High temperature imidazolium ionic polymer for gas chromatography†
Wen-Yueh
Ho
a,
Yu-Nung
Hsieh
b,
Wei-Chao
Lin
a,
Chang Long
Kao
b,
Ping-Chih
Huang
c,
Ching-Fen
Yeh
d,
Chen-Yun
Pan
e and
Chun-Hsiung
Kuei
*b
aDepartment of Cosmetic Science, Chia Nan University of Pharmacy and Science, 60 Erh-Jen Road, Sec.1 Pao-An, Jen-Te Hsiang, Tainan, 717, Taiwan. E-mail: rickho@mail.chna.edu.tw
bDepartment of Chemistry, National Cheng Kung University, No.1, Ta-Hsueh Road, Tainan, 701, Taiwan. E-mail: kuei@mail.ncku.edu.tw; Fax: +886 6 2089092; Tel: +886 6 2089092
cDepartment of Chemical and Materials Engineering, Cheng Shiu University, No.840, Cheng Ching Rd., Niaosong, Kaohsiung, 83347, Taiwan
dDepartment of Medical Chemistry, Chia Nan University of Pharmacy and Science, 60 Erh-Jen Road, Sec.1 Pao-An, Jen-Te Hsiang, Tainan, 717, Taiwan
eGreat Engineering Technology Corp., 14F, No. 392 Yucheng Rd., Zuoying District, Kaohsiung City, 813, Taiwan
First published on 30th March 2010
Abstract
A new main-chain type ionic homopolymer GC stationary phase which has high separation efficiencies for alcohols, phthalates and PAHs and a maximum programmable temperature of 400 °C was prepared and studied.
Ionic liquids (ILs) are salts with relatively low melting points; they are usually liquid around ambient temperature. ILs have recently attracted a lot of interest due to their unique properties. Their minuscule vapor pressure and inflammability make them “green” solvents, in contrast to conventional organic solvents. Moreover, other properties such as solubility, conductivity, and thermal stability can be tuned by changing the substituted functional groups or the combination of cations and anions. These properties make ionic liquids multi-functional and designable. Detailed descriptions of these properties can be found in Ref. 1–7.
Recently, Armstrong et al. demonstrated a series of ionic liquids as the stationary phases for gas chromatography. The ionic liquids have many properties that match the requirements of GC stationary phase, including high solubility, thermal stability, chemical stability, viscosity, wettability, and most important of all, the designability. Many successful applications and detailed discussions are available in the literature.8–10
However, in spite ionic liquids normally having high thermal stability, they are still not comparable to some of the conventionally thermal-stable stationary phases. It could be due to the ion evaporation or decomposition under high temperature. Polymerized ionic liquids or ionic liquid polymers (PILs) show great promise. Ions are tethered to the backbone of the polymer. Despite the loss of ion mobility, the mechanical strength of the whole substrate increases as the does thermal stability.11,12 The comb-like imidazolium based ionic liquid polymers show a decomposition temperature ranging from 280–380 °C. More recently, Armstrong et al. demonstrated a series of dicationic phosphonium and tricationic based ionic liquids which have been proven to have high thermal stability, the thermal stability of these stationary phases achieved 350–400 °C.13–17 Nevertheless, in order to improve the thermal stability of the coated GC stationary phases, sometimes partially or highly cross-linking treatment for the stationary phases should be applied. Highly cross-linking process make stationary phases high temperature resistant, but lose their flexibility. Too rigid a stationary phases will cause poor separation efficiency at low temperature. In this study, we present a new main chain type imidazolium ionic homopolymer as the new stationary phase for GC. The polymer was self-polymerized by a single monomer containing a chloride and a long-paired nitrogen terminals. With combination of NTf2− anion, the polymer has a decomposition temperature around 450 °C (see ESI†). Moreover, the proposed ionic polymer as the new stationary phase for GC shows great separation efficiencies to polar and highly polarizable solutes, including alcohols, phthalates, and PAHs. Also, it does not lose separation efficiency at low temperature.
The polymer used in this study is a homo-polymerization process using 1-(3-chlorohexyl)imidazole (ImC6Cl) as the monomer, followed by self-polymerization reaction to form PImC6Cl, and then a metathesis reaction was carried out to replace the chloride to NTf2− by using LiNTf2 aqueous solution. The processes are described in the ESI.† The structure of the proposed phase is in Fig. 1. Interestingly, another PIL (PImC6PF6) was also prepared. However, both PImC6Cl and PImC6PF6) show poor thermal stability in thermal gravimetric analysis (TGA) which do not match the goal of this study. In TGA results (see ESI†, significant weight loss temperatures of 300, 350, and 450 °C were observed for PImC6Cl, PImC6PF6, and PImC6NTf2, respectively. Of note, previous studies also suggest that the NTf2 containing GC stationary phases not only show high thermal stability, they also have better separation efficiencies.11,12 Therefore, only PImC6NTf2, was selected as the stationary phase in this study.
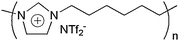 | ||
| Fig. 1 Structure of the proposed ionic polymer PImC6NTf2. | ||
The PIL capillary columns were prepared using the static coating method. The selected PILs were dissolved in acetone at a concentration of 0.32% (w/v). Using the evaluation procedure for the static coating method,14,18 the film thicknesses of the prepared PIL capillary columns were calculated as 0.20 μm.
The thermal stability of the stationary phase is a very important requirement for gas chromatography. Fig. 2 shows a comparison of the baseline test of the PImC6NTf2 column with a commercially available 95% dimethyl, 5% diphenylpolysiloxane column (Rtx-5 ms). In Fig. 2, dot-line describes the FID response for thermal test of Rtx-5 ms column in GC. The FID signal response was low at low temperature. The baseline increased with increases in oven temperature. As the oven temperature reached 330 °C, the baseline rose to higher than 150 pA, which means column bleeding occurred (min. bleeding temp: 330 °C, max. program temp: 360 °C). At a temperature of 360 °C, the FID response increased rapidly. The heating was stopped in order to protect the column. For the PImC6NTf2 column, the FID response remained almost unchanged under 330 °C, after which it started increasing. According to the figure, at a temperature of 380 °C, the baseline is under 100 pA, which means only minor bleeding occurred. The suggested minimum bleeding temperature of the PImC6NTf2 column is around 380 °C. In order to investigate the maximum programmable temperature, the oven temperature was slowly increased to observe the FID response, and a standard solution of naphthalene was routinely tested. After repeatedly heating the column to a temperature of 400 °C, the RSD of retention factor (k′) was 0.75% (n = 6). For the practice application of the PImC6NTf2 column, mixtures of n-alkanes, n-alcohols, phthalates, and PAHs were tested.
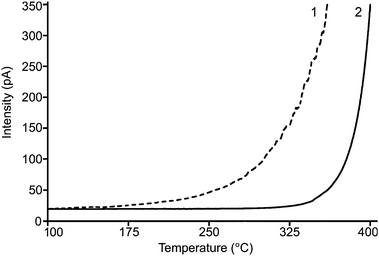 | ||
| Fig. 2 GC-FID response for (1) 95% dimethyl, 5% diphenylpolysiloxane column (Rtx-5 ms; Column dimensions: 30 m ×0.25 mm i.d. ×0.25 μm.); (2) PImC6NTf2 column; (Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm). Oven temperature program: 100 to 360 °C (or 400 °C, for PImC6NTf2) at 15 °C min−1 heating rate, 1 mL min−1 flow rate. | ||
Fig. 3 shows the chromatogram of n-alkanes (C12–C36, omitting C27, C29, C31, C33, and C35). Good resolution was achieved, but poor efficiency. It seems the interactions between n-alkanes with this stationary phase were relatively small. The retention times for the tested n-alkanes were short, all the tested alkanes were eluted out within 16 min. For the n-alkanes (n > 17), front tailing effect were observed, which indicates low interaction with stationary phase. This can be attributed to the large number of imidazolium cations and NTf2− anions presented on the main chain of the polymer, result in making the stationary phase more polar.
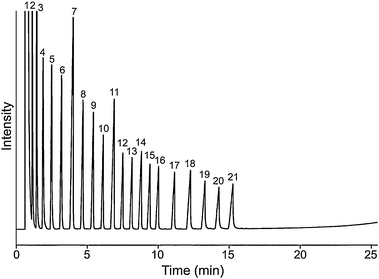 | ||
Fig. 3 Chromatogram for n-alkanes each 100 μg mL−1 in PImC6NTf2 column, peak (1): hexane; peaks (2–16): n-alkanes from C12 to C26; peaks (17–21): n-alkanes C28, C30, C32, C34, and C36; Oven temperature program: 40 to 300 °C at 10 °C min−1; flow rate: N2 1.0 mL min−1; 1 μL, split ratio: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm. 1; Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm. | ||
However, for polar solutes such as those shown in Fig. 4, n-alcohols (1-butanol, 1-hexanol, 1-octanol, 1-nonanol, 1-decanol, and 1-dodecanol) show extraordinary separation efficiency in the column, and the tailing effect for alcohols is pretty minor. Fig. 5 shows the chromatogram of a mixture of six phthalates tested in the column. Excellent separation efficiency was obtained; the peak shapes are symmetric and sharp. In order to emphasize the benefit of the proposed stationary phase, a mixture of fifteen PAHs including very low volatility compounds such as dibenzo[a,i]pyrene and coronene (b.p. 525 °C), were tested. Because these compounds have very low volatility, they require relatively high temperature to be purged out of a GC column. This is a problem if the equipped GC column is not sustainable under high temperature. Normally, for the separation of low-volatility compounds, other separation methods are recommended, such as HPLC. As can be seen in Fig. 6, all of the tested PAHs were eluted out of the column. The peak shapes are symmetrical and the separation efficiencies for the PAHs were high, except for peak (10–12). This is due to the volatilities of (10–12) being similar, and the length of the tested column being only 10 m; better separation can be achieved by extending the length of the column. The results show that the proposed stationary phase has good separation efficiency for polar solutes, especially for aromatic solutes (For naphthalene, the HETP test is around 0.26 mm at 100 °C, N2 flow rate 36 cm s−1). This could be attributed to the structure of the polymer containing a large number of imidazolium rings. The π–π interaction contributed from the imidazolium ring increases the separation efficiency for aromatic solutes. To be worth mentioned, most ultra high temperature-resist stationary phases have poor separation efficiency at low temperature, because of their relatively rigid structure making them less flexibility to interact with solutes under low temperature. The PImC6NTf2 stationary phase not only has high separation efficiency at high temperature, but also can be used at low temperature. However, the straight hexyl chains on the polymer are not long enough for good contact with n-alkanes. A previous study12 suggested that the longer the attached alkyl chain on the comb-like imidazolium based polymer GC stationary phase, the higher the dispersion force it has. This could be result in poor separation efficiencies for non-polar compounds. Two additional PImC6NTf2 columns were produced and tested to prove that these columns are reproducible (results are listed in the ESI†). Good reproducibility was achieved, this can be attributed to the fact that the proposed synthetic procedure is facile; and the coating procedure is simple and reliable. Two of the three columns were routinely tested, after a few months of tests, only a slight retention time shift was observed (RSD < 0.8%).
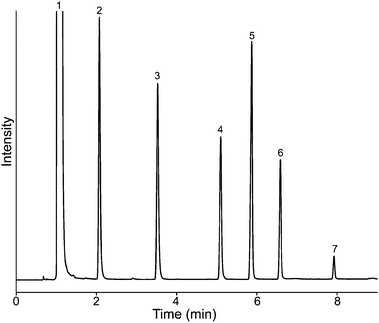 | ||
Fig. 4 Chromatogram for n-alcohols (concentrations of 100–300 μg mL−1) in PImC6NTf2 column, peak (1) solvent: methanol; (2) 1-butanol; (3) 1-hexanol; (4) 1-octanol; (5) 1-nonanol; (6) 1-decanol; (7) 1-dodecanol. Oven temperature program: 40 to 140 °Cat 10 °C min−1; flow rate: N2 1.0 mL min−1; 1 μL, split ratio: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Column dimensions: 10 m × 0.25 mm i.d. × 0.20 μm. 1; Column dimensions: 10 m × 0.25 mm i.d. × 0.20 μm. | ||
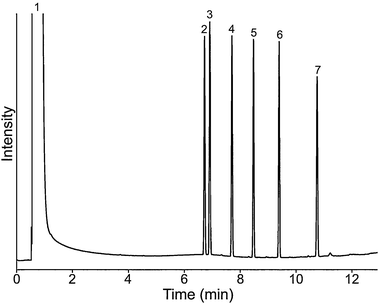 | ||
Fig. 5 Chromatogram for EPA phthalate mix each 100 μg mL−1 in PImC6NTf2 column, peak (1) solvent: hexane; (2) dimethylphthalate; (3) diethylphthalate; (4) di-n-butylphthalate; (5) benzylbutylphthalate; (6) di-n-octylphthalate; (7) bis(2-ethylhexyl)phthalate. Oven temperature program: 40 to 300 °Cat 15 °C min−1; flow rate: N2 1.2 mLmin−1; 1 μL, split ratio: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm. 1; Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm. | ||
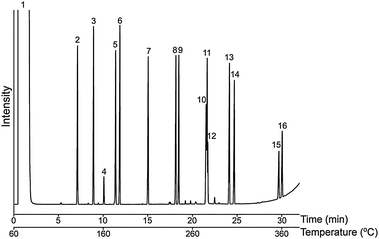 | ||
Fig. 6 Chromatogram for 15 PAHs mix (concentrations of 100–300 μg mL−1) in PImC6NTf2 column, peak (1) solvent: hexane; (2) naphthalene; (3) biphenyl; (4) 1,2-dihydroacenaphthylene; (5) acenaphehylene; (6) fluorene; (7) anthracene; (8) fluoranthene; (9) pyrene; (10) benz(a)anthracene; (11) triphenylene; (12) naphthacene; (13) benz(e)acephenanthrylene; (14) benzo(e)pyrene; (15) dibenzo(a,i)pyrene; (16) coronene. Oven temperature program: 60 to 380 °Cat 10 °C min−1; flow rate: N2 1.2 mL min−1; 1 μL, split ratio: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm. 1; Column dimensions: 10 m ×0.25 mm i.d. ×0.20 μm. | ||
In summary, the proposed ionic polymer GC stationary phase shows high thermal stability, and separation efficiency. The results suggest that PImC6NTf2 has extraordinary separation efficiency and thermal stability for low-volatility and polar solutes. Unlike most conventional polar stationary phases have less thermal stability, the PImC6NTf2 column can be used to analyse very low-volaitilty compounds. Furthermore, the proposed stationary phase has wide operating temperature which can expend the applicability of the GC column. The further work is in progress to design more new ionic polymers, including derivatives of the proposed ionic polymer. The properties and applications of the new materials are being studied.
Notes and references
- J. L. Anderson, D. W. Armstrong and G. T. Wei, Anal. Chem., 2006, 78, 2893–2902 CAS.
- M. Koel, Crit. Rev. Anal. Chem., 2005, 35, 177–192 CrossRef CAS.
- G. A. Baker, S. N. Baker, S. Pandey and F. V. Bright, Analyst, 2005, 130, 800–808 RSC.
- S. Pandey, Anal. Chim. Acta, 2006, 556, 38–45 CrossRef CAS.
- J. F. Liu, J. A. Jonsson and G. B. Jiang, TrAC, Trends Anal. Chem., 2005, 24, 20–27 CrossRef CAS.
- K. J. Fraser, E. I. Izgorodina, M. Forsyth, J. L. Scott and D. R. MacFarlane, Chem. Commun., 2007, 3817–3819 RSC.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS.
- D. W. Armstrong, L. F. He and Y. S. Liu, Anal. Chem., 1999, 71, 3873–3876 CrossRef CAS.
- J. L. Anderson, J. Ding, T. Welton and D. W. Armstrong, J. Am. Chem. Soc., 2002, 124, 14247–14254 CrossRef CAS.
- J. L. Anderson and D. W. Armstrong, Anal. Chem., 2003, 75, 4851–4858 CrossRef CAS.
- Y. N. Hsieh, W. Y. Ho, R. S. Horng, P. C. Huang, C. Y. Hsu, H. H. Huang and C. H. Kuei, Chromatographia, 2007, 66, 607–611 CrossRef CAS.
- Y. N. Hsieh, R. S. Horng, W. Y. Ho, P. C. Huang, C. Y. Hsu, T. J. Whang and C. H. Kuei, Chromatographia, 2008, 67, 413–420 CrossRef CAS.
- J. L. Anderson, R. F. Ding, A. Ellern and D. W. Armstrong, J. Am. Chem. Soc., 2005, 127, 593–604 CrossRef CAS.
- J. L. Anderson and D. W. Armstrong, Anal. Chem., 2005, 77, 6453–6462 CrossRef CAS.
- M. Qi and D. W. Armstrong, Anal. Bioanal. Chem., 2007, 388, 889–899 CrossRef CAS.
- Z. S. Breitbach and D. W. Armstrong, Anal. Bioanal. Chem., 2008, 390, 1605–1617 CrossRef CAS.
- T. Payagala, Y. Zhang, E. Wanigasekara, K. Huang, Z. S. Breitbach, P. S. Sharma, L. M. Sidisky and D. W. Armstrong, Anal. Chem., 2009, 81, 160–173 CrossRef CAS.
- J. Bouche and M. Verzele, J. Gas Chromatogr., 1968, 6, 501–505 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section; Thermal gravimetric analysis TGA result; Column QC tests. See DOI: 10.1039/b9ay00313d |
| This journal is © The Royal Society of Chemistry 2010 |
