A highly sensitive Raman method for selective cyanide detection based on evaporated cuprous iodide substrate†
C. V.
Gopal Reddy‡
a,
Fei
Yan‡
a,
Yan
Zhang
a and
Tuan
Vo-Dinh
*ab
aFitzpatrick Institute for Photonics, Department of Biomedical Engineering, Duke University, Durham, NC 27708, USA. E-mail: tuan.vodinh@duke.edu; Fax: +1-919-613-9145; Tel: +1-919-660-8520
bDepartment of Chemistry, Duke University, Durham, NC 27708, USA
First published on 20th April 2010
Abstract
A strong interaction between cyanide anion and copper(I) cation in combination with non-resonant Raman fingerprinting allows the selective sensing of aqueous free cynanide with high sensitivity to parts per billion (ppb)-level.
Cyanide is a very common pollutant. It is a constituent of vehicle exhaust and cigarette smoke, and is utilized in many industrial processes such as gold mining, electroplating, printing, textiles and leather manufacturing.1 One of the most rapidly acting lethal poisons known to humankind, cyanide can kill within seconds to hours depending on the cyanide levels and exposure patterns. For example, exposure to an airborne cyanide concentration of 90 parts per million (ppm) for 30 min is likely to be incompatible with life, while death may result from minutes of exposure at 300 ppm.2 Long-term exposure to lower concentrations of cyanide in occupational settings can result in a variety of symptoms related to central nervous system effects.3 Guidelines for human exposure to cyanide have been developed by several agencies worldwide. The U.S. Environmental Protection Agency (EPA) requires that the residual cyanide in treated industrial wastewater be less than 1 ppm before they are allowed to be discharged into aquatic ecosystems.4 The concern of a possible terrorist attack using cyanide, as well as the gradual awareness of cyanide poisoning in fire victims, has resulted in a renewed interest in the quick diagnosis of cyanide, which is critical for timely and effective treatment.2 Thus, it is essential to develop rapid, sensitive, selective and accurate methods for aqueous cyanide determination.
Over the years, numerous analytical techniques for the detection of free cyanide have been developed, these include spectrophotometic,5 electrochemical,6 spectroscopic,7 capillary electrophoresis,8 chemiluminescence9 and chromatographic detection10approaches. However, some of the detection strategies either involve the use of synthetic probes that are not easily accessible by other groups, the formation of hydrogen cyanide (HCN), or inherit shortcomings such as low sensitivity, poor reproducibility, and limited selectivity. Further, most of these techniques, while they may well be great analytical tools in a laboratory setting, are generally not suitable for field detection of environmental cyanides due to the requirement of additional reagents and tedious sample pretreatment or measurements.
Here we present a unique approach that employs a non-resonant Raman scattering detection of cyanide after its retention by semiconducting cuprous iodide (CuI) thin films.
In this study, CuI thin films with a variety of thickness ranging from 100 nm to 1μm were deposited on the silicon wafer slides by thermal evaporation, and characterized by SEM and XRD.† Elongated grains with sizes ranging from 20 nm to 1 μm were observed in SEM images of CuI on silicon slides. As demonstrated in Fig. 1, evaporated CuI thin film underwent significant morphological changes after reacting with a 10 ppm cyanide solution for 5 min. CuI has a solubility constant (Ksp) of 1.27 × 10−12, while CN− displaces I− and reacts violently with copper(I) Cu+ ions to form CuCN (Ksp = 3.47 × 10−20). Both CuI and CuCN are essentially insoluble in water, this property makes our approach especially advantageous in determining cyanide contents in complex matrices such as industrial waste water and mining reservoirs etc.
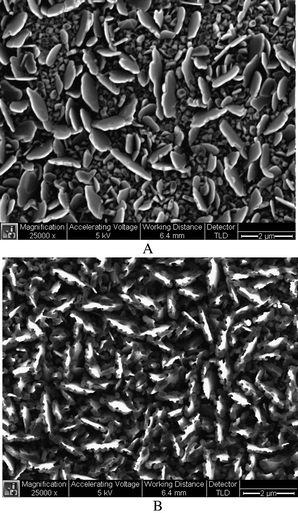 | ||
| Fig. 1 SEM images of evaporated cuprous iodide thin films before (Top) and after (Bottom) reacting with 10 ppm CN− for 5 min. Scale bar: 2 μm. | ||
Raman spectroscopy is well known for its specificity in chemical and biological analyses, and offers some distinct advantages over other spectroscopic methods such as fluorescence and infrared spectroscopy. The vibrational information provided by Raman spectroscopy is very specific for chemical bonds and thus for unique structures in molecules; it can therefore provide an optical fingerprint used to identify a molecule.11 As shown in Fig. 2A, the Raman spectra of cyanide are dominated by an intense, broad peak at 2171 cm−1 attributed to the C≡N stretch. This same mode occurs at 2080 cm−1 in Raman spectra of cyanide solutions, and the frequency shift in Raman spectra is attributed to a strong surface interaction between copper(I) and cyanide. CuCN salt is known to be a white powder, and it does not absorb light in the visible region (400–800 nm), as a matter of fact, it only has two distinct absorbance peaks at 237 nm and 208 nm, respectively.12 The excitation source we used in our Raman measurements is a He–Ne laser (633 nm), hence our observed signals were strictly coming from non-resonant Raman scattering.
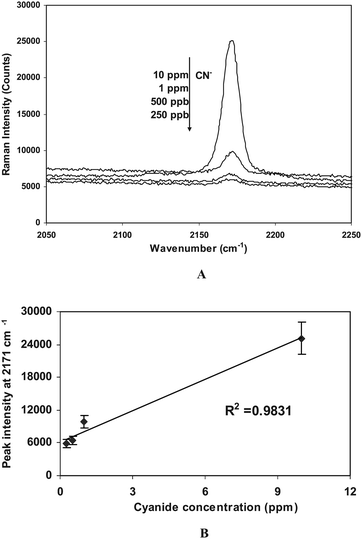 | ||
| Fig. 2 A) Raman spectra of CuI thin film substrates after reacting with CN− of different concentrations, and B) Calibration curve obtained from the peak intensity at 2171 cm−1. Each CuI thin film was incubated with cyanide solutions for 5 min, and rinsed with water before they were examined by Raman scattering. | ||
While the following three copper-cyanide complexes also may form in aqueous solution: [Cu(CN)2]−, [Cu(CN)3]2− and [Cu(CN)4]3−, it has been well established that the distribution of these complexes at equilibrium is highly dependent upon the CN/Cu molar ratio.12 In our case, we were dealing with cyanide ions at ppb to ppm levels, hence, there will not be any excessive cyanide available to form any compounds with higher complexes such as Cu(CN)43− beyond CuCN. This point was also supported by the Raman data we presented, whereby the single cyanide stretching line at 2171 cm−1 indicates that there is only one copper cyanide complex (i.e., CuCN) is present, which agrees well with a previous report.13
Peak intensities of the characteristic C≡N stretching at 2171 cm−1 were used for the quantitative evaluation of cyanide. For a single experimental run, Raman scattering was performed on multiple spots on several cuprous iodide films, which were evaporated at the same time, after dipping them into cyanide solutions of different concentrations for 5 min (while stirring). All Raman spectra were obtained on a Renishaw InVia Raman microscope equipped with an ultra low noise charge-couple device (CCD) detector.† In this system, 632.8 nm radiation from a Helium–Neon laser was used with an excitation power of ∼50 mW. Signal collection is performed at 180° with respect to the incident laser beam. The Raman spectral region of interest for cyanide, 2050–2250 cm−1, was recorded using a 30-s integration time, and 3 accumulations. The calibration curve for potassium cyanide (KCN) was obtained using 0.25, 0.50, 1 and 10 ppm concentrations (Fig. 2B). Each evaporated CuI thin film substrate from the same batch was immersed in a 200-mL Erlenmeyer beaker containing cyanide solutions for 5 min while stirring. Each data point represents the average of triplicate measurements from the same CuI thin film after reacting with the same cyanide solution, with 1σ standard deviation. A nice linear response was found within the concentration range from 250 ppb to 10 ppm. The limit of detection was determined to be ∼100 ppb from 3σ standard deviations above the background. Common interfering anions such as SO42−, ClO4−, and NO3−, and PO43− did not affect the CN− detection even at much high concentration levels.
In summary, we present here the use of cuprous iodide thin films which were evaporated on silicon wafer slides as a capturing element for the direct detection of cyanide without sample pretreatment. The copper(I) ions react with CN− ion, and the resulting CuCN can be easily detected by normal (non-resonant or surface-enhanced) Raman scattering. Even lower detection limit can be expected to achieve by varying the coating thickness of CuI, or the integration of silver nanostructure-based surface-enhanced Raman scattering, as well as increasing the incubation time with the cyanide-containing solutions. Coupled with portable Raman instruments,14 our approach can be easily adapted to be used for the field monitoring of aqueous free cyanide.
The authors are grateful to the Lord Foundation of Duke University for financial support, and to the Shared Materials Instrumentation Facility (SMIF) at Duke University for use of the SEM and XRD. We also thank Michelle Gignac for her technical assistance.
Notes and references
- R. R. Dash, A. Gaur and C. Balomajumder, J. Hazard. Mater., 2009, 163, 1 CrossRef CAS.
- L. Nelson, J. Emerg. Nurs., 2006, 32(4), S8 CrossRef.
- R. Neufeld, J. Greenfield and B. Rieder, Water Res., 1986, 20, 633 CrossRef CAS.
- Cyanide in Water and Soil: Chemistry, Risk, and Management. ed. D. A. Dzombak, D. A. Dzombak, R. S. Ghosh, and G. M. Wong-Chong. Taylor & Francis Group: Boca Raton, FL. 2006 Search PubMed.
- (a) P. Lundquist, H. Rosling and B. Sorbo, Clin. Chem., 1985, 31, 591 CAS; (b) C. Siontorou and D. P. Nikolelis, Anal. Chim. Acta, 1997, 355, 227 CrossRef CAS; (c) H. Sulistyarti, T. J. Cardwell, M. D. Luque de Castro and S. D. Kolev, Anal. Chim. Acta, 1999, 390, 133 CrossRef CAS; (d) G. Drochioiu, Talanta, 2002, 56, 1163 CrossRef CAS; (e) F. H. Zelder and C. Mannel-Croise, Chimia, 2009, 63, 58 CrossRef CAS.
- (a) S. Chung, S. Nam, J. Lim, S. Park and J. Yoon, Chem. Commun., 2009, 2866 RSC; (b) K. Yea, S. Lee, J. B. Kyong, J. Choo, E. K. Lee, S. Joo and S. Lee, Analyst, 2005, 130, 1009 RSC; (c) A. M. Westley and J. Westley, Anal. Biochem., 1989, 181, 190 CAS; (d) A. Taheri, M. Noroozifar and M. Khorasani-Motlagh, J. Electroanal. Chem., 2009, 628, 48 CrossRef CAS.
- (a) L. Shang, L. H. Jin and S. J. Dong, Chem. Commun., 2009, 3077 RSC; (b) P. A. Mosier-Boss and S. H. Lieberman, Appl. Spectrosc., 2003, 57, 1129 CrossRef CAS; (c) W. R. Premasiri, R. H. Clarke, S. Londhe and M. E. Womble, J. Raman Spectrosc., 2001, 32, 919 CrossRef CAS; (d) W. B. Knighton, E. C. Fortner, A. J. Midey, A. A. Viggiano, S. C. Herndon, E. C. Wood and C. E. Kolb, Int. J. Mass Spectrom., 2009, 283, 112 Search PubMed; (e) Y. Yan, H. Li and M. L. Myrick, Appl. Spectrosc., 2000, 54, 1539 CrossRef CAS.
- (a) Q. Lu, G. E. Collins, T. Evans, M. Hammond, J. Wang and A. Mulchandani, Electrophoresis, 2004, 25, 116 CrossRef CAS; (b) S. Chinica, S. akanaka, N. Takayama, N. Tsuji, S. Takou and K. Ueda, Anal. Sci., 2001, 17, 649 CAS.
- (a) A. Amine, M. Alafandy, J. M. Kauffmann and M. N. Pekli, Anal. Chem., 1995, 67, 2822 CrossRef CAS; (b) J. L. Lee and I. Karube, Anal. Chim. Acta, 1995, 313, 69 CrossRef CAS; (c) N. Sadeg and H. Belhadj-Tahar, Toxicol. Environ. Chem., 2009, 91, 419 CrossRef CAS; (d) M. Aguilar, A. Farrán and V. Marti, J. Chromatogr., A, 1997, 778, 397 CrossRef CAS.
- (a) A. Sano, M. Takezewa and S. Takitani, Anal. Chim. Acta, 1989, 225, 351 CrossRef CAS; (b) D. Scilia, S. Rubio, D. Pérez-Bendito, N. Maniasso and E. A. G. Zagatto, Analyst, 1999, 124, 615 RSC; (c) S. Kage, T. Nagata and K. Kudo, J. Chromatogr., B: Biomed. Sci. Appl., 1996, 675, 27 CrossRef CAS.
- (a) T. Vo-Dinh, TrAC, Trends Anal. Chem., 1998, 17, 557 CrossRef CAS; (b) Raman Spectroscopy for Chemical Analysis. Ed. R. L. McCreery, Wiley, 2000 Search PubMed; (c) M. D. King, S. Khadka, G. A. Craig and M. D. Mason, J. Phys. Chem. C, 2008, 112, 11751 CrossRef CAS.
- G. C. Lukey, J. S. J. van Deventer, S. T. Huntington, R. L. Chowdhury and D. C. Shallcross, Hydrometallurgy, 1999, 53, 233 CrossRef CAS.
- G. Laufer, T. F. Schaaf and J. T. Huneke, J. Chem. Phys., 1980, 73, 2973 CrossRef CAS.
- (a) F. Yan and T. Vo-Dinh, Sens. Actuators, B, 2007, 121, 61 CrossRef; (b) L. X. Quang, C. Lim, G. H. Seong, J. Choo, K. J. Dob and S. Yoo, Lab Chip, 2008, 8, 2214 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Further experimental details. SEM, and XRD characterization of evaporated cuprous iodide films, and Raman measurement conditions. See DOI: 10.1039/c0ay00085j |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |
