Microwave-assisted digestion in closed vessels: effect of pressurization with oxygen on digestion process with diluted nitric acid
Cezar Augusto
Bizzi
a,
Érico
Marlon de Moraes Flores
a,
Rochele
Sogari Picoloto
a,
Juliano
Smanioto Barin
b and
Joaquim
Araújo Nóbrega
*cd
aDepartamento de Química, Universidade Federal de Santa Maria, Instituto Nacional de Ciência e Tecnologia de Bioanalítica, 97105-900, Santa Maria, RS, BrazilCampinas, SP, Brazil
bDepartamento de Tecnologia e Ciência dos Alimentos, Universidade Federal de Santa Maria, 97105-900Santa Maria, RS, Brazil
cDepartamento de Química, Universidade Federal de São Carlos, 13565-905, São Carlos, SP, Brazil
dInstituto Nacional de Ciências e Tecnologias Analíticas Avançadas, Campinas, SP, Brazil. E-mail: djan@terra.com.br; Fax: +55-16-3351-8088; Tel: +55-16-3351-8088
First published on 7th April 2010
Abstract
The efficiency of diluted nitric acid solutions under oxygen pressure for decomposition of bovine liver was evaluated using microwave-assisted wet digestion. Calcium, Cu, Fe, Mg, Mn and Zn were determined by inductively coupled plasma optical emission spectrometry (ICP OES). Efficiency was evaluated by determining the residual carbon content (RCC) by ICP OES and residual acidity in digests. Samples (up to 500 mg) were digested using nitric acid solutions (0.1, 0.5, 1, 2, 3, 7, and 14 mol L−1 HNO3) and the effect of oxygen pressure was evaluated using pressures of 0.5, 1, 1.5 and 2 MPa. It was demonstrated that 2 and 0.5 mol L−1 nitric acid solutions may be used for efficient digestion of 500 and 100 mg of bovine liver, respectively, with oxygen pressures ranging from 0.5 to 2 MPa. Using these conditions, less than 0.86 and 0.21 mL of concentrated nitric acid were necessary to digest 500 and 100 mg of sample, respectively. Similar digestion efficiencies for both conditions were obtained under pressures of O2 ranging from 0.5 to 2 MPa. The residual acidities in final digests were lower than 24% when compared to the initial amount of acid used for digestion. The accuracy of the proposed procedure was evaluated using certified reference materials of bovine liver and bovine muscle. Using a solution of 2 mol L−1 with oxygen pressure of 0.5 MPa for 500 mg of sample, the agreement with certified values ranged from 96 to 105% (n = 5). Using the proposed procedure with diluted nitric acid it was possible to obtain RCC values lower than 15% that is important for minimizing the generation of laboratory residues and improving limits of detection.
1. Introduction
The use of concentrated acids for conversion of solid samples to solutions has been adopted for more than five centuries in chemical analysis. Nowadays, starting in the last decades of the 20th century, modern sample preparation for inorganic analysis has been gradually modified by using microwave-assisted heating.1,2 Currently, many procedures for digestion of biological samples are based on the use of closed vessels in cavity-oven systems.1–5 Both the evolution of the design of these reaction vessels and the availability of new materials chemically, mechanically and thermally resistant led to the development of some digestion procedures at high pressure and temperature (13 MPa and 320 °C, respectively).6The possibility of working at high pressures causes an increase of the boiling point of the reagents and this aspect has important thermodynamic and kinetic implications. For instance, the oxidant properties of nitric acid are improved at high temperatures and due to this condition the digestion of samples containing high content of organics can be carried out without addition of auxiliary reagents, such as sulfuric or perchloric acids.1 The use of concentrated acids may be necessary when looking for drastic reaction conditions. However, the use of concentrated reagents is hazardous, requires dilution of the digests before analytes determination, and generates high volumes of concentrated acids as effluents.7,8
Performing reactions at high pressure and temperature may allow a reduction of the acid concentration without decreasing the efficiency of digestion. This hypothesis has been investigated in recent studies and the feasibility of using diluted solutions containing as low as 2 mol L−1 of nitric acid has been evaluated.9–12 It was experimentally demonstrated that the efficiency of using diluted acids for digestion derived from the temperature gradient inside the reaction vessel during the initial steps of sample digestion and due to the presence of oxygen in the closed-vessel atmosphere. The former aspect is related to the non-absorption of microwave radiation by the gas phase and the latter one implies that oxygen in gas phase can improve the oxidation processes. The combination of both aspects results in that reaction products can be oxidized also at the upper atmosphere of the reaction vessel and further reabsorbed in the liquid phase. The temperature gradient causes a less pronounced pressure increment in the initial digestion steps and consequently this avoids a too fast increase of temperature of the liquid phase. Again, it implies that the absorption of formed gaseous products will be more effective during these steps. It was experimentally demonstrated that the simultaneous occurrence of these processes led to a regeneration of nitric acid according to the following reactions:12
| (CH2)n + HNO3(aq) → CO2(g) + NO(g) + H2O(l) | (Eq. 1) |
| 2 NO(g) + O2(g) → 2 NO2(g) | (Eq. 2) |
| 2 NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq) | (Eq. 3) |
| 2 HNO2(aq) → H2O(aq) + NO2(g) + NO(g) | (Eq. 4) |
The occurrence of processes described in eqn (1–4) allows the regeneration of nitric acid and the reaction cycle remains effective when two conditions are simultaneously obeyed:13i) there are organic compounds in the sample being decomposed and generating NO (eqn (1)), and ii) there is oxygen in the gas phase to increase NO2 regeneration (eqn (2)). In a subsequent step, water reacts with NO2 generating HNO3 and HNO2 (eqn (3)). Furthermore, HNO2 decomposes to NO2 and NO which follows the sequence described by eqn (2) in the gaseous phase. This means that the availability of oxygen as a reagent causes an important effect on the efficiency of digestion process.
Based on these processes, it can be supposed that the pressurization of the digestion vessel with oxygen could improve the efficiency of digestion when using diluted nitric acid as reagent. This hypothesis was systematically investigated in the present work. In addition, the reduction as much as possible of the amount of nitric acid needed for the digestion process, in order to minimize the blank values, and also the decrease of the consumption of reagents and generation of laboratory residues was tried.
2. Experimental
2.1 Instrumentation
A microwave oven (Multiwave 3000 microwave sample preparation system, Anton Paar, Graz, Austria) equipped with 8 high-pressure quartz vessels was used in the experiments. The internal volume of vessels was 80 mL and the maximum operational temperature and pressure was set at 280 °C and 8 MPa, respectively. Pressure and temperature were monitored in each vessel for all the runs.Analytes were determined by ICP OES using an axial view configuration spectrometer (Spectro Ciros CCD, Spectro Analytical Instruments, Kleve, Germany). Nebulization was performed through a cross-flow nebulizer coupled to a Scott double path type nebulization chamber. Plasma operating conditions and selected wavelengths used for analytes determination are listed in Table 1, and they were used as recommended by the instrument manufacturer.14 For the determination of residual carbon content (RCC),15 digested solutions were sonicated with a ultrasonic probe16 (VCX 130 PB, 130 W, 20 kHz, Sonics and Materials Inc., Newton, CT, USA) before the determination by ICP OES in order to remove the volatile carbon compounds.17 Argon 99.996% (White Martins-Praxair, São Paulo, SP, Brazil) was used in ICP OES determinations for plasma generation, nebulization and also as auxiliary gas.
| Parameter | ICP OES |
|---|---|
| Radio-frequency power (W) | 1600 |
| Plasma gas flow rate (L min−1) | 14.0 |
| Auxiliary gas flow rate (L min−1) | 1.0 |
| Nebulizer gas flow rate (L min−1) | 0.85 |
| Spray chamber | double path, Scott type |
| Nebulizer | crossflow |
| Observation view | axial |
| Emission line (nm) | |
| C (I) | 193.091 |
| Ca (II) | 393.366 |
| Cu (I) | 324.752 |
| Fe (I) | 238.204 |
| Mg (I) | 285.213 |
| Mn (II) | 257.610 |
| Zn (I) | 213.857 |
Results for residual acidity were obtained using a titration system (Titrando 836, Metrohm, Herisau, Switzerland) equipped with a magnetic stirrer (module 803 Ti Stand), 20 mL burette (Dosino 800) and pH electrode (LL Electrode plus, model 6.0262.100).
2.2 Sample, reagents and standards
Preliminary experiments were carried out using a bovine liver sample that was purchased in a local market. This sample was freeze-dried using a Model LH 2000/3 Lyophilizer (Terrone Fauvel, São Carlos, SP, Brazil) and ground using a cryogenic mill (Spex Certi Prep, model 6750, Metuchen, NJ, USA). The final particle diameter was below 102 μm. The accuracy was evaluated using standard reference materials (SRM) of bovine liver (SRM NIST 1577) and bovine muscle (SRM NIST 8414) both produced by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). Samples were accurately weighed using an analytical balance (model AY 220, max. 220 g, 0.1 mg of resolution, Shimadzu, Kyoto, Japan).Distilled-deionized water (Milli-Q, 18.2 MΩ cm, Millipore. Bedford, MA, USA) and analytical-grade nitric acid (Merck, Darmstadt, Germany) were used to prepare samples and standards. Reference solutions containing 25 to 500 mg L−1 of C were prepared by dissolution of citric acid for RCC determinations (Merck) in water. A 0.1 mol L−1 of KOH (Merck) solution was used to residual acidity determination. Glass and quartz material were soaked in 1.4 mol L−1 HNO3 for 24 h and thoroughly washed with water before use.
2.3 Microwave-assisted acid digestion
Sample aliquots of 100 and 500 mg were directly inserted into the closed quartz vessels. The digestion efficiency of nitric acid solutions (6 mL) was evaluated in the following concentrations: 0.1, 0.5, 1, 2, 3, 7, and 14 mol L−1. After closing and capping the rotor, vessels were pressurized with 0.5, 1, 1.5 and 2 MPa of oxygen using the valve originally designed for pressure release after conventional acid sample digestion. The same procedure was carried out without oxygen pressure and, additionally, under argon pressure (2 MPa). Then the rotor was placed inside the oven, and the microwave heating program was started by applying (i) 1000 W with a ramp of 5 min, (ii) 1000 W for 10 min, and (iii) 0 W for 20 min (cooling step).18 The maximum temperature observed for all procedures ranged from 230 to 250 °C. Temperature and pressure curves presented the same behaviour and practically no differences were observed in their shape. After digestion, the pressure of each vessel was carefully released inside a fume hood. In this work, each run was performed with 4 vessels. The resulting solutions were transferred to 30 mL polypropylene vials and diluted up to the mark with water. Cleaning of vessels was carried out with 6 mL of concentrated HNO3 in the microwave oven at 1000 W for 10 min and 0 W for 20 min for cooling. Final digests were analyzed by ICP OES for both RCC and analyte determination. Residual acidities were evaluated by titration.3. Results and discussion
Preliminary experiments were performed in order to evaluate the influence of oxygen pressure on sample digestion when using nitric acid solution with and without oxygen at 2 MPa. This pressure was arbitrarily selected and because of safety reasons higher pressures were not tested. Sample masses of 100 and 500 mg were employed and both RCC and residual acidity were determined in digests. Results for 500 mg of bovine liver are shown in Fig. 1. Without using oxygen pressure, a lower RCC (< 9%) was only obtained if a nitric acid solution containing at least 7 mol L−1 was used at maximum temperature and pressure available in the microwave oven, 280 °C and 8 MPa, respectively. Under these conditions, a clear solution was always observed. However, using 3 mol L−1 HNO3, final digests presented a yellow colour and RCC values were about 20%. The digestion was not effective when using 2 mol L−1 HNO3 and solid residues remained as suspended particles in a brown coloured digest. As expected, a similar behaviour was observed by using 1 mol L−1 HNO3 and a RCC as high as 50% was obtained. On the other hand, for 100 mg of sample (results not shown), colourless final digests were obtained for digestions using concentrated HNO3 (RCC = 4.2%) and also when using solutions from 1 to 7 mol L−1 (RCC = 8.7 and 4.9%, respectively). For 100 mg of sample, more diluted nitric acid solutions were also tested (0.1 and 0.5 mol L−1) and a yellow colour was observed with corresponding values of RCC higher than 32%.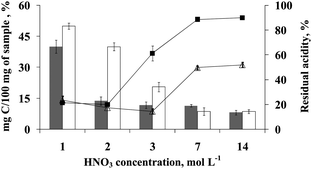 | ||
| Fig. 1 Effect of nitric acid concentration on digestion of 500 mg of sample mass; effectiveness of organic matter digestion performed under oxygen pressure (gray bars) and without oxygen pressure (white bars). Lines represent the residual acidity obtained from digestion performed under oxygen pressure (-■-) and without oxygen pressure (-△-). | ||
When using 2 MPa of oxygen and concentrated nitric acid or 7 mol L−1 (500 mg of sample), no significant difference was observed in RCC values when compared with results without using O2. In this case, the presence of O2 has no detectable effect and these results may be explained by the high oxidant environment even without O2. However, a more effective digestion was obtained with 2 MPa oxygen pressure and nitric acid solutions containing 2 and 3 mol L−1 (RCC = 13.8 and 11.7, respectively) could be used resulting in a colourless digests. Therefore, it was possible to reduce the nitric acid concentration about 3.5 times but still obtaining a similar digestion efficiency (2 mol L−1 HNO3, RCC = 13.8%). The effect of O2 was less pronounced when using lower HNO3 concentrations and this effect may be explained by the low oxidation power of a solution containing 1 mol L−1 HNO3 even associated with 2 MPa oxygen. Similar behaviour was observed when working with 100 mg of sample. However, for 100 mg of sample, even a nitric acid solution as diluted as 0.5 mol L−1 led to RCC values (7.5%) similar to those obtained using more concentrated nitric acid solutions but without oxygen pressure.
As reported in previous studies,9,10,12 the oxidant action of nitric acid may be improved if a regenerating process occurs, which is mainly dependent on the amount of oxygen available in gas phase during the oxidation of the organic matter. This reaction could be effective with diluted nitric acid if digestion vessels were pressurized with oxygen. This hypothesis was reinforced by the results obtained for residual acidity shown in Fig. 1. It may be seem that residual acidity in the same reaction conditions was higher with oxygen pressure and may be explained due to a regeneration process. In addition, after digestion it was possible to observe final digests with colourless fumes inside the reaction vessel when digestion was performed under oxygen pressure. This may be due to the absence of NO2 in the gas phase, probably owing to the regeneration process of nitric acid promoted by oxygen. The opposite behaviour was observed in final digests obtained from experiments performed without oxygen pressure, where brown fumes of NO2 were observed inside the reaction vessels.
In order to confirm if this process was related to oxygen or it was only caused by a pressure effect, digestion was performed with diluted nitric acid under an inert gas pressure. In this way, a mass of 500 mg of bovine liver was digested under 2 MPa of argon using a 2 mol L−1 HNO3 solution. It was possible to observe a yellowish colour in digests with solid residues remaining as suspended particles (RCC values were higher than 45%). These values are higher than those obtained when using the same pressure but with oxygen (colourless digests without solids in suspension, RCC = 13.8%) which confirms the effect of oxygen on digestion efficiency.
3.1 Influence of oxygen pressure
For evaluating the effect of oxygen pressure inside the digestion vessel on the efficiency of digestion, bovine liver was digested under different oxygen pressures, ranging from 0 to 2 MPa. For this test, solutions of 7 and 2 mol L−1 HNO3 were selected to digest 500 mg of sample and solutions of 7 and 0.5 mol L−1 HNO3 were chosen to digest 100 mg of sample.As it can be seen in Fig. 2, using sample masses of 500 mg the efficiency of digestion was not too dependent on the oxygen pressure from 0.5 to 2 MPa (for 2 or 7 mol L−1 HNO3). Using a solution of 2 mol L−1 HNO3 (Fig. 2, white bars) a suitable efficiency of digestion (RCC < 15%) was observed only when adding oxygen at pressures of 0.5, 1, 1.5, and 2 MPa. Without oxygen pressurization, the RCC of final digests was higher than 40% and solution presented a yellow aspect with solid residues. Digestions performed with 100 mg of sample presented a similar behaviour. During digestion using a solution of 0.5 mol L−1 HNO3 values of RCC were below 14% when digestion vessel was pressurized with oxygen (RCC = 12.8, 12.6, 13.8 and 7.5% for pressures of 0.5, 1, 1.5 and 2 MPa of oxygen, respectively). However, when this experiment was performed without adding oxygen the digestion presented a poor efficiency, resulting in yellowish colour solutions with solid residues and RCC higher than 32%.
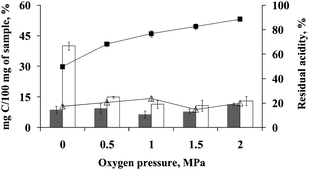 | ||
| Fig. 2 Effect of oxygen pressure on digestion of 500 mg of bovine liver. Digestions performed using 7 mol L−1 HNO3 (gray bars) or 2 mol L−1 HNO3 (white bars). Lines represent the residual acidity obtained from digestion performed with 7 mol L−1 HNO3 (-■-) and 2 mol L−1 HNO3 (-△-). | ||
The residual acidity after digestion carried out under different oxygen pressures was also determined when the digestion was performed with 7 mol L−1 HNO3 for sample mass of 500 mg. It was possible to observe a decrease in acidity correspondent to the decrease of HNO3 concentration (Fig. 2). The residual acidity was lower than 24% when using 2 mol L−1 HNO3 under oxygen pressure ranging from 0 to 2 MPa. However, without oxygen pressure the digestion efficiency was lower and a yellowish final solution was observed. During digestions with sample masses of 100 mg, a decrease in residual acidity was observed only with lower concentrations of nitric acid. Using a solution of 7 mol L−1 HNO3, values of residual acidity were higher than 93% for both conditions of pressure (residual acidity of 93.3, 98, 98.9, 97 and 97.4% from digestions under pressures of 0, 0.5, 1, 1.5 and 2 MPa). Using a solution of 2 mol L−1 HNO3 for digestion under 2 MPa O2, the residual acidity was higher than 90%. However, for oxygen pressure of 0.5, 1 and 1.5 MPa, values of residual acidity were lower than 43%. In spite of a relatively low residual acidity obtained for digestion without oxygen (20.8%) the digestion efficiency was only 35% and a yellowish final solution was observed. Therefore, in the range of oxygen pressure evaluated in this work, no significant changes were observed and 0.5 MPa was selected for subsequent experiments.
3.2 Determination of Ca, Cu, Fe, Mg, Mn and Zn by ICP OES in bovine liver and bovine muscle
Accuracy was evaluated by employing SRMs of bovine liver and bovine muscle. Digestions of both SRMs were performed using sample masses of 500 mg (2 mol L−1 HNO3 solutions and 0.5 MPa O2) and results obtained for Ca, Cu, Fe, Mg, Mn and Zn determinations are presented in Table 2. Results obtained with digestion performed with the proposed procedure presented a good agreement with certified values (higher than 96%), even if digestion was performed with a solution of 2 mol L−1 HNO3. As observed in previous works19–21 the use of diluted nitric acid and vessels pressurized with oxygen resulted in low blank values. For 500 mg and using 2 mol L−1 HNO3 and 0.5 MPa O2, low blank values were obtained allowing limits of detection of 12, 18, 18, 12, 10, and 12 ng g−1 for Ca, Cu, Fe, Mg, Mn, and Zn, respectively.| Analyte | SRM NIST 1577a | SRM NIST 8414b | ||
|---|---|---|---|---|
| certified | found | certified | found | |
| a Bovine liver. b Bovine muscle. | ||||
| Ca | 124 ± 6 | 129 ± 6 | 145 ± 20 | 143 ± 8 |
| Cu | 193 ± 10 | 185 ± 2 | 2.84 ± 0.45 | 2.75 ± 0.11 |
| Fe | 268 ± 8 | 260 ± 7 | 71.2 ± 9.2 | 70.8 ± 1.8 |
| Mg | 604 ± 9 | 592 ± 3 | 960 ± 95 | 944 ± 23 |
| Mn | 10.3 ± 1.0 | 9.90 ± 0.52 | 0.37 ± 0.09 | 0.377 ± 0.013 |
| Zn | 130 ± 13 | 137 ± 2 | 142 ± 14 | 139 ± 1 |
4. Conclusions
The use of diluted nitric acid associated with addition of oxygen was proven to be a feasible and recommendable sample preparation procedure, reducing the volume of the reagents and the amount of digestion residues. Using digestion vessels under oxygen pressure (from 0.5 to 2 MPa), it was possible to bring into solution sample masses of up to 500 mg with only an equivalent amount of 0.86 mL of concentrated nitric acid. For digestion performed in the same conditions, but without oxygen pressure, 3 mL of concentrated nitric acid were necessary. These results represent a decrease of about 3.5-fold of the volume of concentrated nitric acid. A similar behaviour was observed during digestion sample mass of 100 mg. For procedures performed under oxygen pressure, only an equivalent volume of 0.21 mL of HNO3 was necessary as opposed to 0.43 mL of concentrated nitric acid needed in procedure performed without oxygen pressure. These results could be compared with those obtained in microwave-assisted UV digestion, under high temperature, where digestion of 75 mg of skimmed milk was performed with only 0.05 mL of concentrated nitric acid and with 1 mL of H2O2.22 In addition, a considerable reduction (about 20%) in residual acidity was observed, in relation to the initial nitric acid used during sample digestion. Another advantage could be related to unnecessary use of others reagents for sample digestion, such as H2O2, HCl and H2SO4, which represent a significant reduction on residues generation. It is important to mention that these aspects are in agreement with the recommendations of green chemistry. Finally, experimental data suggesting the formation of NO, its conversion to NO2 by O2 action, and regeneration of HNO3 may explain the chemical processes involved during the digestion and, additionally, improve the effectiveness of digestion using diluted nitric acid solutions.Acknowledgements
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Instituto Nacional de Ciência e Tecnologia de Bioanalítica and Instituto Nacional de Ciências e Tecnologias Analíticas Avançadas, Proc. Nr. 573672/2008-3 and 471436/2008-9), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Processes 2006/59083-9 and 2008/57808-1) for grants and fellowships.References
- G. Knapp, A. S. Panholzer and P. Kettisch, Pressure-controlled microwave-assisted wet digestion systems, in H. M. Kingston; S. J. Haswell; Microwave-enhanced chemistry. Fundamentals, sample preparation and applications, American Chemical Society, Washington, ( 1988) 423–451 Search PubMed.
- H. Matusiewicz, Wet digestion methods, in: Z. Mester and R. Sturgeon, Comprehensive analytical chemistry. Sample preparation for trace element analysis, vol. XLI, Elsevier, Amsterdam, 2003, 193–233 Search PubMed.
- E. M. M. Flores, A. P. F. Saidelles, J. S. Barin, S. R. Mortari and A. F. Martins, Hair sample decomposition using polypropylene vials for determination of arsenic by hydride generation atomic absorption spectrometry, J. Anal. At. Spectrom., 2001, 16, 1419–1423 RSC.
- F. E. Smith and E. A. Arsenault, Microwave-assisted sample preparation in analytical chemistry, Talanta, 1996, 43, 1207–1268 CrossRef CAS.
- A. Zlotorzynski, The application of microwave radiation to analytical and environmental chemistry, Crit. Rev. Anal. Chem., 1995, 25, 43–76 CrossRef CAS.
- G. Knapp, Der weg zu leistungsfähigen methoden der elementspurenanalyse in umweltproben, Fresenius' Z. Anal. Chem., 1984, 317, 213–219 CrossRef CAS.
- S. Ashoka, B. M. Peake, G. Bremner, K. J. Haegman and M. R. Reid, Comparison of digestion methods for ICP-MS determination of trace elements in fish tissues, Anal. Chim. Acta, 2009, 653, 191–199 CrossRef CAS.
- N. C. Peixoto, T. Rosa, E. M. M. Flores and M. E. Pereira, Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats, Toxicol. Lett., 2003, 146, 17–25 CrossRef CAS.
- G. C. L. Araújo, M. H. Gonzalez, A. G. Ferreira, A. R. A. Nogueira and J. A. Nóbrega, Effect of acid concentration on closed-vessel microwave-assisted digestion of plant material, Spectrochim. Acta, Part B, 2002, 57, 2121–2132 CrossRef.
- M. H. Gonzalez, G. B. Souza, P. V. Oliveira, L. A. Forato, J. A. Nóbrega and A. R. A. Nogueira, Microwave-assisted digestion procedures for biological samples with diluted nitric acid: identification of reaction products, Talanta, 2009, 79, 396–401 CrossRef CAS.
- M. A. Z. Arruda, Trends in sample preparation, Nova Science Publishers, New York, 2006, 304 p Search PubMed.
- J. T. Castro, E. C. Santos, W. P. C. Santos, L. M. Costa, J. A. Nóbrega and M. G. A. Korn, A critical evaluation of digestion procedures for coffee samples using diluted nitric acid in closed vessels for inductively coupled plasma optical emission spectrometry, Talanta, 2009, 78, 1378–1382 CrossRef CAS.
- F. A. Cotton, G. Wilkinson, C. A. Murilo and M. Bochmann, Advanced inorganic chemistry, 6th ed., Wiley-Interscience, New York, 1999, 1355p Search PubMed.
- Spectro Ciros CCD, software version 01/March 2003, Spectro Analytical Instruments GmbH & Co. KG: Kleve, Germany.
- A. Krushevska, R. M. Barnes, C. J. Amarasiriwaradena, H. Foner and L. Martines, Determination of the residual carbon content by inductively coupled plasma atomic emission spectrometry after decomposition of biological samples, J. Anal. At. Spectrom., 1992, 7, 845 RSC.
- E. M. M. Flores, M. F. Mesko, D. P. Moraes, J. S. F. Pereira, P. A. Mello, J. S. Barin and G. Knapp, Determination of halogens in coal after digestion using the microwave-induced combustion technique, Anal. Chem., 2008, 80, 1865–1870 CrossRef CAS.
- S. T. Gouveia, F. V. Silva, L. M. Costa, A. R. A. Nogueira and J. A. Nóbrega, Determination of residual carbon by inductively-coupled plasma optical emission spectrometry with axial and radial view configurations, Anal. Chim. Acta, 2001, 445, 269–275 CrossRef CAS.
- Anton Paar GmbH, Multiwave 3000®, Microwave Sample Preparation System; Software version v1.27-Synt, Graz, Austria, 2003.
- E. M. M. Flores, J. S. Barin, M. F. Mesko and G. Knapp, Sample preparations techniques based on combustion reactions in closed vessels–A brief overview and recent applications, Spectrochim. Acta, Part B, 2007, 62, 1051–1064 CrossRef.
- D. P. Moraes, M. F. Mesko, P. A. Mello, J. N. G. Paniz, V. L. Dressler, G. Knapp and E. M. M. Flores, Application of microwave induced combustion in closed vessels for carbon black-containing elastomers decomposition, Spectrochim. Acta, Part B, 2007, 62, 1065–1071 CrossRef.
- M. F. Mesko, D. P. Moraes, J. S. Barin, V. L. Dressler, G. Knapp and E. M. M. Flores, Digestion of biological materials using the microwave-assisted sample combustion technique, Microchem. J., 2006, 82, 183–188 CrossRef CAS.
- D. Florian and G. Knapp, High-temperature, microwave assisted UV digestion: a promising sample preparation technique for trace element analysis, Anal. Chem., 2001, 73, 1515–1520 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
