGL were measured by back titration on various mono and dicaprylate PEGs in which the number of ethylene oxide unit ranged from 6 to 32 as previously reported.23,24 Dispersions were prepared using 500 mg of either PEG ester with 15.0 mL of 150 mM NaCl, 4 mM NaTDC, 1.4 mM CaCl2, 1 mM Tris-HCl. The enzymes were then incubated for 3 min at pH 6.0 with the dispersion under mechanical stirring. Using a pH-stat and a 0.1 M NaOH solution, the FFAs released were measured by performing back-titration at pH 9.0, as described in section 2.4.As shown in Fig. 1, PEG esters were more suitable substrates on the whole for CEH and rHPLRP2 than for rHPL and rDGL. As a matter of fact, PEG esters are amphiphilic molecules that strongly interact with water and hence are poor substrates for true lipases such as rHPL and rDGL. On the other hand more versatile esterases such as CEH and rHPLRP2 were able to hydrolyze all of the PEG esters with a significant specific activity. CEH showed a preference for PEG monocaprylates with 6 or 8 ethylene oxide units versus those with longer PEG chain. In addition, CEH exhibited similar levels of activity on PEG dicaprylates whatever the number of ethylene oxide units. The highest specific activity measured with CEH was 326 ± 26 U/mg on PEG-8 monocaprylates. rHPLRP2 showed higher activities on PEG monocaprylates than on the corresponding PEG dicaprylates, and higher activities on PEG esters with 6 or 8 ethylene oxide units than on PEG esters with larger PEG chains. The highest specific activities measured with rHPLRP2 were 94 ± 7 U/mg, 88 ± 1 U/mg, and 91 ± 3 U/mg on PEG-6, PEG-8, and PEG-20 monocaprylates, respectively. CEH activities on PEG dicaprylates range from 221 to 293 U/mg.
The ratio of specific activities of rHPL, rHPLRP2 or rDGL on CEH for PEG monocaprylates and PEG dicaprylates (Table 2) indicated that the most appropriate substrates to develop a selective assay method for CEH were PEG-20 and PEG-32 dicaprylates because the lowest rHPLRP2 to CEH specific activity ratio (0.14) was obtained with these substrates. Among these two compounds, PEG-20 dicaprylate was the preferred substrate because a better specificity versus the other lipases, especially rDGL, was observed. The DGL to CEH specific activity ratio were 0.00 and 0.09 for PEG-20 dicaprylate and PEG-32 dicaprylate, respectively. Moreover, CEH displayed a higher specific activity on PEG-20 dicaprylate than on PEG-32 dicaprylate. Since the specific activity of CEH on PEG-20 dicaprylate was much higher (289 ± 1 U/mg) than the specific activities of other enzymes (9 ± 5 U/mg for rHPL, 40 ± 5 U/mg for rHPLRP2, and 1 ± 1 U/mg for rDGL), PEG-20 dicaprylate was chosen as the best substrate to develop a specific assay of CEH.
| rHPL/CEH | rHPLRP2/CEH | rDGL/CEH | |
|---|---|---|---|
| PEG-6 monocaprylate | 0.02 | 0.36 | 0.25 |
| PEG-6 dicaprylate | 0.03 | 0.29 | 0.06 |
| PEG-8 monocaprylate | 0.01 | 0.27 | 0.01 |
| PEG-8 dicaprylate | 0.05 | 0.21 | 0.05 |
| PEG-20 monocaprylate | 0.06 | 0.70 | 0.03 |
| PEG-20 dicaprylate | 0.03 | 0.14 | 0.00 |
| PEG-32 monocaprylate | 0.02 | 0.76 | 0.15 |
| PEG-32 dicaprylate | 0.02 | 0.14 | 0.09 |
Although the back-titration method23,24 is a good method to determine lipase activities, it is long and tedious to use. A direct titration method using PEG-20 dicaprylate as substrate was therefore developed. The apparent activities of CEH were determined by performing both direct and back-titration lipase activity measurements and found to be equivalent when the pH of the assay solution was equal to or greater than 7.0. This finding indicates that the FFA released from PEG-20 dicaprylate are totally ionised at pH 7.0. Hence no correction factor is needed to assay directly the exact amount of FFA released. From the ratios between the direct and back-titration of lipase activities at various pH values below 7.0, which correspond to the fatty acid ionisation levels, it is possible to calculate a correction factor which can be used to estimate the real activity through direct FFA measurements. At pH 6.0 and 6.5, extents of ionisation of FFA are 40% and 71% and the corresponding correction factors are 2.50 and 1.41, respectively.
3.2. Optimization of the CEH assay method
Table 1 shows the specific activities of bovine CEH and rHPLRP2 measured by direct titration in conditions defined by the design of experiments. The specific activities of CEH showed greater variations over the explored domain than those of rHPLRP2. Specific activities of CEH on PEG-20 dicaprylate ranged from 0 to 273 U/mg whereas those of rHPLRP2 only varied from 6 to 71 U/mg. The highest specific activity measured with CEH was obtained at pH 6.0, 0.67 mM NaCl and 7.5 mM NaTDC. On the other hand, CEH showed no activity at pH 9.0, whatever the concentrations of NaCl and NaTDC.Experimental design was used to generate two mathematical models describing the variations in the specific activities of CEH (eqn (1)) and rHPLRP2 (eqn (2)) as functions of pH, NaCl and NaTDC concentrations.
| CEH specific activity = −1874.9 + 568.627*pH + 462.071*NaCl − 0.184*NaTDC − 29.216*pH*NaCl + 0.908*pH*NaTDC − 0.446*NaCl*NaTDC − 40.138*pH2 − 187.182*NaCl2 − 0.643*NaTDC2 (R2 = 0.881) | (Equation 1) |
| rHPLRP2 specific activity = −676.597 + 188.957*pH + 9.757*NaCl + 1.261*NaTDC − 3.525*pH*NaCl + 0.414*pH*NaTDC − 12.529*pH2 + 15.556*NaCl2 − 0.146*NaTDC2 (R2 = 0.969) | (Equation 2) |
The variance of these two models was analyzed by ANOVA (Table 3) and the probabilities for the regression of the CEH and rHPLRP2 models were significant at 95%. These two models were therefore suitable and were further analysed.
| CEH | Degrees of freedom | Sum of squares | Mean squares | F | p |
|---|---|---|---|---|---|
| Total | 16 | 144 280 | 9 017.47 | — | — |
| Regression | 9 | 127 136 | 14 126.2 | 5.76789 | 0.015 |
| Residual | 7 | 17 143.8 | 2 449.11 | — | — |
| rHPLRP2 | Degrees of freedom | Sum of squares | Mean squares | F | p |
|---|---|---|---|---|---|
| Total | 16 | 6 874.94 | 429.684 | — | — |
| Regression | 9 | 6 663.7 | 740.412 | 24.5361 | 0.001 |
| Residual | 7 | 211.235 | 30.1764 | — | — |
Fig. 2 shows the modelling of CEH (top panels) and rHPLRP2 (lower panels) specific activities as a function of the concentration of NaTDC (0.5, 7.75, and 15 mM). The specific activity of bovine CEH was maximal at low concentrations of NaTDC whereas rHPLRP2 specific activity increased with the concentration of NaTDC. Concerning the pH effects, the specific activity of CEH was maximal at low pH values ranging from 6.0 to 7.0, whereas for rHPLRP2, this response was maximal at pH values ranging from 7.0 to 8.0. The ionic strength induced by the concentration of NaCl had no significant effect on the specific activity of rHPLRP2 and a low effect on the specific activity of CEH, which decreased at high salt concentrations.
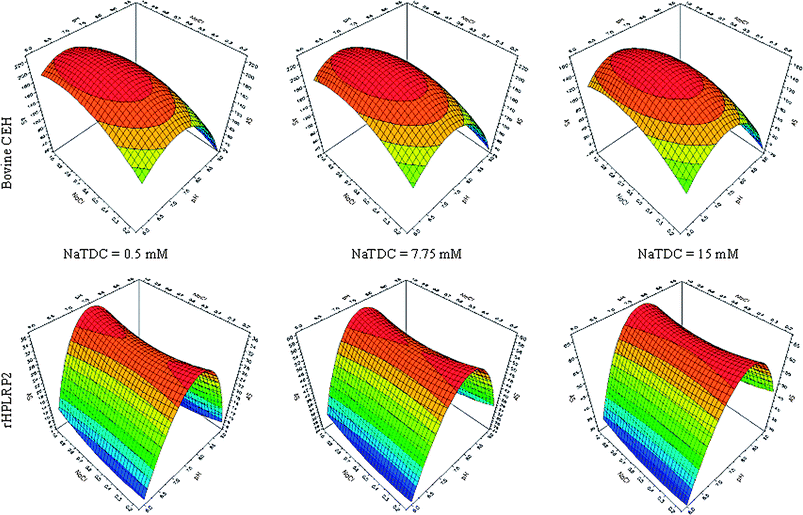 | ||
| Fig. 2 Modelling of the specific activities (AS, U/mg) of bovine CEH (top panels) and rHPLRP2 (lower panels) as a function of pH, concentration of NaCl (M), and concentration of NaTDC (mM). | ||
From modelling, the most adequate conditions to obtain the highest activity for CEH and the lowest one for rHPLRP2 on PEG-20 dicaprylate were pH 6.0, 0.5 mM NaTDC, and 0.575 M NaCl. Under these conditions the specific activities of bovine CEH and rHPLRP2 calculated by the two models were 191 U/mg and 6 U/mg, respectively.
The accuracy of the models and the specificity of the CEH assay method were then tested by measuring the specific activities of bovine CEH, rHPLRP2, rDGL, and rHPL on PEG-20 dicaprylate under the optimum conditions predicted by the models. The specific activities measured for bovine CEH, rHPLRP2, rDGL, and rHPL were 196 U/mg, 10 U/mg, 0 U/mg, and 0 U/mg, respectively, which confirmed the specificity of the assay method for CEH and the good prediction ability of the two models generated by experimental design.
3.3. Validation of the CEH assay method
| Volume of enzymatic solution engaged/μL | Average slope/μmol min−1 | Fatty acid release/μmol min−1 calculated from eqn (3) | Deviation to the value obtained from eqn (3) | Activity (U/mL) | Specific activity (U/mg) |
|---|---|---|---|---|---|
| 5 | 0.88 | 1.10 | −20% | 175 | 159 |
| 10 | 1.69 | 1.70 | −1% | 169 | 153 |
| 30 | 4.29 | 4.10 | 5% | 143 | 130 |
| 45 | 6.25 | 5.90 | 6% | 139 | 126 |
| 60 | 7.50 | 7.70 | −3% | 125 | 114 |
| 80 | 10.00 | 10.10 | −1% | 125 | 114 |
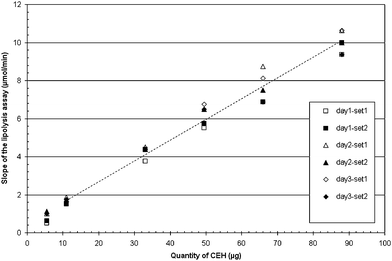 | ||
| Fig. 3 Linearity of the assay method. Injections were repeated twice a day for 3 days. day1-set1: first day-first injection, day1-set2: first day-second injection, day2-set1: second day-first injection, day2-set2: second day-second injection, day3-set1: third day-first injection, and day3-set2: third day-second injection. The dotted line is the linear regression of all six sets of injection for a range of 11 to 88 μg (eqn (3)). | ||
The regression line does not include the zero point which is surprising because in absence of CEH one could suppose that the enzyme activity would be zero. However the linear regression established with data points from 11 to 88 μg CEH allows a good prediction of experimental values obtained with CEH amounts in this range (see Table 4). It reflects the fact that the CEH specific activity slightly decreases with CEH amounts and this raises several questions. We checked that these results are not due to limiting amounts of substrate. Moreover, the validation of other lipase assays has shown that most lipases show the same features and the assays have often to be limited to narrow ranges of enzyme amounts (0.5 to 3 μg in the case of human pancreatic lipase assayed with tributyrin as substrate) where the variation in the specific activity is not significant compared to the precision of the assay. We can force the linear regression to include the zero point but the resulting equation will not fit with the experimental data given in Table 4.
| y = 0.1091 * x + 0.4999 R2 = 0.9958 | (Equation 3) |
| GIF volume/μL | CEH/μg | rHPLRP2 (μg) | rDGL/μg | rHPL/μg | Theoretical activity of CEH (U)a | Theoretical activity of rHPLRP2(U) b | Activity measured (U) c | CV (%) vs. theoretical CEH activity | CV (%) vs. theoretical CEH+rHPLRP2 activities |
|---|---|---|---|---|---|---|---|---|---|
| a activities estimated using a specific activity of 196 U/mg for CEH. b activities estimated using a specific activity of 10 U/mg for rHPLRP2. c number of experiments, n = 3. | |||||||||
| 100 | 12.5 | 1.6 | 1.6 | 25 | 2.45 | 0.02 | 2.53 ± 0.12 | 3.4% | 2.7% |
| 400 | 50 | 6.3 | 6.3 | 100 | 9.80 | 0.06 | 10.33 ± 0.58 | 5.4% | 4.8% |
| 700 | 87.5 | 10.9 | 10.9 | 175 | 17.15 | 0.11 | 17.50 ± 0.87 | 2.0% | 1.4% |
Conclusions
Carboxyl Ester Hydrolase is a non-specific esterase able to hydrolyze many substrates. From its abundance, 4% of the total proteins in the human pancreatic juice7 and 1% in mother milk,8,9 CEH should have a major role in lipid digestion in adult humans and newborn children.We developed a sensitive and selective assay to quantify CEH based on its enzyme activity on PEG-20 dicaprylate used as substrate. This assay with the pH-stat technique is easy and fast to perform. Using experimental conditions optimized by the design of experiments (0.5 mM NaTDC, 0.575 M NaCl, 1.4 mM CaCl2, 1 mM Tris-HCl at pH 6.0), the specific activity of CEH on PEG-20 dicaprylate measured by direct titration of the FFA was higher than those measured on p-nitrophenylacetate (50–60 U/mg20) or triolein (100–110 U/mg22). This assay is therefore more sensitive than the previous ones. It is also more specific based on the very low activities of gastric lipase, classic pancreatic lipase and pancreatic lipase-related protein 2 on PEG-20 dicaprylate, whereas all these lipases display a significant activity on triolein for instance.37,32,38
The method developed should allow quantifying CEH contained in biological samples such as pancreatic secretion, intestinal contents and human milk. So far, all the significant lipolytic activities measured in human gastrointestinal fluids could be associated with human enzymes. For instance, a good correlation was observed between the amounts of gastric and pancreatic lipases deduced from activity measurements and those obtained by ELISA.39 This is probably due to the fact that the human pancreas produces huge amounts of enzymes (80– 400 mg pancreatic lipase, 40–200 mg CEH as mentioned in the introduction). Therefore, we do not think that microbial enzymes could significantly interfere with CEH determination in intestinal contents.
This method can have many applications such as the quantification of human CEH secreted during a meal in healthy humans or patients suffering pancreatic insufficiency, or assay the quality control of infant formula containing CEH/BSSL for the substitution of mother milk.
References
- D. Lombardo, J. Fauvel and O. Guy, Biochim. Biophys. Acta, 1980, 611, 136–146 CAS.
- O. Hernell and T. Olivecrona, Biochim. Biophys. Acta, 1974, 369, 234–244 CAS.
- J. H. Mueller, J. Biol. Chem., 1915, 22, 1–9.
- J. H. Mueller, J. Biol. Chem., 1916, 27, 463–480.
- E. A. Rudd and H. L. Brockman, in Lipases, ed. B. Borgström and H. L. Brockman, Elsevier, Amsterdam, 1st edn, 1984, pp. 185–204.
- H. Van Den Bosch, A. J. Aarsman, J. G. N. De Jong and L. L. M. Van Deenen, Biochim. Biophys. Acta, 1973, 296, 94–104 CAS.
- O. Guy and C. Figarella, Scand. J. Gastroenterol. Suppl., 1981, 15, 59–61 Search PubMed.
- L. Blackberg and O. Hernell, Eur. J. Biochem., 1981, 116, 221–225 CrossRef CAS.
- L. Blackberg, K. A. Angquist and O. Hernell, FEBS Lett., 1993, 217, 37–41.
- D. Y. Hui and A. Kissel, FEBS Lett., 1990, 276, 131–134 CrossRef CAS.
- J. Nilsson, L. Blackberg, P. Carlsson, S. Enerbaeck, O. Hernell and G. Bjursell, Eur. J. Biochem., 1990, 192, 543–550 CrossRef CAS.
- K. Reue, J. Zambaux, H. Wong, G. Lee, T. H. Leete, M. Ronk, J. E. Shively, B. Sternby, B. Borgström and D. Ameis, J. Lipid Res., 1991, 32, 267–276 CAS.
- J. Hyun, H. Kothari, E. Herm, J. Mortenson, C. R. Treadwell and G. V. Vahouny, J. Biol. Chem., 1969, 244, 1937–1945 CAS.
- D. Lombardo and O. Guy, Biochim. Biophys. Acta, 2008, 611, 147–155.
- C. Erlanson, Scand. J. Gastroenterol., 1975, 10, 401–408 CAS.
- K. R. Lynn, C. A. Chuaqui and N. A. Clevette-Radford, Bioorg. Chem., 1982, 11, 19–23 CrossRef CAS.
- D. E. Breithaupt, A. Bamedi and U. Wirt, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2002, 132, 721–728 CrossRef.
- O. Hemell and L. Blackberg, Pediatr. Res., 1982, 16, 882–885.
- C. S. Wang, J. Biol. Chem., 1981, 256, 10198–10202 CAS.
- C. S. Wang, A. Kuksis and F. Manganaro, J. Biol. Chem., 1983, 258, 9197–9202 CAS.
- C. S. Wang, A. Dashti and D. Downs, in Lipases and Phospholipase Protocols, ed. M. Doolittle and K. Reue, Humana Press, Totowa, 1st edn, 1998, pp. 71–79.
- A. Murasugi, Y. Asami and Y. Mera-Kikuchi, Protein Expression Purif., 2001, 23, 282–288 CrossRef CAS.
- S. Fernandez, V. Jannin, J. D. Rodier, N. Ritter, B. Mahler and F. Carriere, Biochim. Biophys. Acta, 2007, 1771, 633–640 CAS.
- S. Fernandez, J. D. Rodier, N. Ritter, B. Mahler, F. Demarne, F. Carrière and V. Jannin, Biochim. Biophys. Acta, 2008, 1781, 367–375 CAS.
- F. Li and D. Y. Hui, J. Biol. Chem., 1997, 272, 28666–28671 CrossRef CAS.
- F. W. Holtsberg, L. E. Ozgur, D. E. Garsetti, J. Myers, R. W. Egan and M. A. Clark, Biochem. J., 1995, 309, 141–144 CAS.
- L. Chen and R. Morin, Biochim. Biophys. Acta, 1971, 231, 194–197 CAS.
- D. Lombardo, Biochim. Biophys. Acta, 2001, 1533, 1–28 CAS.
- O. Hernell and L. Blackberg, J. Pediatr., 1994, 125, S56–S61 CrossRef CAS.
- C. Eydoux, A. Aloulou, J. De Caro, P. Grandval, R. Laugier, F. Carriere and A. De Caro, Biochim. Biophys. Acta, 2006, 1760, 1497–1504 CrossRef CAS.
- V. Belle, A. Fournel, M. Woudstra, S. Ranaldi, V. Prieri, V. Thome, J. Currault, R. Verger, B. Guigliarelli and F. Carriere, Biochemistry, 2007, 46, 2205–2214 CrossRef CAS.
- C. Eydoux, J. De Caro, F. Ferrato, P. Boullanger, D. Lafont, R. Laugier, F. Carriere and A. De Caro, J. Lipid Res., 2007, 48, 1539–1549 CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- A. Aloulou, J. A. Rodriguez, D. Puccinelli, N. Mouz, J. Leclaire, Y. Leblond and F. Carriere, Biochim. Biophys. Acta, 2007, 1771, 228–237 CAS.
- Y. Gargouri, G. Pieroni, C. Riviere, P. A. Lowe, J. F. Sauniere, L. Srada and R. Verger, Biochim. Biophys. Acta, 1986, 879, 419–423 CAS.
- F. Carriere, C. Renou, J. Lopez, J. De Caro, F. Ferrato, H. Lengsfeld, A. De Caro, R. Laugier and R. Verger, Gastroenterology, 2000, 119, 949–960 CrossRef CAS.
- F. Carriere, E. Rogalska, C. Cudrey, F. Ferrato, R. Laugier and R. Verger, Bioorg. Med. Chem., 1997, 5, 429–435 CrossRef CAS.
- E. Rogalska, S. Ransac and R. Verger, J. Biol. Chem., 1990, 265, 20271–20276 CAS.
- F. Carriere, C. Renou, S. Ransac, V. Lopez, J. De Caro, F. Ferrato, A. De Caro, A. Fleury, P. Sandwal-Ducray, H. Lengsfeld, C. Beglinger, P. Hadvary, R. Verger and R. Laugier, Am. J. Physiol., 2001, 281, G16–G28 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
